Abstract
Objectives
To optimize conversion of rutin to isoquercetin by commercial α-l-rhamnosidase using high hydrostatic pressure (HHP).
Results
The de-rhamnosylation activity of α-l-rhamnosidase for isoquercetin production was maximal at pH 6.0 and 50 °C using HHP (150 MPa). The enzyme showed high specificity for rutin. The specific activity for rutin at HHP was 1.5-fold higher than that at atmospheric pressure. The enzyme completely hydrolysed 20 mM rutin in tartary buckwheat extract after 2 h at HHP, with a productivity of 10 mM h−1. The productivity and conversion were 2.2- and 1.5-fold higher at HHP than at atmospheric pressure, respectively.
Conclusions
This is the first report concerning the enzymatic hydrolysis of isoquercetin in tartary buckwheat at HHP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rutin, isoquercetin, and quercetin are plant flavonoids with various biological activities. The antioxidant, anti-inflammatory, anti-carcinogenic, antidiabetic, and anti-allergic biological activities of isoquercetin (quercetin-3-O-glucoside) are higher than those of quercetin or rutin due to its higher water solubility and better intestinal absorption, respectively, but natural levels of isoquercetin are very low (Amado et al. 2009; Zhang et al. 2011; Makino et al. 2009). However, isoquercetin can be produced by de-rhamnosylation of rutin, which is present in relatively large amounts in tartary buckwheat, buckwheat, bracken ferns, red grapes, and various teas (Lee et al. 2006).
α-l-Rhamnosidase (EC3.2.1.40) cleaves α-l-rhamnose from glycosides such as rutin, naringin, and hesperidin (Park et al. 2005). De-rhamnosylation of rutin to produce isoquercetin using α-l-rhamnosidase from Bacillus litoralis strain C44 (Lu et al. 2012), Bifidobacterium breve (Zhang et al. 2015), and Aspergillus niger (You et al. 2010) has been reported.
High hydrostatic pressure (HHP) has been used for food processing including extraction of functional compounds from plants, inactivation of microorganisms, and denaturation of several enzymes. In enzymology, HHP is used to improve the stability and activity of some enzymes, including the commercial enzymes naringinase, Viscozyme, pectinase, cellulase, amylase, and arabinofuranosidase, over their activities at atmospheric pressure (atmospheric pressure) (Palaniyandi et al. 2015).
All of the previous studies on isoquercetin bioconversion from rutin were performed at atmospheric pressure and not under HHP conditions. Therefore, in the present study, we investigated the effect of HHP on the activity of commercial α-l-rhamnosidase for production of isoquercetin from rutin and buckwheat extract.
Methods
Materials
Rutin, isoquercetin, and quercetin were purchased from Sigma Aldrich. α-l-Rhamnosidase was purchased from Megazyme. Tartary buckwheat (Fagopyrum tataricum Gaertn.) was purchased from a local market (Daejeon, Korea). All other regents were purchased from Sigma Aldrich.
Enzyme assay
α-l-Rhamnosidase activity was assayed using 1 mM rutin with 5 % (v/v) dimethyl sulphoxide (DMSO) at 30–70 °C and pH 4–8 for 30 min. The reactions were performed at atmospheric pressure (0.1 MPa) and HHP (0.1–400 MPa) using an HHP instrument (TFS-2L, Toyo-Koatsu Innoway Co. Ltd., Hiroshima, Japan). One unit of the enzyme (U) was defined as the amount of enzyme releasing 1 µmol p-nitrophenol from p-nitrophenyl-α-l-rhamnopyranoside per min. All assays were performed in triplicate.
Rutin extraction from tartary buckwheat
100 g dried tartary buckwheat was extracted by steaming in 1000 ml of 70 % (v/v) ethanol at 80 °C for 2 h prior to filtering. The extract was evaporated at low pressure using a rotary vacuum evaporator at 60 °C, and the remaining water was removed by lyophilisation.
Analytical methods
Rutin, isoquercetin, and quercetin contents were determined using HPLC with a Zorbax Eclipse XDB-C18 column (4.6 × 250 mm, 5 µm) and eluted at 30 °C and 0.8 ml min−1 with a gradient of solvent A (3 % v/v acetic acid in water) and solvent B [3 % acetic acid in water/acetonitrile (1:1) v/v] from 75:25 to 40:60 for 24 min and then from 40:60 to 75:25 for 6 min. The eluate was monitored with a diode array detector at 350 nm.
Results and discussion
Effects of pH and temperature on enzyme activity at atmospheric pressure and HHP
The maximum activity of commercial α-l-rhamnosidase was at pH 6 and 50 °C under both atmospheric pressure and HHP conditions (Fig. 1); however, the relative activity at HHP was higher than at atmospheric pressure. [The maximum activities of α-l-rhamnosidase from B. breve (Zhang et al. 2015) was at pH 6.5 and 55 °C.]
Effect of pH (a) and temperature (b) on the commercial α-l-rhamnosidase activity at high hydrostatic pressure (open circles) and atmospheric pressure (filled circles). a The reactions were performed in 50 mM McIlvaine buffer (pH 4–8) containing 1 mM rutin with 5 % DMSO at 50 °C for 30 min. b The reactions were performed in 50 mM McIlvaine buffer (pH 6.0) containing 1 mM rutin with 5 % DMSO at 30–70 °C for 30 min. Error bars indicate the standard deviations of triplicate samples
The thermostability of α-l-rhamnosidase for isoquercetin production was determined at atmospheric pressure and HHP (Fig. 2). The half-lives of the enzyme reaction at atmospheric pressure at 30, 40, 50, 60, and 70 °C were 17.3, 12.6, 7.0, 4.1, and 2.1 h, respectively, and those under HHP were 48.5, 36.6, 28.5, 17.7, 10.3 h, respectively, two to fourfold higher under atmospheric pressure. These results indicated that the thermostability of the enzyme was enhanced at HHP. At 60 °C, recombinant α-l-rhamnosidase from B. breve retained more than 90 % of its initial activity after 20 h (Zhang et al. 2015), and the thermostabilities of some enzymes including β-galactosidase from Escherichia coli (Real et al. 2007), commercial naringinase (Pedro et al. 2007), and pectin methylesterase from A. aculeatus (Dirix et al. 2005) were two to fivefold increased using HHP. The activity and/or stability of over 25 enzymes were stimulated at HHP because of changes in enzyme structure through hydrogen bonding and hydrophobic interactions, changes in the reaction mechanisms, and changes in the substrate or solvent physical properties (Eisenmenger and Reyes-De-Corcuera 2009a, b).
Thermal inactivation of commercial α-l-rhamnosidase at 30 (open circles), 40 (open triangles), 50 (open squares), 60 (filled circles), and 70 °C (filled triangles) at atmospheric pressure (a) or high hydrostatic pressure (b). The enzymes were preincubated at 30–70 °C for varying time. At each time interval, the enzyme activity of a sample was assayed in 50 mM McIlvaine buffer (pH 6.0) containing 1 mM rutin with 5 % DMSO at 50 °C for 30 min. The experimental data for thermal deactivation were fit to a first-order curve, and the half-lives of the enzyme were calculated using Sigma Plot 10.0 Software. Error bars indicate the standard deviations of triplicate samples
Substrate specificity at atmospheric pressure and HHP
The substrate specificity of α-l-rhamnosidase was investigated using aryl-glycosides and flavonoids under atmospheric pressure and HHP conditions. The specific activities of commercial α-l-rhamnosidase for rhamnose-containing pNP-Rha, rutin (α-1,6 linkage), hesperidin (α-1,6 linkage), and naringin (α-1,2 linkage) at atmospheric pressure were 176, 96, 65, and 24 U ml−1, respectively, and there was no measurable activity for other aryl glycosides (Table 1). Thus, the commercial α-l-rhamnosidase hydrolyses only the rhamnose moiety and has activity for both α-1,2 and α-1,6 linkages with a preference for α-1,6 linkages. Furthermore, these results indicate a high specificity for rutin and production of only isoquercetin. The specific enzyme activities for pNP-Rha and rutin at HHP were 1.8- and 1.5-fold higher than atmospheric pressure, but the substrate specificity was unchanged. The activities of commercial hydrolases including naringinase, Viscozyme, pectinase, cellulase, amylase, and α-l-arabinofuranosidase were enhanced 1.2–2.3-fold at HHP compared to activity at atmospheric pressure (Palaniyandi et al. 2015; Real et al. 2007).
Optimization of enzyme reaction at HHP
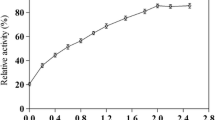
Production of isoquercetin was evaluated from 0 to 400 MPa with maximum production at 150 MPa (Fig. 3). Isoquercetin production decreased above 200 MPa; the commercial α-l-rhamnosidase was deactivated at 400 MPa. The effects of enzyme and substrate concentration were investigated at 150 MPa, with maximal isoquercetin production occurring at 3 U α-l-rhamnosidase ml−1 and 20 mM rutin (data not shown).
Effect of pressure on isoquercetin (open circles) production from rutin (filled circles). The reactions were performed in 50 mM McIlvaine buffer (pH 6.0) containing 0.1 U enzyme ml−1 and 1 mM rutin with 5 % DMSO at 50 °C and 0.1–400 MPa for 30 min using an HHP instrument. Error bars indicate the standard deviations of triplicate samples
The production of isoquercetin by commercial α-l-rhamnosidase was performed using 20 mM reagent-grade rutin at atmospheric pressure and HHP (Fig. 4). Under atmospheric pressure and HHP, the enzyme completely hydrolysed 20 mM reagent-grade rutin to isoquercetin within 150 and 90 min, respectively, corresponding to a productivity of 8 and 13.3 mM h−1, respectively. With HHP conditions, the productivity was 1.6-fold higher than with atmospheric pressure condition. The highest previously reported conversion yield and productivity from reagent-grade rutin were 97 % and 10 mM h−1, respectively, using recombinant α-l-rhamnosidase from B. breve (Zhang et al. 2015).
Time course of isoquercetin (open circles) production from regent-grade rutin (filled circles) by commercial α-l-rhamnosidase at atmospheric pressure (a) and high hydrostatic pressure (b). The reactions were performed in 50 mM McIlvaine buffer (pH 6.0) containing 3 U enzyme ml−1 and 20 mM rutin with 5 % DMSO at 50°C for 3 h. Error bars indicate the standard deviations of triplicate samples
Hydrolysis of rutin in tartary buckwheat extract to isoquercetin at atmospheric pressure and HHP
We extracted rutin from tartary buckwheat, and the amounts of rutin and quercetin in extract powder were 102 and 2.4 mg g−1; isoquercetin was not detected in the extract. The concentration of tartary buckwheat extract powder was adjusted to 12 %, which yielded 20 mM rutin. Isoquercetin was produced from tartary buckwheat extract using commercial α-l-rhamnosidase under the optimum conditions at atmospheric pressure and HHP (Fig. 5). At atmospheric pressure, the commercial α-l-rhamnosidase produced 13.4 mM isoquercetin within 180 min, with a productivity of 4.56 mM h−1 and conversion of 67 % (mol/mol). However, at HHP, the commercial α-l-rhamnosidase completely hydrolysed 20 mM rutin from tartary buckwheat within 120 min, corresponding to a productivity of 10 mM h−1, 2.2-fold higher than at atmospheric pressure.
Time course of isoquercetin (open circles) production from tartary buckwheat extract (rutin, filled circles) by commercial α-l-rhamnosidase at atmospheric pressure (a) and high hydrostatic pressure (b). The reactions were performed in 50 mM McIlvaine buffer (pH 6.0) containing 3 U enzyme ml−1 and 20 mM rutin with 5 % DMSO at 50 °C for 3 h. Error bars indicate the standard deviations of triplicate samples
At atmospheric pressure, the commercial α-l-rhamnosidase completely hydrolysed 20 mM reagent-grade rutin to isoquercetin; however, the enzyme hydrolysed only 67 % of extracted rutin from tartary buckwheat extract. There are several inhibitors of α-l-rhamnosidase such as l-rhamnose, glucose, and citric acid (Yadav et al. 2010). Tartary buckwheat extract contains some of these inhibitors, such as sugars or organic acids, which decrease the conversion yield of rutin. With HHP, the commercial α-l-rhamnosidase completely hydrolysed 20 mM rutin from tartary buckwheat, although it required longer than the reaction with regent-grade rutin. β-Galactosidase inhibition can be decreased via mutation of the inhibitor binding site or enzyme immobilization (Kim et al. 2011; Mateo et al. 2004); however, there are no reports of the relationship between enzyme inhibition and pressure. These results suggested that increasing pressure might affect the enzyme structure by strengthening the hydrogen bonding and hydrophobic interactions, thereby increasing activity and thermostability and decreasing inhibition.
In summary, we optimized the production of isoquercetin from rutin in tartary buckwheat extract using commercial α-l-rhamnosidase. Enzyme activity and stability were stimulated 1.3–4-fold by HHP. Under the optimal conditions, 20 mM isoquercetin was produced by commercial α-l-rhamnosidase after 2 h with a conversion yield of 100 % and a productivity of 10 mM h−1, which is 2.2-fold higher than that at atmospheric pressure. Thus, HHP increased the enzyme activity, stability, and productivity relative to atmospheric pressure. Our results suggest a new approach for the production of isoquercetin from rutin in tartary buckwheat extract by commercial α-l-rhamnosidase using an HHP system.
References
Amado NG, Cerqueira DM, Menezes FS, Mendes da Silva JF, Vivaldo MN, Abreu JG (2009) Isoquercitrin isolated from hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization. Anticancer Drugs 20:543–552
Dirix C, Duvetter T, Loey AV, Hendrickx M, Heremans K (2005) The in situ observation of the temperature and pressure stability of recombinant Aspergillus aculeatus pectin methylesterase with Fourier transform IR spectroscopy reveals an unusual pressure stability of beta-helices. Biochem J 392:565–571
Eisenmenger MJ, Reyes-De-Corcuera JI (2009a) High pressure enhancement of enzymes: a review. Enz Microb Technol 45:331–347
Eisenmenger MJ, Reyes-De-Corcuera JI (2009b) High hydrostatic pressure increased stability and activity of immobilized lipase in hexane. Enz Microb Technol 45:118–125
Kim YS, Yeom SJ, Oh DK (2011) Reduction of galactose inhibition via the mutation of β-galactosidase from Caldicellulosiruptor saccharolyticus for lactose hydrolysis. Biotechnol Lett 33:353–358
Kuntić V, Pejić N, Ivković B, Vujić Z, Ilić K, Mićić S, Vukojević V (2007) Isocratic RP-HPLC method for rutin determination in solid oral dosage forms. J Pharm Biomed Anal 43:718–721
Lee HS, Park CH, Park BJ, Kwon SM, Chang KJ, Kim SL (2006) Rutin, catechin, derivatives, and chemical components of tartary buckwheat (Fagopyrum tataricum Gaertn.) sprouts. Korean J Crop Sci 51:277–282
Lu Z, Wang J, Lin S, Zhang Y (2012) Degradation of rutin into isoquercitrin by Bacillus litoralis strain C44. IOSR J Eng 2:1154–1161
Makino T, Shimizu R, Kanemaru M, Suzuki Y, Moriwaki M, Mizukami H (2009) Enzymatically modified isoquercitrin, α-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol Pharm Bull 32:2034–2040
Mateo C, Monti R, Pessela BC, Fuentes M, Torres R, Guisán JM, Fernández-Lafuente R (2004) Immobilization of lactase from Kluyveromyces lactis greatly reduces the inhibition promoted by glucose. full hydrolysis of lactose in milk. Biotechnol Prog 20:1259–1262
Palaniyandi SA, Damodharan K, Lee KW, Yang SH, Suh JW (2015) Enrichment of ginsenoside Rd in Panax ginseng extract with combination of enzyme treatment and high hydrostatic pressure. Biotechnol Bioprocess Eng 20:608–613
Park SY, Kim JH, Kim DH (2005) Purification and characterization of quercitrin-hydrolyzing α-l-rhamnosidase from Fusobacterium K-60, a human intestinal bacterium. J Microbiol Biotechnol 15:519–524
Pedro HAL, Alfaia AJ, Marques J, Vila-Real HJ, Calado A, Ribeiro MHL (2007) Design of an immobilized enzyme system for naringin hydrolysis at high-pressure. Enzyme Microb Technol 40:442–446
Real HJV, Alfaia AJ, Calado ART, Ribeiro MHL (2007) High pressure–temperature effects on enzymatic activity: naringin bioconversion. Food Chem 102:565–570
Yadav V, Yadav PK, Yadav S, Yadav KDS (2010) α-l-Rhamnosidase: a review. Proc Biochem 45:1226–1235
You HJ, Ahn HJ, Ji GE (2010) Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspercillus niger. J Agric Food Chem 58:10886–10892
Zhang R, Yao Y, Wang Y, Ren G (2011) Antidiabetic activity of isoquercetin in diabetic KK-Ay mice. Nutr Metab (Lond) 8:85
Zhang R, Zhang BL, Xie T, Li GC, Tuo Y, Xiang YT (2015) Biotransformation of rutin to isoquercirtin using recombinant α-l-rhamnosidase from Bifidobacterium breve. Biotechnol Lett 37:1257–1264
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number 2015R1D1A1A01059570).
Supplementary Information
Supplementary Fig. 1—Effect of enzyme concentration on isoquercetin production by commercial α-l-rhamnosidase. The reactions were performed in 50 mM McIlvaine buffer (pH 6.0) containing 0.5–4 U enzyme ml −1 and 20 mM rutin with 5 % DMSO at 50 °C for 3 h. Error bars indicate the standard deviations of triplicate samples.
Supplementary Fig. 2—Effect of substrate (rutin) concentration on isoquercetin production by commercial α-l-rhamnosidase. The reactions were performed in 50 mM McIlvaine buffer (pH 6.0) containing 3 U enzyme ml −1 and 1–20 mM rutin with 5 % DMSO at 50 °C for 3 h. Error bars indicate the standard deviations of triplicate samples.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, DY., Yeom, SJ., Park, CS. et al. Effect of high hydrostatic pressure treatment on isoquercetin production from rutin by commercial α-l-rhamnosidase. Biotechnol Lett 38, 1775–1780 (2016). https://doi.org/10.1007/s10529-016-2157-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2157-5