Abstract
Investigation of the development of cartilage degeneration after ACL reconstruction is important for improving current surgical treatment of ACL injuries to prevent long-term knee joint degeneration. This pilot study examined the relationship between the changes in weight-bearing knee contact kinematics 6 months after ACL reconstruction and the biochemical composition changes in the knee cartilage measured using T2 relaxation values 3 years after the surgery in seven patients. The analysis indicated that the change of the knee contact kinematics in short-term after ACL reconstruction is associated with an increase of T2 values of the cartilage in longer follow up times. The data of this study could provide preliminary data to power future studies that use prospective, longitudinal research and large patient populations to establish prognostic biomechanical markers for determination of long-term cartilage degeneration after ACL reconstruction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
ACL reconstruction is a popular treatment for unstable ACL deficient knees. Although satisfactory clinical outcomes regarding the anterior stability of the knee have been reported,35 recent mid- to long-term follow-up studies have revealed prevalent radiographic knee osteoarthritis (OA) in ACL reconstructed patients.1,3,6,7,15,17,19,27,29,30,32,33,–34,38,39,–40,42 It is important to improve current treatment techniques to delay or prevent post-operative cartilage degeneration of ACL reconstruction patients.
Magnetic resonance (MR) imaging is widely used for analysis of early knee OA. Considerable progress has been made to explore biochemical composition changes in cartilage using T1ρ or T2 mapping sequences.4,13,36,41 Both T1ρ and T2 techniques reported that the superficial layer of the cartilage is more susceptible to early cartilage degenerative changes. The majority of relevant literature assumes that altered kinematics after ACL reconstruction play a crucial role in cartilage degeneration.8,10,12,14,45 However, a paucity of data exists on the quantitative relationship between the post-operative kinematic alteration and cartilage degeneration after ACL reconstruction. This information is necessary for development of biomechanical markers that can predict long-term cartilage degeneration after the surgery.
We previously investigated contact kinematics of the knee after ACL reconstruction28 and measured T2 relaxation values of the ACL reconstructed and intact contralateral knees,2,20 where patients were investigated for early post-operative contact kinematics and T2 values 3 years after surgery. In this paper, we present a pilot study of a small sample size patient cohort that analyzed the relationship between the contact kinematics of the knee during weight-bearing full extension standing 6 months after ACL reconstruction and the cartilage status measured using T2 relaxation values 3 years after the surgery. Our objective is to provide preliminary data to power future studies that use prospective, longitudinal research and large patient populations to establish prognostic biomechanical markers for determination of long-term cartilage degeneration after ACL reconstruction. We hypothesized that the changed knee joint kinematics at short-term after ACL reconstruction was associated with longer-term cartilage degeneration.
Materials and Methods
Patients
Seven patients (sex: 3M, 4F; age: 20–43 years; height: 65–72 inches; BW: 150–190 lbs; BMI: 23.6–27.4 kg/m2) with a unilateral ACL injured knee were investigated with the IRB approval. This patient group was a sample of convenience obtained from our previous study.23 These patients were diagnosed with no other ligamentous injuries and gross cartilage or meniscus damage (confirmed by MRI and arthroscopy) that required surgery, and no history of contralateral knee injury or symptoms. All patients underwent ACL reconstruction within 6 weeks after injury. Written consent was obtained from all subjects before participation in the study.
Contact Kinematics
Both knees were scanned using a 3-Tesla MR scanner (MAGNETOM Trio, Siemens, Malvern, PA) before ACL reconstruction. The MR images were used to construct 3 dimensional (3D) models of the knee, including the femur, tibia, patella and their cartilage surfaces. All patients then performed a step-up motion (14 cm high) before and 6 months after surgery using both knees.23 The step-up motion was chosen because it represents a strenuous motion of the knee during daily life activity. The knee motion was imaged using a dual fluoroscopic imaging system (DFIS) with a frame rate of 30 Hz (Fig. 1a). In this study, we only analyzed the knee kinematics at the end of the step-up motion after a standing position had been achieved.
The fluoroscopic images were imported into solid modeling software (Rhinoceros, Robert McNeel and Assoc, Seattle, WA) to construct a virtual DFIS based on the positions of the actual DFIS setup. The knee positions along the motion path were reproduced using a 2D–3D matching method that has been previously validated with an error of 0.08 mm and a repeatability of < 0.38° in measurement of the position and orientation of the knee, respectively.9,16,26 To analyze the tibiofemoral cartilage contact locations, the cartilage models of the femur and tibia were mapped to the corresponding bony models at each knee position. The cartilage contact area at a given knee position was determined by overlapping of the tibial and femoral cartilage surfaces, where the centroid of the overlapping area was defined as the cartilage contact location.43 A contact axis was defined by connecting the medial and lateral contact locations. The position of the midpoint of the contact axis was defined as the contact location in the tibial coordinate system (Fig. 1b).24,28 The tibial long axis (z) was selected parallel to the posterior wall of the tibial shaft. The medial-lateral axis (x) of the coordinate system was defined as a line connecting the centroids of the two circles fit to the medial and lateral tibial plateau surfaces.24 The anterior-posterior axis (y) was perpendicular to the other two axes. The angle between the contact axis and the x-axis was used to describe the internal(+)/external(−) rotation of the contact axis.
To compare the contact kinematics between the ACL reconstruction and intact contralateral knees, only the data corresponding to the full weight-bearing, single-legged standing position (representing the knee position at the end of the step-up motion) was analyzed, since the ACL mainly functions at low flexion angles.18,46 The changes in cartilage contact kinematics at the standing position 6 months after ACL reconstruction was calculated by subtracting the cartilage contact data of the intact contralateral knee measured before surgery.21
T2 Mapping
At least three years (36–39 months) after ACL reconstruction, both knees of each patient were scanned using a 3T MR scanner. A multiple-TE fast-spin echo sagittal pulse sequence (a repetition time: 1700 ms; ten echo times: 10.6, 21.2, 31.8, 42.4, 53.0, 63.6, 74.2, 84.8, 95.4, 106 ms; matrix: 384 × 384; field of view: 18 × 18 cm; slice thickness: 3.0 mm; slice gap: 0 mm; number of slices: 26–30; bandwidth: 250 Hz/pixel; and total scan time: 11 min per knee) was used for T2 relaxometry images.2 Both knees were scanned using the same imaging parameters at the same session.
For quantification of the T2 relaxation time, the MR images were imported into OsiriX software (Pixmeo Sarl, Bernex, Switzerland). Six compartments of the articular cartilage of the knee were investigated: medial femoral condyle (FM), lateral femoral condyle (FL), medial tibial plateau (TM), lateral tibial plateau (TL), trochlear grove (Tro), and patella (Pat). The femoral condyle cartilage was divided into five sub-compartments (Fig. 2a) (FM1 or FL1, FM2 or FL2, FM3 or FL3, FM4 or FL4, and FM5 or FL5); the tibial plateau into three sub-compartments (TM1 or TL1, TM2 or TL2, and TM3 or TL3)2; and the patellar and femoral trochlear cartilage evenly into three regions in coronal plane (medial, central and lateral regions).20 Further, each region was evenly divided into superficial and deep zones since the cartilage could respond to early degeneration differently along the thickness direction2 (Fig. 2b). The change of T2 value at each region was calculated by subtracting the T2 value of the contralateral knee from that of the ACL reconstruction knee. A higher T2 value of the ACL reconstruction knee compared to the intact contralateral knee indicates early biochemical composition changes of the cartilage.41 In this study, we aimed to investigate the superficial weight-bearing cartilage layer of the tibiofemoral joint by combining FM2 with FM3, FL2 with FL3, TM1 with TM2, and TL1 with TL2.
(a) The cartilage of femur and tibia is divided into the sub-compartments with regard to the anterior and posterior horns of the meniscus. The femoral condyle has five sub-compartments (F-1, 2, 3, 4, 5) and the tibia plateau has three sub-compartments (T-1, 2, 3). (b) The articular cartilage is divided into the superficial and deep zones. S superficial zone, D deep zone.
Statistical Analysis
An ANOVA was used to compare the contact kinematics and cartilage T2 values between the ACL reconstruction and intact contralateral knees. A General Linear Model5,37 was used to test the relationship between the changes in the cartilage contact kinematics 6 months after ACL reconstruction (independent variables) and the cartilage T2 value changes 3 years post-operatively (dependent variables). The output variables were r2 (representing how close the data are to the fitted regression line), β (representing the weight of change in kinematic variable values in response to T2 variable) and SE (the standard deviation of the estimate of β). Significant difference was set when p < 0.05.
Results
Six months after surgery, no statistically significant difference in contact kinematics was observed between the ACL reconstruction and intact contralateral knees among this small patient cohort (p > 0.08) (Table 1). Three years after surgery, no statistically significant differences in cartilage T2 values were observed between the ACL reconstructed and intact contralateral knees in this patient cohort (p > 0.06) (Table 2).
Overall, there is no statistically significant correlation between the changes in the contact locations in anterior-posterior and medial-lateral directions 6 months and the changes in T2 values of the cartilage 3 years after ACL reconstruction in this small patient cohort (p > 0.11) (Table 3). For example, the increased T2 values of the medial femoral and tibial cartilage were not significantly associated with the anterior-posterior contact location changes after ACL reconstruction (r2 = 0.26, β= − 1.4, p= 0.24; r2 = 0.30, β= − 0.67, p= 0.20); the lateral trochlea and medial patellar cartilage were not significantly correlated with the anterior-posterior contact location changes (r2 = 0.43, β= 6.76, p= 0.11; r2 = 0.04, β= 1.94, p= 0.19).
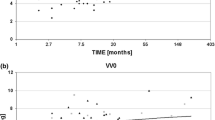
The increased T2 values of the femoral cartilage were not significantly associated with reduced internal (increased external) rotation angle of the contact axis (ΔIE) for the medial side (ΔFM: r2 = 0.51, β= − 0.64, p= 0.07), but were significantly associated for the lateral side (ΔFL: r2 = 0.64, β= − 0.47, p= 0.03) (Fig. 3). No significant correlations were observed for the tibial cartilage (r2 = 0.41, β= − 0.26, p= 0.12; r2 = 0.22, β= − 0.31, p= 0.28, respectively for the medial (ΔTM) and lateral (ΔTL) sides). The increased T2 value of the medial trochlea (ΔMed Tro) was correlated with the reduced internal (increased external) rotation angle of the contact axis (ΔIE) (r2 = 0.71, β= − 1.02, p= 0.02). The T2 value increase of the lateral patellar cartilage (ΔLat Pat) was not significantly correlated with the external rotation angle change of the contact axis (r2 = 0.34, β= − 0.72, p= 0.17).
Discussion
This pilot study examined the relationship of longer-term cartilage biochemical composition changes and short-term contact kinematics of the knee after ACL reconstruction using a small sample size patient cohort. The data indicated that for weight-bearing regions of the knee cartilage, there was an association between the contact kinematics changes during the weight-bearing single-legged standing 6 months and the changes in the T2 relaxation values 3 years after ACL reconstruction. The data only partially supported our hypothesis that the changed knee joint kinematics at short-term after ACL reconstruction was associated with longer-term cartilage degeneration.
The results of this pilot study revealed interesting implications for future investigation of post-operative cartilage degeneration after ACL reconstruction. While marginal correlations emerged between the short-term contact location changes and longer-term cartilage biochemical composition changes in this small sample size patient cohort, we found that the reduced internal (increased external) rotation of the contact axis 6 months after ACL reconstruction were correlated with the increased T2 values 3 years after the surgery. An increased internal rotation of the contact axis compared to the intact contralateral knee corresponds to less changes in T2 values, and we therefore speculate that this could be beneficial for maintenance of the cartilage after the surgery. For example, at the patellofemoral cartilage, a reduced internal contact axis rotation angle (increased internal tibial rotation) was shown to correspond to increased T2 values in the medial patellofemoral cartilage. This is consistent with the biomechanics observation that an increased internal tibial rotation is associated with an increase of the contact pressure at the medial compartment of the patellofemoral joint.22
One of the primary goal of ACL reconstruction is to restore anterior stability of the knee. The data of this pilot study indicate that to prevent long-term changes in cartilage biochemical composition, more biomechanics research, such as during dynamic gait, is necessary to understand how kinematics changes after the surgery could affect the long-term cartilage homeostasis, althogh other factors besides biomechanical ones could also play a role. For example, the correlation analyses implied that for knees having similar contact kinematics of the intact contralateral knees at 6 months after ACL reconstruction (i.e., ΔIE ~ 0 in Fig. 3), their cartilage could experience higher T2 values in certain regions than the contralateral knees 3 years after the surgery. Previous in-vitro cadaveric studies indicated that contemporary ACL reconstructions could restore normal knee stability, but the graft forces were larger than the intact ACL.25 We therefore speculate that the increased ACL graft forces could be beneficial for restoration of knee stability, but could also increase the cartilage contact force.44 In addition, altered muscle strength31 and different ACL reconstruction techniques11 could also cause changes of the kinematics and consequently the cartilage contact loadings of the ACL reconstruction knees. We speculate that eventually, these factors could be a biomechanical cause for long-term cartilage degeneration. Therefore, more prospective, longitudinal studies using larger patient cohorts are warranted to determine if there is a threshold for restoration of knee kinematics after the surgery that corresponds to minimal changes in biochemical compositions of the cartilage long-term after ACL reconstruction.
Diagnosis of early cartilage biochemical composition changes is critical for prevention or treatment of post-operative cartilage degeneration. While T1ρ and T2 mapping are sensitive and feasible to detect early biochemical composition changes in the cartilage, this indicates that the cartilage has already lost some structural integrity and has started to degenerate. It would be ideal if there is a biomarker that could predict cartilage biochemical composition changes before it is initiated. This pilot study implies that an early detection of altered tibiofemoral contact kinematics of the knee after ACL reconstruction compared to the intact contralateral side might serve as a prognostic marker of long-term cartilage biochemical composition changes. With such a predictive tool, early intervention could be developed, such as patient-specific muscle training and rehabilitation regimen, to improve the knee joint contact biomechanics to potentially impede long-term cartilage degeneration.
This is a pilot study that investigated the correlation between the contact kinematic changes in short-term (at weight-bearing, full extension of the knee) and cartilage biochemical composition changes in longer-term after ACL reconstruction using a small sample size patient cohort. There are several limitations when interpreting these data. The correlation analysis was based on a patient cohort of 7. A sample analysis using the data of Table 1 indicated that to detect a statistically significant change in contact axis rotation angles after ACL reconstruction with 80% power, 44 patients would be needed. Therefore, future studies should include a large patient population in a prospective, longitudinal investigation to confirm the association between longer-term cartilage biochemical composition changes and short-term kinematics measurements of the knee after ACL reconstruction. We only analyzed the knee kinematics at the weight-bearing, single-legged standing position and corresponding weight-bearing contact locations of the cartilage due to the retrospective nature of the study. To evaluate the cartilage of the entire knee, the knee kinematics during functional daily activities, such as gait, jumping, etc., that include various flexion angles and loading ranges should be investigated. Our early study only used T2 relaxation values to examine the cartilage biochemical composition changes. Future studies should also use other validated techniques, such as T1ρ sequence, cartilage thickness change, to detect cartilage degeneration. Despite these various limitations, the results of this pilot study provide insights for designing future research for prediction of long-term cartilage degeneration using short-term knee kinematics after ACL reconstruction.
Conclusion
Despite the small sample size, several relationships between changes in contact kinematics and T2 values were identified in this study. For example, for knees having similar contact kinematics of the intact contralateral sides at 6 months after ACL reconstruction, their cartilage could still experience higher T2 values in certain regions than the contralateral sides 3 years after the surgery. These observations could provide the basis for future studies that use prospective, longitudinal research and large patient cohorts to further explore these novel, yet preliminary, findings.
References
Ahn, J. H., J. G. Kim, J. H. Wang, C. H. Jung, and H. C. Lim. Long-term results of anterior cruciate ligament reconstruction using bone-patellar tendon-bone: an analysis of the factors affecting the development of osteoarthritis. Arthroscopy 28:1114–1123, 2012.
Bae, J. H., A. Hosseini, Y. Wang, M. Torriani, T. J. Gill, A. J. Grodzinsky, and G. Li. Articular cartilage of the knee 3 years after ACL reconstruction. A quantitative T2 relaxometry analysis of 10 knees. Acta Orthop. 86:605–610, 2015.
Barenius, B., S. Ponzer, A. Shalabi, R. Bujak, L. Norlen, and K. Eriksson. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am. J. Sports Med. 42:1049–1057, 2014.
Bolbos, R. I., C. B. Ma, T. M. Link, S. Majumdar, and X. Li. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Investig. Radiol. 43:782–788, 2008.
Christensen, R. Plane Answers to Complex Questions: The Theory of Linear Models. New York: Springer, 2002.
Chu, C. R., B. D. Beynnon, J. A. Buckwalter, W. E. Garrett, Jr., J. N. Katz, S. A. Rodeo, K. P. Spindler, and R. A. Stanton. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am. J. Sports Med. 39:1569–1578, 2011.
Claes, S., L. Hermie, R. Verdonk, J. Bellemans, and P. Verdonk. Is osteoarthritis an inevitable consequence of anterior cruciate ligament reconstruction? A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 21:1967–1976, 2012.
Dare, D., and S. Rodeo. Mechanisms of post-traumatic osteoarthritis after ACL injury. Curr. Rheumatol. Rep. 16:448, 2014.
Defrate, L. E., R. Papannagari, T. J. Gill, J. M. Moses, N. P. Pathare, and G. Li. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am. J. Sports Med. 34:1240–1246, 2006.
Felson, D. T., R. C. Lawrence, P. A. Dieppe, R. Hirsch, C. G. Helmick, J. M. Jordan, R. S. Kington, N. E. Lane, M. C. Nevitt, Y. Zhang, M. Sowers, T. McAlindon, T. D. Spector, A. R. Poole, S. Z. Yanovski, G. Ateshian, L. Sharma, J. A. Buckwalter, K. D. Brandt, and J. F. Fries. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann. Intern. Med. 133:635–646, 2000.
Gadikota, H. R., A. Hosseini, P. Asnis, and G. Li. Kinematic analysis of five different anterior cruciate ligament reconstruction techniques. Knee Surg. Relat. Res. 27:69–75, 2015.
Gao, B., M. L. Cordova, and N. N. Zheng. Three-dimensional joint kinematics of ACL-deficient and ACL-reconstructed knees during stair ascent and descent. Hum. Mov. Sci. 31:222–235, 2012.
Guermazi, A., H. Alizai, M. D. Crema, S. Trattnig, R. R. Regatte, and F. W. Roemer. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr. Cartil. 23:1639–1653, 2015.
Guilak, F., A. Ratcliffe, N. Lane, M. P. Rosenwasser, and V. C. Mow. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J. Orthop. Res. 12:474–484, 1994.
Holm, I., B. E. Oiestad, M. A. Risberg, R. Gunderson, and A. K. Aune. No differences in prevalence of osteoarthritis or function after open versus endoscopic technique for anterior cruciate ligament reconstruction: 12-year follow-up report of a randomized controlled trial. Am. J. Sports Med. 40:2492–2498, 2012.
Hosseini, A., S. Van de Velde, T. J. Gill, and G. Li. Tibiofemoral cartilage contact biomechanics in patients after reconstruction of a ruptured anterior cruciate ligament. J. Orthop. Res. 30:1781–1788, 2012.
Janssen, R. P., A. W. du Mee, J. van Valkenburg, H. A. Sala, and C. M. Tseng. Anterior cruciate ligament reconstruction with 4-strand hamstring autograft and accelerated rehabilitation: a 10-year prospective study on clinical results, knee osteoarthritis and its predictors. Knee Surg. Sports Traumatol. Arthrosc. 21:1977–1988, 2012.
Jordan, S. S., L. E. DeFrate, K. W. Nha, R. Papannagari, T. J. Gill, and G. Li. The in vivo kinematics of the anteromedial and posterolateral bundles of the anterior cruciate ligament during weightbearing knee flexion. Am. J. Sports Med. 35:547–554, 2007.
Kessler, M. A., H. Behrend, S. Henz, G. Stutz, A. Rukavina, and M. S. Kuster. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee Surg. Sports Traumatol. Arthrosc. 16:442–448, 2008.
Kim, C. W., A. Hosseini, L. Lin, Y. Wang, M. Torriani, T. J. Gill, A. J. Grodzinsky, and G. Li. Quantitative analysis of T2 relaxation times of the patellofemoral joint cartilage 3 years after anterior cruciate ligament reconstruction. J. Orthop. Transl. 12:85–92, 2017.
Kozanek, M., S. K. Van de Velde, T. J. Gill, and G. Li. The contralateral knee joint in cruciate ligament deficiency. Am. J. Sports Med. 36:2151–2157, 2008.
Li, G., L. E. DeFrate, S. Zayontz, S. E. Park, and T. J. Gill. The effect of tibiofemoral joint kinematics on patellofemoral contact pressures under simulated muscle loads. J. Orthop. Res. 22:801–806, 2004.
Li, J. S., A. Hosseini, L. Cancre, N. Ryan, H. E. Rubash, and G. Li. Kinematic characteristics of the tibiofemoral joint during a step-up activity. Gait Posture 38:712–716, 2013.
Li, C., A. Hosseini, T. Y. Tsai, Y. M. Kwon, and G. Li. Articular contact kinematics of the knee before and after a cruciate retaining total knee arthroplasty. J. Orthop. Res. 33:349–358, 2015.
Li, G., R. Papannagari, L. E. DeFrate, J. D. Yoo, S. E. Park, and T. J. Gill. Comparison of the ACL and ACL graft forces before and after ACL reconstruction: an in-vitro robotic investigation. Acta Orthop. 77:267–274, 2006.
Li, G., S. K. Van de Velde, and J. T. Bingham. Validation of a non-invasive fluoroscopic imaging technique for the measurement of dynamic knee joint motion. J. Biomech. 41:1616–1622, 2008.
Liden, M., N. Sernert, L. Rostgard-Christensen, C. Kartus, and L. Ejerhed. Osteoarthritic changes after anterior cruciate ligament reconstruction using bone-patellar tendon-bone or hamstring tendon autografts: a retrospective, 7-year radiographic and clinical follow-up study. Arthroscopy 24:899–908, 2008.
Lin, L., J. S. Li, W. A. Kernkamp, A. Hosseini, C. Kim, P. Yin, L. Wang, T. Y. Tsai, P. Asnis, and G. Li. Postoperative time dependent tibiofemoral articular cartilage contact kinematics during step-up after ACL reconstruction. J. Biomech. 49:3509–3515, 2016.
Lohmander, L. S., A. Ostenberg, M. Englund, and H. Roos. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 50:3145–3152, 2004.
Louboutin, H., R. Debarge, J. Richou, T. A. Selmi, S. T. Donell, P. Neyret, and F. Dubrana. Osteoarthritis in patients with anterior cruciate ligament rupture: a review of risk factors. Knee 16:239–244, 2009.
Lynch, A. D., D. S. Logerstedt, H. Grindem, I. Eitzen, G. E. Hicks, M. J. Axe, L. Engebretsen, M. A. Risberg, and L. Snyder-Mackler. Consensus criteria for defining ‘successful outcome’ after ACL injury and reconstruction: a Delaware–Oslo ACL cohort investigation. Br. J. Sports Med. 49:335–342, 2015.
Murray, J. R., A. M. Lindh, N. A. Hogan, A. J. Trezies, J. W. Hutchinson, E. Parish, J. W. Read, and M. V. Cross. Does anterior cruciate ligament reconstruction lead to degenerative disease? Thirteen-year results after bone-patellar tendon-bone autograft. Am. J. Sports Med. 40:404–413, 2011.
Neuman, P., I. Kostogiannis, T. Friden, H. Roos, L. E. Dahlberg, and M. Englund. Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury: a prospective cohort study. Osteoarthr. Cartil. 17:284–290, 2009.
Oiestad, B. E., I. Holm, L. Engebretsen, and M. A. Risberg. The association between radiographic knee osteoarthritis and knee symptoms, function and quality of life 10–15 years after anterior cruciate ligament reconstruction. Br. J. Sports Med. 45:583–588, 2011.
Okafor, E. C., G. M. Utturkar, M. R. Widmyer, E. S. Abebe, A. T. Collins, D. C. Taylor, C. E. Spritzer, C. T. Moorman, 3rd, W. E. Garrett, and L. E. DeFrate. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J. Biomech. 47:96–101, 2014.
Samosky, J. T., D. Burstein, W. Eric Grimson, R. Howe, S. Martin, and M. L. Gray. Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. J. Orthop. Res. 23:93–101, 2005.
Scott, M., D. Flaherty, and J. Currall. Statistics: general linear models (a flexible approach). J. Small Anim. Pract. 55:527–530, 2014.
Struewer, J., T. Efe, T. M. Frangen, T. Schwarting, B. Buecking, S. Ruchholtz, K. F. Schuttler, and E. Ziring. Prevalence and influence of tibial tunnel widening after isolated anterior cruciate ligament reconstruction using patella-bone-tendon-bone-graft: long-term follow-up. Orthop. Rev. 4:e21, 2012.
Struewer, J., T. M. Frangen, B. Ishaque, C. Bliemel, T. Efe, S. Ruchholtz, and E. Ziring. Knee function and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using bone-patellar tendon-bone graft: long-term follow-up. Int. Orthop. 36:171–177, 2012.
Struewer, J., E. Ziring, T. M. Frangen, T. Efe, S. Meissner, B. Buecking, C. Bliemel, and B. Ishaque. Clinical outcome and prevalence of osteoarthritis after isolated anterior cruciate ligament reconstruction using hamstring graft: follow-up after two and ten years. Int. Orthop. 37:271–277, 2013.
Taylor, C., J. Carballido-Gamio, S. Majumdar, and X. Li. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magn. Reson. Imaging 27:779–784, 2009.
Tourville, T. W., R. J. Johnson, J. R. Slauterbeck, S. Naud, and B. D. Beynnon. Assessment of early tibiofemoral joint space width changes after anterior cruciate ligament injury and reconstruction: a matched case-control study. Am. J. Sports Med. 41:769–778, 2013.
Van de Velde, S. K., J. T. Bingham, A. Hosseini, M. Kozanek, L. E. DeFrate, T. J. Gill, and G. Li. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 60:3693–3702, 2009.
Wang, L., L. Lin, Y. Feng, T. L. Fernandes, P. Asnis, A. Hosseini, and G. Li. Anterior cruciate ligament reconstruction and cartilage contact forces: a 3D computational simulation. Clin. Biomech. 30:1175–1180, 2015.
Wen, C., and L. S. Lohmander. Osteoarthritis: does post-injury ACL reconstruction prevent future OA? Nat. Rev. Rheumatol. 10:577–578, 2014.
Wu, J. L., A. Hosseini, M. Kozanek, H. R. Gadikota, T. J. T. Gill, and G. Li. Kinematics of the anterior cruciate ligament during gait. Am. J. Sports Med. 38:1475–1482, 2010.
Acknowledgments
The authors would like to thank the National Institutes of Health (NIH) (R01 AR055612) for the support.
Author information
Authors and Affiliations
Contributions
GL: research idea, data analysis and interpretation, drafting manuscript. J-SL: research idea, data analysis and interpretation, drafting manuscript. MT: data interpretation, assisting in paper writing. AH: data interpretation, assisting in paper writing. All authors have read and approved the final submitted manuscript.
Corresponding author
Additional information
Associate Editor Michael R. Torry oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Li, G., Li, JS., Torriani, M. et al. Short-Term Contact Kinematic Changes and Longer-Term Biochemical Changes in the Cartilage After ACL Reconstruction: A Pilot Study. Ann Biomed Eng 46, 1797–1805 (2018). https://doi.org/10.1007/s10439-018-2079-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-2079-6