Abstract
Advances in various imaging modalities for breast lesions have improved diagnostic capabilities not only for tumors but also for non-tumorous lesions. Contrast-enhanced ultrasound (CEUS) plays a crucial role not only in the differential diagnosis of breast lesions, identification of sentinel lymph nodes, and diagnosis of lymph node metastasis but also in assessing the therapeutic effects of neoadjuvant chemotherapy (NAC). In CEUS, two image interpretation approaches, i.e., qualitative analysis and quantitative analysis, are employed and applied in various clinical settings. In this paper, we review CEUS for breast lesions, including its various applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, with the inclusion of testing for hereditary breast and ovarian cancer syndrome (HBOC) under insurance coverage, there has been an increase in the number of patients diagnosed with HBOC. The cumulative incidence risk of breast cancer by age 80 is reported to be 72% for BRCA1 mutation carriers and 69% for BRCA2 mutation carriers, while the cumulative incidence risk of ovarian cancer is 44% for BRCA1 and 17% for BRCA2 [1]. Magnetic resonance imaging (MRI) screening is recommended for follow-up of BRCA-positive breast cancer patients and screening of asymptomatic individuals [2], yet the inability of certain patients to undergo MRI for various reasons remains problematic. Triple-negative breast cancer is prevalent in BRCA1 mutation carriers, whereas estrogen receptor-positive, HER2-negative luminal breast cancer is more common in BRCA2 mutation carriers [3]. While mammography detects calcifications in BRCA2-related breast cancer, reports indicate a lack of calcifications in BRCA1-related breast cancer [4, 5]. Due to the difficulty in diagnosing HBOC-related breast cancer using mammography or conventional ultrasound (US), there is a potential increase in the importance of contrast-enhanced US (CEUS) as an alternative imaging modality to MRI [6]. CEUS, being capable of clearly demonstrating the microvascular perfusion within and around tumors, represents an innovative diagnostic technique allowing for more accurate real-time evaluation of microvascular structures of breast lesions [7,8,9]. Additionally, CEUS can depict small vessels that may not be detected on MRI [10].
In CEUS, either qualitative analysis through contrast-enhanced pattern analysis or quantitative analysis through contrast-enhanced kinetic analysis, or both, are employed for differentiation between benign and malignant lesions [8]. CEUS proves beneficial in various aspects of breast cancer management, including differential diagnosis of breast lesions, assessment of tumor spread, staging of invasive cancer, evaluation of the effectiveness of neoadjuvant chemotherapy (NAC), and diagnosis of axillary lymph node metastasis.
In recent years, the availability of new therapeutic agents tailored to the biology and intrinsic subtype of breast cancer, as evidenced by clinical trial results, has led to a diversified treatment approach, necessitating personalized treatment strategies. Selection of available therapeutic agents is determined based on pre-treatment evaluations such as the recurrence score derived from the 21-gene breast cancer assay and the number of metastatic lymph nodes, even within the same intrinsic subtype of breast cancer [11, 12]. Evaluation of lesions, including lymph node metastasis status, is crucial across all patient groups, and accurate determination of treatment strategies through CEUS-based assessment and diagnosis may lead to improved treatment outcomes. The aim of this review is to discuss the various utilities of CEUS with the objective of elucidating its effectiveness.
History of CEUS
Contrast-enhanced methods in US imaging had their origins in 1969 when Gramiak et al. first utilized indocyanine green (ICG) as a contrast agent in cardiovascular US imaging [13]. By the late 1970s, it was discovered that the source of echo enhancement in CEUS was microbubbles [14]. In 1982, Matsuda et al. initiated CEUS of liver tumors using the CO2 microbubble injection method, establishing diagnostic criteria and paving the way for its widespread adoption [15,16,17,18,19]. Subsequently, the availability of Levovist, a contrast agent suitable for intravenous administration at high mechanical index (MI) values, became feasible [20]. Furthermore, the development of second-generation contrast agents such as Optison, Definity, SonoVue, and Sonazoid [21,22,23,24,25] enabled visualization of blood flow signals at low to moderate MI values, expanding the application of CEUS to areas including the liver, biliary tract, pancreas, and breast regions.
Differences between CEUS in the liver and breast regions
The liver is characterized by a dual vascular supply from the artery and portal vein, resulting in two distinct vascular phases: the arterial-dominant phase and the portal-dominant phase. Moreover, in CEUS using Sonazoid, unlike other contrast agents such as Definity or SonoVue, Kupffer cells in the liver’s reticuloendothelial system uptake Sonazoid, resulting in the presence of two contrast enhancement phases: the vascular phase and the post-vascular phase (Kupffer phase, typically observed 10 min after contrast agent injection) [26,27,28].
While blood flow in normal liver tissue is abundant, it is relatively less so in normal breast tissue. However, relatively strong enhancement effects may be observed in premenopausal breast tissue. Therefore, in contrast-enhanced MRI, it is recommended to consider the menstrual cycle and perform imaging between days 5 and 12 after the onset of menstruation. Due to the relatively deeper imaging depth in the liver, probes with frequencies around 3.5 MHz are used, whereas for breast imaging, probes with relatively higher frequencies are utilized as lesions may be present up to 3–5 cm deep under the skin.
Advances in CEUS imaging, coupled with the development of second-generation contrast agents, have endowed CEUS with the ability to sensitively and accurately depict tumor vasculature. Consequently, significant improvements have been achieved in the diagnosis of focal liver lesions, including hepatocellular carcinoma (HCC) [29]. By utilizing Sonazoid, which does not contain Kupffer cells, the diagnostic sensitivity for malignant liver tumors is increased. Sonazoid CEUS enables the detection of small malignant liver lesions, including metastatic liver tumors, determination of the surgical approach for liver resection, appropriate guidance for non-surgical ablation techniques such as radiofrequency ablation, and accurate evaluation of treatment response to drug therapies, including those for HCC [30,31,32] (Fig. 1).
In the Kupffer phase of Sonazoid CEUS, it is possible to scan the entire liver, allowing for the detection of HCC even when lesions are relatively small, making it valuable for HCC surveillance [29,30,31,32]. Furthermore, utilizing reperfusion techniques involving reinjection of contrast agents enables the diagnosis of newly detected lesions in the Kupffer phase [30].
CEUS Diagnostic criteria for breast lesions
Contrast agents
When using SonoVue as the contrast agent, imaging is typically performed at a low MI value, generally around 0.06–0.08. On the other hand, when using Sonazoid, imaging is usually conducted at a moderate MI value, typically around 0.2. SonoVue is composed of sulfur hexafluoride and is a stable aqueous suspension of microbubbles encapsulated by a lipid shell [33]. Sonazoid, on the other hand, is considered to exhibit higher stability than SonoVue due to its stable outer shell containing hydrogenated egg phosphatidylserine, which enables it to withstand pressure and minimize bubble collapse and signal loss [22, 33, 34].
The gas in microbubbles can pass through pulmonary capillary filters and be exhaled through lung respiration. Long-term safety has been confirmed in the liver and breast regions [21, 24, 25, 35]. The size of second-generation contrast agents is comparable to that of red blood cells, preventing them from passing through the vessel wall into the interstitial space. As a result, these contrast agents can directly and accurately reflect the microcirculation perfusion of lesions, thereby improving the diagnostic agreement rate of breast lesions [36].
Qualitative diagnostic evaluation
The following diagnostic criteria have been utilized in clinical trials of Sonazoid CEUS for breast lesions and have been widely adopted clinically due to their relatively straightforward application and favorable diagnostic performance [25]:
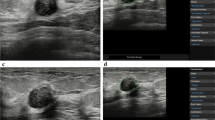
Enhancement patterns indicative of benign lesions include strong or homogeneous enhancement of the entire lesion, or lack of enhancement of the entire lesion. Enhancement patterns indicative of malignant lesions include heterogeneous enhancement with or without clear defects (Fig. 2, Fig. 3), rapid washout from the lesion compared to washout from the surrounding mammary tissues, and the degree of enhancement being greater than that of the surrounding tissue, and the area of enhancement being larger than the hypoechoic lesion on the precontrast (or conventional US) images (Fig. 4, 5, 6).
Several scoring systems and qualitative evaluation criteria have been proposed, generally focusing on similar evaluation parameters. Specifically, benign lesions typically exhibit synchronous or delayed enhancement, homogeneous or low enhancement, clear margins, and regular shapes, whereas malignant lesions often show early heterogeneous over-enhancement, indistinct margins, irregular shapes, and an expanded area of enhancement compared to conventional US tumor size [37,38,39]. Regarding expansion of tumor size, it is defined as enlargement when either the length or width increases by more than 3 mm compared to conventional US measurements [40].
Other findings indicative of malignant lesions through qualitative evaluation include heterogeneous and centripetal enhancement, as well as the presence of peripheral radial or penetrating vessels, whereas benign lesions predominantly demonstrate homogeneous and centrifugal enhancement [8]. Regarding the characteristics of vascular architecture assessed qualitatively, malignant lesions typically exhibit tortuous and irregular vessels, whereas benign lesions show gently curved vessels along the tumor margins. Arteriovenous shunting is observed in malignant lesions but not in benign lesions [41, 42]. Additionally, malignant lesions often show heterogeneous distribution of vessels and frequently exhibit local perfusion defects [8, 41,42,43] (Fig. 7).
Quantitative diagnostic evaluation
Quantitative parameters obtained from the time-intensity curve (TIC) include the following: peak intensity (%), which is defined as the maximum intensity value in the time-intensity curve; time-to-peak (TTP) (sec), defined as the duration between the moment when the contrast medium first reaches the lesion and the time of maximum signal intensity after contrast medium administration; mean transit time (MTT) (sec), defined as the duration of enhancement of the lesion; regional blood volume (RBV) (mL), which represents the area under the TIC, reflecting the total volume of contrast medium passing through the lesion of interest; and regional blood flow (RBF) (mL/sec), calculated as the fraction area under the curve divided by MTT, reflecting the relative blood flow in the selected lesion’s area [44].
Regarding quantitative parameters, malignant lesions typically exhibit significantly shorter TTP, higher peak intensity, and increased wash-in slope. While the TICs of malignant lesions are predominantly plateau and wash-out types, benign lesions mainly demonstrate plateau and slow-rise types [39, 45]. Factors involved in the differentiation between benign and malignant lesions using CEUS with Sonazoid include not only enhancement patterns but also the slope of the tangent at the starting point of the TIC (Axk value), as demonstrated by Fujimitsu et al. [46]. The Axk value is defined as the slope of the tangent at the beginning of the TIC.
Characteristics of CEUS in non-mass abnormalities
With respect to terminology related to non-mass abnormalities, other terms such as non-mass lesions, non-mass-like lesions, and non-mass breast lesions may be used interchangeably with similar definitions. Guidelines for non-mass abnormalities (NMAs) in conventional US were established by the Japan Society of Ultrasonics in Medicine in 2023, and several review articles have been published [47,48,49,50,51,52,53]. However, reports on the diagnosis of NMAs using CEUS are currently limited [54,55,56]. Malignant NMAs are characterized by early wash-in time, hyper-enhancement degree, unclear enhancement margin, enlarged enhancement area, and early wash-out time. Conversely, benign NMAs are primarily characterized by the absence of radial or penetrating vessels and perfusion defects [45, 56, 57]. In non-mass abnormalities, CEUS can clearly demonstrate the area of the lesion and the status of internal vessels, enabling accurate guidance for biopsy sites [38]. Factors predicting malignant ductal lesions among non-mass abnormalities include the presence of microcalcifications and enlargement of the enhancement area [58] (Figs. 8, 9,10).
Characteristics of CEUS in pathological prognostic factors
The majority of HER2-positive breast cancers exhibit heterogeneous enhancement, while ER-negative breast cancers often demonstrate centripetal enhancement. Perfusion defects are frequently observed in cancers with high malignancy grades such as HER2-positive, ER-negative, and Ki-67-positive tumors, serving as indicators of increased microvessel density (MVD). Radial vessels or perforator vessels are commonly found in lesions with high histological grades of breast cancer or those accompanied by lymph node metastasis [39, 44, 59,60,61]. In metastatic lymph nodes, heterogeneous enhancement within the lymph nodes is a characteristic feature [44] (Fig. 11). All four subtypes of breast cancer show an increase in area post-enhancement, but the rate of increase in the transverse diameter allows for the prediction of the histological malignancy of malignant tumors [62, 63]. Regarding quantitative parameters, studies have shown that the upward slope serves as the best discriminator for proliferative activity [64].
CEUS assessment of treatment response to neoadjuvant chemotherapy (NAC)
Patients achieving pathological complete response (pCR) after NAC generally exhibit improved disease-free survival and overall survival compared to non-pCR patients [65]. CEUS enables early prediction of pCR and recurrence-free survival (RFS) in patients with locally advanced breast cancer undergoing NAC therapy by assessing changes in microvascular perfusion [30].
CEUS allows for easy dynamic observation and quantification of tumor perfusion [66] (Fig. 12), enabling differentiation between fibrosis and residual tumor after NAC treatment without exposing patients to the risks of radiation [67,68,69,70,71,72,73].
Contrast-enhanced ultrasound (CEUS) before and after neoadjuvant chemotherapy (NAC). a Arrival time parametric imaging before NAC. b CEUS imaging before NAC. c Arrival time parametric imaging after two cycles of NAC. d CEUS imaging after two cycles of NAC. e Time-intensity curve before (blue-line) and after NAC (red-line)
Qualitative and quantitative assessment of changes in tumor blood flow during NAC therapy is feasible with CEUS [18,19,20, 22, 24, 25], and it demonstrates a strong correlation with pathological response outcomes [66,67,68, 74,75,76,77,78,79].
The time-to-peak (TTP) on CEUS at the 5th week of NAC is significantly prolonged in responders compared to non-responders, thus serving as a useful tool for evaluation of early response to NAC [67, 80].
Combining conventional US with qualitative CEUS evaluation methods allows for accurate prediction of axillary lymph node status after NAC in breast cancer patients. Studies have shown associations between lymph node medulla boundary, lymph node aspect ratio, CEUS pattern, and post-NAC lymph node pCR [81].
CEUS for lymph nodes
US evaluation is useful for assessing axillary lymph nodes, but performing CEUS provides more perfusion information, thereby improving the diagnostic accuracy for lymph node metastasis [81,82,83,84,85,86,87,88,89,90].
Lymph nodes consist of two vascular systems: lymphatic circulation and blood circulation. In the evaluation of axillary lymph nodes, two CEUS techniques, namely perfusion CEUS and lymphatic CEUS, are utilized.
Lymphatic CEUS
Lymphatic CEUS identifies sentinel lymph nodes through percutaneous contrast agent administration via lymphatics to diagnose lymph node metastasis. Studies have classified lymph node CEUS enhancement into four enhancement groups, leading to reduced false-positive rates and improved specificity. Niu et al. categorized sentinel lymph nodes into four enhancement patterns, showing that Patterns I & II had a 91.7% negative metastasis rate (Fig. 13, 14), while Patterns III & IV had a higher probability of metastasis [91].
-
Pattern I: Homogeneous
-
Pattern II: Featured inhomogeneous
-
Pattern III: Focal defect
-
Pattern IV: No enhancement
Perfusion CEUS
The primary aim of perfusion CEUS is to diagnose lymph node metastasis through contrast agent administration via veins, similar to diagnosing lesions in the breast (Fig. 15). When performing perfusion CEUS after lymphatic CEUS, microbubbles within lymphatic vessels and lymph nodes are shattered using high acoustic pressure before perfusion CEUS is performed. Du et al. concluded that combining conventional US with CEUS enables the appropriate evaluation of axillary lymph nodes.
The arterial phase begins when the contrast agent reaches the lymph nodes, while the venous phase (delayed phase) begins approximately 30–45 s after contrast agent administration [18].
Perfusion CEUS is classified into the following two types:
-
Type A: Homogeneous enhancement in the arterial phase and homogeneous regression in the venous phase.
-
Type B: Inhomogeneous regression in the venous phase.
Discussion
While evaluating minimal blood flow using color Doppler imaging can be challenging [81], CEUS allows for the assessment of blood flow as small as 100 μm [68, 92]. Compared to contrast-enhanced MRI and CT, CEUS offers superior spatial and temporal resolution, enabling real-time observation of tumor microcirculation [25, 45, 93]. Miyamoto et al. evaluated enhancement patterns and found that a diagnosis could be made with the detection of one or a few patterns, demonstrating significantly higher accuracy and specificity with CEUS for lesions both less than 1 cm and over 1 cm in size compared to conventional US and contrast-enhanced MRI [25].
Breast cancer relies on angiogenesis, but the number of vessels, blood flow velocity, and intratumoral vascular resistance do not clearly distinguish malignant from benign breast lesions [94,95,96]. Angiogenesis is a characteristic pathological process common to most solid tumors, including breast cancer, and is associated with tumor growth, invasion, metastasis, and prognosis [40, 45, 80, 97,98,99,100,101,102,103,104].
The diagnostic accuracy of CEUS varies depending on the size of the lesion [34], which is closely related to vascular density [39, 103, 105,106,107]. Microvessel density (MVD) and vascular endothelial growth factor (VEGF) expression serve as prognostic markers for breast cancer [108,109,110,111].
Benign lesions typically exhibit normal vascular caliber, while in malignant lesions, the distribution of nutrient vessels may be disrupted, sometimes accompanied by arteriovenous shunts [112].
The occurrence rates of high enhancement and heterogeneous enhancement are higher in malignant lesions, whereas low enhancement and homogeneous enhancement are more common in benign lesions. Centripetal enhancement reflects the actual density and distribution of microvessels in malignant lesions [40].
A significant increase in the extent of tumor enhancement after contrast administration is a crucial indicator of malignant lesions and correlates with histopathological findings [40, 64, 113,114,115]. Additionally, preoperative assessment of tumor spread with CEUS enables accurate evaluation of the extent of resection [113, 116].
While benign tumors mostly exhibit a normal vascular caliber with minimal neovascularization and homogeneous distribution, malignant tumors tend to have more neovascularization and disrupted distribution of nutrient vessels, and may be associated with arteriovenous shunts [40, 112].
Furthermore, in malignant tumors, microvessel density (MVD) and expression of vascular endothelial growth factor (VEGF) tend to concentrate at the tumor periphery [47, 64]. Some low-grade ductal carcinoma in situ (DCIS) may be nourished by normal peripheral vessels without the formation of abnormal peripheral vessels [59, 115, 117,118,119,120,121,122,123].
CEUS offers short examination times and can be safely performed in patients with contraindications to gadolinium administration, claustrophobia, or implanted pacemakers. Additionally, it enables more accurate prediction of malignant lesions, thus reducing the need for false-positive biopsies [118, 124, 125].
While benign breast lesions may sometimes yield false-positive CEUS images, this could be attributed to cellular proliferation, hyperplasia, and inflammatory reactions [37, 54, 56, 126,127,128,129]. Research on background parenchymal enhancement (BPE) on CEUS has shown similar patterns between patients with malignant tumors and those with benign tumors in each menstrual phase. Cases where BPE exhibits higher enhancement than breast tumors at the peak of contrast are all benign [130].
Although reports suggest that generally similar diagnostic criteria as for tumors can be applied to classifying malignant non-mass abnormalities (NMAs), due to the high proportion of hypoechoic areas in malignant NMAs, current criteria may not be sufficient. It is hoped that future research will elucidate evaluation criteria and diagnostic standards for CEUS based on NMA classification.
Clinical trial results regarding post-NAC treatment in cases where pCR is not achieved are informative [131, 132] as predicting the effectiveness of NAC plays a crucial role in treatment selection, including surgical methods [82].
Due to the difficulty in interpreting extracellular volume changes on DCE-MRI, there is often overestimation or underestimation of the response to NAC [133,134,135]. Since the contrast agents used in CEUS are present only in the vascular bed, it is possible to obtain results comparable to those of DCE-MRI [67, 73].
Huang et al. performed CEUS before and after two cycles of NAC, but various studies have examined CEUS after the first cycle or after four cycles, etc. [38, 68, 73].
In CEUS studies, various approaches have been taken for setting regions of interest (ROIs), including ROIs encompassing tumor margins, ROIs placed on tumor contours, and ROIs based on hotspots of a certain diameter [75, 112, 136]. Determining appropriate ROIs requires further analysis based on a large number of cases with various ROI settings tailored to the lesion characteristics and objectives.
Regarding sentinel lymph node biopsy using CEUS, Omoto et al. conducted a basic study using a 25% albumin contrast agent in 2002 [137], followed by the first report of identifying sentinel lymph nodes in breast cancer patients in 2006 [138]. Since 2009, evaluation of sentinel lymph nodes using Sonazoid CEUS has been widely performed, with favorable outcomes reported [139,140,141]. CEUS can provide high diagnostic accuracy in detecting metastatic sentinel lymph nodes, with the risk of metastasis in sentinel lymph nodes showing heterogeneous enhancement being approximately six times higher than those showing homogeneous enhancement [39, 90, 142, 143].
In breast cancer management, diagnosis and treatment of hepatic metastases may be necessary, and CEUS is useful for assessing the efficacy of treatments such as drug therapy due to its simplicity and low patient burden. Evaluation of metastatic liver tumors in the Kupffer phase with Sonazoid CEUS is extremely straightforward and useful [29, 30].
In cases of BRCA-positive breast cancer and BRCA-positive unaffected individuals, contrast-enhanced MRI is recommended for follow-up, but there are patients who cannot undergo contrast-enhanced MRI [3]. Although data accumulation and analysis are needed in the future, diagnosis with CEUS is considered important as an alternative diagnostic modality to contrast-enhanced MRI.
Regarding the assessment of treatment efficacy for new minimally invasive treatment of breast cancer using microwave ablation, there are reports comparing CEUS and MRI, showing comparable results [144]. US-guided radiofrequency ablation therapy for small breast cancer, which became eligible for insurance coverage in Japan in 2023, may be widely performed [145,146,147,148]. Similar to radiofrequency ablation therapy for liver cancer [149,150,151], CEUS is expected to be used for preoperative diagnosis and assessment of treatment efficacy.
In recent years, diagnosis using artificial intelligence (AI) has been increasingly utilized in various fields [152,153,154]. In the future, combining CEUS with AI diagnosis is expected to further enhance the diagnostic capabilities for breast cancer.
Conclusion
For the diagnosis of breast lesions, both qualitative and quantitative evaluations are utilized with CEUS. CEUS has a wide range of applications, including distinguishing between benign and malignant breast lesions, identifying sentinel lymph nodes, diagnosing lymph node metastasis, assessing the efficacy of NAC, and preoperative assessment of breast cancer spread. Additionally, CEUS is a useful diagnostic modality for evaluating treatment efficacy in liver metastases and for treatments such as radiofrequency ablation therapy, with no radiation exposure and minimal patient burden.
References
Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–16.
Tozaki M, Nakamura S. Current status of breast cancer screening in high-risk women in Japan. Breast Cancer. 2021;28:1181–7.
Nakamura S, Takahashi M, Tozaki M, et al. Prevalence and diferentiation of hereditary breast and ovarian cancers in Japan. Breast Cancer. 2015;22:462–8.
Murakami W, Tozaki M, Nakamura S, et al. The clinical impact of MRI screening for BRCA mutation carriers: the first report in Japan. Breast Cancer. 2019;26:552–61.
Schrading S, Kuhl CK. Mammographic, US and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58–70.
Riedl C, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015;33:1128–35.
Sridharan A, Eisenbrey JR, Dave JK, et al. Quantitative nonlinear contrast-enhanced ultrasound of the breast. AJR Am J Roentgenol. 2016;207:274–81.
Wan C, Du J, Fang H, et al. Evaluation of breast lesions by contrast-enhanced ultrasound: qualitative and quantitative analysis. Eur J Radiol. 2012;81:e444–50.
Janu E, Krikavova L, Little J, et al. Prospective evaluation of contrast-enhanced ultrasound of breast BI-RADS 3–5 lesions. BMC Med Imaging. 2020;20:66.
Kanazawa S, Mitsuzuka Y, Ogata H, et al. Comparison of CEUS with enhanced MR-mammography in patients with breast cancer. Ultrasound Med Biol. 2011;37:S47.
Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;378:111–21.
Kalinsky K, Barlow WE, Gralow JR, et al. 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med. 2021;385:2336–47.
Gramiak R, Shah PM, Kramer DH. Ultrasound cardiography: contrast studies in anatomy and function. Radiology. 1969;92:939–47.
Meltzer RS, Tickner EG, Sahines TP, et al. The source of ultrasound contrast effect. J Clin Ultrasound. 1980;8:121–7.
Matsuda Y, Yabuuchi I. Hepatic tumors: US contrast enhancement with CO2 microbubbles. Radiology. 1986;161:701–5.
Kudo M, Tomita S, Tochio H, et al. Hepatic nodular hyperplasia: Specific findings at dynamic contrast-enhanced US with carbon dioxide microbubbles. Radiology. 1991;179:377–82.
Kudo M, Tomita S, Tochio H, et al. Small hepatocellular carcinoma: diagnosis with US angiography with intraarterial CO2 microbubbles. Radiology. 1992;182:155–60.
Nomura Y, Matsuda Y, Yabuuchi I, et al. Hepatocellular carcinoma in adenomatous hyperplasia: detection with contrast-enhanced US with carbon dioxide microbubbles. Radiology. 1993;187:353–6.
Matsuda Y, Ito T, Oguchi Y, et al. Rationale of surgical management for recurrent hepatocellular carcinoma. Ann Surg. 1993;217:28–34.
Matsuda Y, Yabuuchi I, Ito T, et al. Classification of ultrasonographic images of hepatocellular carcinoma using galactose-based contrast agent: relation between image patterns and histologic features. J Med Ultrason. 2004;31:111–20.
Moriyasu F, Itoh K. Efficacy of perflubutane microbubble–enhanced ultrasound in the characterization and detection of focal liver lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2009;193:86–95.
Sontum PC. Physicochemical characteristics of Sonazoid, a new contrast agent for ultrasound imaging. Ultrasound Med Biol. 2008;34:824–33.
Watanabe R, Matsumura M, Munemasa T, et al. Mechanism of hepatic parenchyma-specific contrast of microbubble-based contrast agent for ultrasonography microscopic studies in rat liver. Invest Radiol. 2007;42:643–51.
Miyamoto Y, Ito T, Takada E, et al. Phase II clinical study of DD-723 (perflubutane): dose–response study in patients with breast tumors. J Med Ultrason. 2012;39:79–86.
Miyamoto Y, Ito T, Takada E, et al. Efcacy of sonazoid (Perfubutane) for contrast-enhanced ultrasound in the diferentiation of focal breast lesions: phase 3 multicenter clinical trial. AJR Am J Roentgenol. 2014;202:W400–7.
Kim TK. Contrast-enhanced us incorporating Kuppfer-phase findings for the diagnosis of hepatocellular carcinoma. Radiology. 2023;308:e231494.
Minami Y, Kudo M. Contrast-enhanced ultrasonography with Sonazoid in hepatocellular carcinoma diagnosis. Hepatoma Res. 2020;6:46.
Lee JY, Minami Y, Choi BI, et al. The AFSUMB consensus statements and recommendations for the clinical practice of contrast-enhanced ultrasound using Sonazoid. Ultrasonography. 2020;39:191–220.
Minami Y. Understanding the differences between Japanese and U.S. guidelines on clinical practice for contrast-enhanced ultrasound of the liver. J Med Ultrason. 2023;50:1–3.
Kudo M, Ueshima K, Osaki Y, et al. B-Mode ultrasonography versus contrast-enhanced ultrasonography for surveillance of hepatocellular carcinoma: a prospective multicenter randomized controlled trial. Liver Cancer. 2019;8:271–80.
Arita J, Hasegawa K, Takahashi M, et al. Correlation between contrast-enhanced intraoperative ultrasound using Sonazoid and histologic grade of resected hepatocellular carcinoma. AJR Am J Roentgenol. 2011;196:1314–21.
Arita J, Takahashi M, Hata S, et al. Usefulness of contrast-enhanced intraoperative ultrasound using Sonazoid in patients with hepatocellular carcinoma. Ann Surg. 2011;254:992–9.
Zhang Q, Liang X, Zhang Y, et al. A review of contrast-enhanced ultrasound using SonoVue® and Sonazoid™ in non-hepatic organs. Eur J Radiol. 2023;167:111060.
Kang HJ, Lee JM, Kim SW. Sonazoid-enhanced ultrasonography for noninvasive imaging diagnosis of hepatocellular carcinoma: special emphasis on the 2022 KLCA-NCC guideline. Ultrasonography. 2023;42:479–89.
Morin SH, Lim AK, Cobbold JF, et al. Use of second generation contrast-enhanced ultrasound in the assessment of focal liver lesions. World J Gastroenterol. 2007;13:5963–70.
Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics. 2004;24:921–35.
Guo W, Li F, Jia C, et al. The clinical value of conventional ultrasound combined with contrast-enhanced ultrasound in the evaluation of BI-RADS 4 lesions detected by magnetic resonance imaging. Br J Radiol. 2022;95:20220025.
Gu LS, Zhang R, Wang Y, et al. Characteristics of contrast-enhanced ultrasonography and strain elastography of locally advanced breast cancer. J Thorac Dis. 2019;11:5274–89.
Zhao YX, Liu S, Hu YB, et al. Diagnostic and prognostic values of contrast-enhanced ultrasound in breast cancer: a retrospective study. Onco Targets Ther. 2017;10:1123–9.
Wang Y, Fan W, Zhao S, et al. Qualitative, quantitative and combination score systems in differential diagnosis of breast lesions by contrast-enhanced ultrasound. Eur J Radiol. 2016;85:48–54.
Raza S, Baum JK. Solid breast lesions: evaluation with power Doppler US. Radiology. 1997;203:164–8.
Kedar RP, Cosgrove D, McCread VR, et al. Microbubble contrast agent for color Doppler US: effect on breast masses—work in progress. Radiology. 1996;198:679–86.
Less JR, Skalak TC, Sevick EM, et al. Microvascular architecture in a mammary carcinoma: branching patterns and vessel dimensions. Cancer Res. 1991;51:265–73.
Vraka I, Panourgias E, Sifakis E, et al. Correlation between contrast-enhanced ultrasound characteristics (qualitative and quantitative) and pathological prognostic factors in breast cancer. In Vivo. 2018;32:945–54.
Liu W, Zong M, Gong HY, et al. Comparison of diagnostic efficacy between contrast-enhanced ultrasound and DCE-MRI for mass- and non-mass-like enhancement types in breast lesions. Cancer Manag Res. 2020;12:13567–78.
Fujimitsu R, Shimakura M, Urakawa H, et al. Homogeneously enhancing breast lesions on contrast enhanced US: differential diagnosis by conventional and contrast enhanced US findings. Jpn J Radiol. 2016;34:508–14.
Ito T, Ueno E, Endo T, et al. The Japan society of ultrasonics in medicine guidelines on non-mass abnormalities of the breast. J Med Ultrason. 2023;50:331–9.
Uematsu T. Non-mass on breast ultrasound: why doesn’t the ACR BI-RADS breast ultrasound lexicon add the terminology? J Med Ultrason. 2023;50:341–6.
Watanabe T. Features of ductal carcinoma in situ ultrasound images. J Med Ultrason. 2023;50:347–50.
Izumori A, Kokubu Y. Ultrasound diagnosis of non-mass lesions at MRI detected lesions. J Med Ultrason. 2023;50:351–60.
Kubota K, Mori M, Fujioka T, et al. Magnetic resonance imaging diagnosis of non-mass enhancement of the breast. J Med Ultrason. 2023;50:361–6.
Sakakibara J, Nagashima T, Fujimoto H, et al. A review of MRI (CT)/US fusion imaging in treatment of breast cancer. J Med Ultrason. 2023;50:367–73.
Yamaguchi R, Watanabe H, Mihara Y, et al. Histopathology of non-mass-like breast lesions on ultrasound. J Med Ultrason. 2023;50:375–80.
Zhang W, Xiao X, Xu X, et al. Non-mass breast lesions on ultrasound: feature exploration and multimode ultrasonic diagnosis. Ultrasound Med Biol. 2018;44:1703–11.
Xu P, Yang M, Liu Y, et al. Breast non-mass-like lesions on contrast-enhanced ultrasonography: feature analysis, breast image reporting and data system classification assessment. World J Clin Cases. 2020;8:700–12.
Zhang F, Jin L, Li G, et al. The role of contrast-enhanced ultrasound in the diagnosis of malignant non-mass breast lesions and exploration of diagnostic criteria. Br J Radiol. 2021;94:20200880.
Cai YY, Du YC, Zhao L. The kinetic quantitative characteristics of non-mass breast lesions with contrast-enhanced ultrasound: a prospective study. Br J Radiol. 2023;96:20221002.
Wang B, Yang D, Zhang X, et al. The diagnostic value of contrast-enhanced ultrasonography in breast ductal abnormalities. Cancer Imaging. 2023;23:25.
Zhao LX, Liu H, Wei Q, et al. Contrast-enhanced ultrasonography features of breast malignancies with different sizes: correlation with prognostic factors. Biomed Res Int. 2015;2015:613831.
Cao XL, Bao W, Zhu SG, et al. Contrast-enhanced ultrasound characteristics of breast cancer: correlation with prognostic factors. Ultrasound Med Biol. 2014;40:11–7.
Li C, Gong H, Ling L, et al. Diagnostic performance of contrast-enhanced ultrasound and enhanced magnetic resonance for breast nodules. J Biomed Res. 2018;32:198–207.
Wen B, Kong W, Zhang Y, et al. Association between contrast-enhanced ultrasound characteristics and molecular subtypes of breast cancer. J Ultrasound Med. 2022;41:2019–31.
Jia C, Niu Q, Liu L, et al. Value of an expanded range of lesions on contrast-enhanced ultrasound for the diagnosis of hypervascular breast masses. Gland Surg. 2023;12:824–33.
Wan CF, Du J, Fan H, et al. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012;262:450–9.
Spring LM, Bar Y, Isakoff SJ, et al. The evolving role of neoadjuvant therapy for operable breast cancer. J Natl Compr Canc Netw. 2022;20:723–34.
Wan CF, Liu XS, Wang L, et al. Quantitative contrast-enhanced ultrasound evaluation of pathological complete response in patients with locally advanced breast cancer receiving neoadjuvant chemotherapy. Eur J Radiol. 2018;103:118–23.
Saracco A, Szabó BK, Tánczos E, et al. Contrast-enhanced ultrasound (CEUS) in assessing early response among patients with invasive breast cancer undergoing neoadjuvant chemotherapy. Acta Radiol. 2017;58:394–402.
Huang Y, Le J, Miao A, et al. Prediction of treatment responses to neoadjuvant chemotherapy in breast cancer using contrast-enhanced ultrasound. Gland Surg. 2021;10:1280–90.
Peng J, Pu H, Jia Y, et al. Early prediction of response to neoadjuvant chemotherapy using contrast-enhanced ultrasound in breast cancer. Medicine. 2021;100:e25908.
Wang B, Jiang T, Huang M, et al. Evaluation of the response of breast cancer patients to neoadjuvant chemotherapy by combined contrast-enhanced ultrasonography and ultrasound elastography. Exp Ther Med. 2019;17:3655–63.
Jia WR, Tang L, Wang DB, et al. Three-dimensional contrast-enhanced ultrasound in response assessment for breast cancer: a comparison with dynamic contrast-enhanced magnetic resonance imaging and pathology. Sci Rep. 2016;6:33832.
Wang JW, Zheng W, Liu JB, et al. Assessment of early tumor response to cytotoxic chemotherapy with dynamic contrast-enhanced ultrasound in human breast cancer xenografts. PLoS ONE. 2013;8:e58274.
Wan C, Zhou L, Li H, et al. Multiparametric contrast-enhanced ultrasound in early prediction of response to neoadjuvant chemotherapy and recurrence-free survival in breast cancer. Diagnostics. 2023;13:2378.
Lee YJ, Kim SH, Kang BJ, et al. Contrast-enhanced ultrasound for early prediction of response of breast cancer to neoadjuvant chemotherapy. Ultraschall Med. 2019;40:194–204.
Amioka A, Masumoto N, Gouda N, et al. Ability of contrast-enhanced ultrasonography to determine clinical responses of breast cancer to neoadjuvant chemotherapy. Jpn J Clin Oncol. 2016;46:303–9.
Kim Y, Kim SH, Song BJ, et al. Early prediction of response to neoadjuvant chemotherapy using dynamic contrast-enhanced MRI and ultrasound in breast cancer. Korean J Radiol. 2018;19:682–91.
Zhang Q, Yuan C, Dai W, et al. Evaluating pathologic response of breast cancer to neoadjuvant chemotherapy with computer-extracted features from contrast-enhanced ultrasound videos. Phys Med. 2017;39:156–63.
Saracco A, Aspelin P, Leifland K, et al. Bolus compared with continuous infusion of microbubble contrast agent using real-time contrast harmonic imaging ultrasound in breast tumors. Acta Radiol. 2009;50:854–9.
Han MR, Park AY, Seo BK, et al. Association between vascular ultrasound features and DNA sequencing in breast cancer: a preliminary study. Discov Oncol. 2023;14:52.
Cao X, Xue J, Zhao B. Potential application value of contrast-enhanced ultrasound in neoadjuvant chemotherapy of breast cancer. Ultrasound Med Biol. 2012;38:2065–71.
Han X, Jin S, Yang H, et al. Application of conventional ultrasonography combined with contrast-enhanced ultrasonography in the axillary lymph nodes and evaluation of the efficacy of neoadjuvant chemotherapy in breast cancer patients. Br J Radiol. 2021;94:20210520.
Kim R, Chang JM, Lee HB, et al. Predicting axillary response to neoadjuvant chemotherapy: breast MRI and US in patients with node positive breast cancer. Radiology. 2019;293:49–57.
Eun NL, Son EJ, Gweon HM, et al. Prediction of axillary response by monitoring with ultrasound and MRI during and after neoadjuvant chemotherapy in breast cancer patients. Eur Radiol. 2020;30:1460–9.
Hotton J, Salleron J, Henrot P, et al. Pre-operative axillary ultrasound with fine-needle aspiration cytology performance and predictive factors of false negatives in axillary lymph node involvement in early breast cancer. Breast Cancer Res Treat. 2020;183:639–47.
Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850–60.
Agliata G, Valeri G, Argalia G, et al. Role of contrast-enhanced sonography in the evaluation of axillary lymph nodes in breast carcinoma: a monocentric study. J Ultrasound Med. 2017;36:505–11.
Zhao J, Zhang J, Zhu Q, et al. The value of contrast-enhanced ultrasound for sentinel lymph node identification and characterisation in pre-operative breast cancer patients: a prospective study. Eur Radiol. 2018;28:1654–61.
Matsuzawa F, Einama T, Abe H, et al. Accurate diagnosis of axillary lymph node metastasis using contrast-enhanced ultrasonography with Sonazoid. Mol Clin Oncol. 2015;3:299–302.
Bailey A, Layne G, Shahan C, et al. Comparison between ultrasound and pathologic status of axillary lymph nodes in clinically node-negative breast cancer patients. Am Surg. 2015;81:865–9.
Wang L, Li J, Qiao J, et al. Establishment of a model for predicting sentinel lymph node metastasis in early breast cancer based on contrast-enhanced ultrasound and clinicopathological features. Gland Surg. 2021;10:1701–12.
Niu Z, Gao Y, Xiao M, et al. Contrast-enhanced lymphatic US can improve the preoperative diagnostic performance for sentinel lymph nodes in early breast cancer. Eur Radiol. 2023;33:1593–602.
Cha JH, Moon WK, Cho N, et al. Differentiation of benign from malignant solid breast masses: conventional US versus spatial compound imaging. Radiology. 2005;237:841–6.
Lang M, Liang P, Shen H, et al. Head-to-head comparison of perfuorobutane contrast-enhanced US and multiparametric MRI for breast cancer: a prospective, multicenter study. Breast Cancer Res. 2023;25:61.
Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. JAMA. 2015;314:1599–614.
Brem RF, Lenihan MJ, Lieberman J, et al. Screening breast ultrasound: past, present, and future. AJR Am J Roentgenol. 2015;204:234–40.
Kuhl CK, Schrading S, Strobel K, et al. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32:2304–10.
Maeda H, Wu J, Sawa T, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84.
Aalders KC, Tryfonidis K, Senkus E, et al. Anti-angiogenic treatment in breast cancer: facts, successes, failures and future perspectives. Cancer Treat Rev. 2017;53:98–110.
Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005;23:1782–90.
Srivastava A, Laidler P, Hughes LE, et al. Neovascularization in human cutaneous melanoma: a quantitative morphological and Doppler ultrasound study. Eur J Cancer Clin Oncol. 1986;22:1205–9.
Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8.
Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9:216.
Folkman J. Angiogenesis and breast cancer. J Clin Oncol. 1994;12:441–3.
Hida K, Maishi N, Matsuda A, et al. Beyond starving cancer: anti-angiogenic therapy. J Med Ultrason. 2023. https://doi.org/10.1007/s10396-023-01310-1.
Chen Y, Tang L, Du Z, et al. Factors influencing the performance of a diagnostic model including contrast-enhanced ultrasound in 1023 breast lesions: comparison with histopathology. Ann Transl Med. 2019;7:647.
Goubran HA, Kotb RR, Stakiw J, et al. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis. 2014;7:9–18.
Balleyguier C, Opolon P, Mathieu MC, et al. New potential and applications of contrast-enhanced ultrasound of the breast: own investigations and review of the literature. Eur J Radiol. 2009;69:14–23.
Delli Carpini J, Karam AK, Montgomery L. Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian, and cervical cancer. Angiogenesis. 2010;13:43–58.
Uzzan B, Nicolas P, Cucherat M, et al. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–55.
Szabó BK, Saracco A, Tánczos E, et al. Correlation of contrast-enhanced ultrasound kinetics with prognostic factors in invasive breast cancer. Eur Radiol. 2013;23:3228–36.
Mori N, Mugikura S, Takahashi S, et al. Quantitative analysis of contrast-enhanced ultrasound imaging in invasive breast cancer: a novel technique to obtain histopathologic information of microvessel density. Ultrasound Med Biol. 2017;43:607–14.
Li J, Guo L, Yin L, et al. Can different regions of interest influence the diagnosis of benign and malignant breast lesions using quantitative parameters of contrast-enhanced sonography? Eur J Radiol. 2018;108:1–6.
Shima H, Okuno T, Nakamura T, et al. Comparing the extent of breast cancer tumors through contrast-enhanced ultrasound vs B-mode, opposed with pathology: evergreen study. Breast Cancer. 2021;28:405–13.
Park AY, Kwon M, Woo OH, et al. A prospective study on the value of ultrasound microflow assessment to distinguish malignant from benign solid breast masses: association between ultrasound parameters and histologic microvessel densities. Korean J Radiol. 2019;20:759–72.
Xiao X, Ou B, Yang H, et al. Breast contrast-enhanced ultrasound: is a scoring system feasible? A preliminary study in China. PLoS ONE. 2014;9:e105517.
Noro A, Nakamura T, Hirai T, et al. Impact of parametric imaging on contrast-enhanced ultrasound of breast cancer. J Med Ultrason. 2016;43:227–35.
Niu RL, Li SY, Wang B, et al. Papillary breast lesions detected using conventional ultrasound and contrast-enhanced ultrasound: imaging characteristics and associations with malignancy. Eur J Radiol. 2021;141:109788.
Wang W, Zheng Y, Wu XF, et al. Value of contrast-enhanced ultrasound area ratio in identifying benign and malignant small breast masses. Gland Surg. 2020;9:1486–94.
Zhang Y, Zhang B, Fan X, et al. Clinical value and application of contrast-enhanced ultrasound in the differential diagnosis of malignant and benign breast lesions. Exp Ther Med. 2020;20:2063–9.
Li W, Zhou Q, Xia S, et al. Application of contrast-enhanced ultrasound in the diagnosis of ductal carcinoma in situ: analysis of 127 cases. J Ultrasound Med. 2020;39:39–50.
Quan J, Hong Y, Zhang X, et al. The clinical role of contrast-enhanced ultrasound in diferential diagnosis of BI-RADS 4 breast disease. Clin Hemorheol Microcirc. 2019;72:293–303.
Zhao H, Xu R, Ouyang Q, et al. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur J Radiol. 2010;73:288–93.
Liberman L, Morris EA, Dershaw DD, et al. Ductal enhancement on MR imaging of the breast. AJR Am J Roentgenol. 2003;181:519–25.
Luo J, Chen JD, Chen Q, et al. Contrast-enhanced ultrasound improved performance of breast imaging reporting and data system evaluation of critical breast lesions. World J Radiol. 2016;8:610–7.
Xiao X, Jiang Q, Wu H, et al. Diagnosis of sub-centimetre breast lesions: combining BI-RADS-US with strain elastography and contrast-enhanced ultrasound—a preliminary study in China. Eur Radiol. 2017;27:2443–50.
Liu G, Zhang MK, He Y, et al. Bi-Rads 4 breast lesions: could multi-mode ultrasound be helpful for their diagnosis? Gland Surg. 2019;8:258–70.
Lehotska V, Rauova K, Vanovcanova L. Pitfalls of contrast enhanced ultrasound (CEUS) in determination of breast tumor biological dignity. Neoplasma. 2018;65:124–31.
Zhang JX, Cai LS, Chen L, et al. CEUS helps to rerate small breast tumors of BI-RADS category 3 and category 4. Biomed Res Int. 2014;2014:572532.
Tang L, Chen Y, Du Z, et al. A multicenter study of a contrast-enhanced ultrasound diagnostic classification of breast lesions. Cancer Manag Res. 2019;11:2163–70.
Haga M, Hirai T, Nakai T, et al. Evaluation of background parenchymal enhancement in breast contrast-enhanced ultrasound with Sonazoid. J Med Ultrason. 2020;47:591–601.
Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–59.
von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28.
Prevos R, Smidt ML, Tjan-Heijnen VC, et al. Pretreatment differences and early response monitoring of neoadjuvant chemotherapy in breast cancer patients using magnetic resonance imaging: a systematic review. Eur Radiol. 2012;22:2607–16.
Marinovich ML, Macaskill P, Irwig L, et al. Meta-analysis of agreement between MRI and pathologic breast tumour size after neoadjuvant chemotherapy. Br J Cancer. 2013;109:1528–36.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62.
Lee YJ, Kim SH, Kang BJ, et al. Contrast-enhanced ultrasound for early prediction of response of breast cancer to neo-adjuvant chemotherapy. Ultraschall Med. 2019;40:194–204.
Omoto K, Mizunuma H, Ogura S, et al. New method of sentinel node identification with ultrasonography using albumin as contrast agent: a study in pigs. Ultrasound Med Biol. 2002;28:1115–22.
Omoto K, Hozumi Y, Omoto Y, et al. Sentinel node detection in breast cancer using contrast-enhanced sonography with 25% albumin—Initial clinical experience. J Clin Ultrasound. 2006;34:317–26.
Omoto K, Matsunaga H, Take N, et al. Sentinel node detection method using contrast-enhanced ultrasonography with sonazoid in breast cancer: preliminary clinical study. Ultrasound Med Biol. 2009;35:1249–56.
Shimazu K, Ito T, Uji K, et al. Identification of sentinel lymph nodes by contrast-enhanced ultrasonography with Sonazoid in patients with breast cancer: a feasibility study in three hospitals. Cancer Med. 2017;6:1915–22.
Miyake T, Shimazu K, Tanei T, et al. Hookwire-guided sentinel lymph node biopsy using contrast-enhanced ultrasonography followed by a one-step nucleic acid amplification (OSNA) assay for breast cancer. Anticancer Res. 2019;39:6183–92.
Du LW, Liu HL, Gong HY, et al. Adding contrast-enhanced ultrasound markers to conventional axillary ultrasound improves specificity for predicting axillary lymph node metastasis in patients with breast cancer. Br J Radiol. 2021;94:20200874.
Mori N, Mugikura S, Miyashita M, et al. Perfusion contrast-enhanced ultrasound to predict early lymph-node metastasis in breast cancer. Jpn J Radiol. 2019;37:145–53.
Liu S, Cai W, Luo Y, et al. CEUS versus MRI in evaluation of the effect of microwave ablation of breast cancer. Ultrasound Med Biol. 2022;48:617–25.
Ito T, Oura S, Nagamine S, et al. Radiofrequency ablation of breast cancer: a retrospective study. Clin Breast Cancer. 2018;18:e495–500.
Kinoshita T. RFA experiences, indications and clinical outcomes. Int J Clin Oncol. 2019;24:603–7.
Izzo F, Thomas R, Delrio P, et al. Radiofrequency ablation in patients with primary breast carcinoma : a pilot study in 26 patients. Cancer. 2001;92:2036–44.
Fornage BD, Sneige N, Ross MI, et al. Small breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology. 2004;231:215–24.
Minami Y, Nishida N, Kudo M. Therapeutic response assessment of RFA for HCC: contrast-enhanced US. CT and MRI World J Gastroenterol. 2014;20:4160–6.
Inoue T, Kudo M, Hatanaka K, et al. Usefulness of contrast-enhanced ultrasonography to evaluate the post-treatment responses of radiofrequency ablation for hepatocellular carcinoma: comparison with dynamic CT. Oncology. 2013;84:51–7.
Minami Y, Kudo M. Review of dynamic contrast-enhanced ultrasound guidance in ablation therapy for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4952–9.
Minami Y, Nishida N, Kudo M. Imaging diagnosis of various hepatocellular carcinoma subtypes and its hypervascular mimics: differential diagnosis based on conventional interpretation and artificial intelligence. Liver Cancer. 2023;12:103–15.
Nishida N, Yamakawa M, Shiina T, et al. Artificial intelligence (AI) models for the ultrasonographic diagnosis of liver tumors and comparison of diagnostic accuracies between AI and human experts. J Gastroenterol. 2022;57:309–21.
Nishida N, Yamakawa M, Shiina T, et al. Current status and perspectives for computer-aided ultrasonic diagnosis of liver lesions using deep learning technology. Hepatol Int. 2019;13:416–21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ito, T., Manabe, H., Kubota, M. et al. Current status and future perspectives of contrast-enhanced ultrasound diagnosis of breast lesions. J Med Ultrasonics (2024). https://doi.org/10.1007/s10396-024-01486-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10396-024-01486-0