Abstract
Purpose
Breast cancer is the most common cancer among females worldwide. Axillary lymph node involvement is an important prognostic factor in pre-operative evaluation. The aim of this study was to evaluate the sensitivity and accuracy of AUS during the initial breast cancer diagnosis and the contribution of ultrasound with guided FNAC (AUS + FNAC) in cases of suspicious node.
Methods
A retrospective study was conducted at the Lorraine Cancer Institute between 1 January and 31 December 2015. It included patients with early breast cancer, all of whom received AUS. If axillary node involvement was suspected, FNAC was performed. Sentinel lymph node biopsy (SLNB) and/or axillary lymph node dissection (ALND) were performed depending on FNAC results.
Results
In total, 292 patients were included. 88 patients (30.1%) had a suspicious lymph node on ultrasound and had FNAC, of whom 53 tested positive for axillary node involvement (60.2%). Among the 35 patients who tested negative with FNAC, 15 had axillary metastatic involvement. Performance of AUS + FNAC was better than that of AUS alone, with sensitivity, specificity, positive predictive and negative predictive values of approximately 44.5%, 100%, 100% and 72.4%, respectively, and accuracy of approximately 77.4%. Luminal A subgroup, axillary involvement of less than two positive nodes or nodal tumor of less than 7 mm are independent factors of false negative rate.
Conclusions
AUS performance would seem to be improved by FNAC, with a false negative rate of approximately 26%. It may be possible to reduce the false negative rate of AUS if its contributing factors are taken into consideration, along with the impact of specific echographic signs as revealed by experienced radiologists.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer among females in France. In 2018, its incidence in women in France was approximately 56,162 new cases (33.5% of all cancers in women), and its mortality rate reached 13,353 deaths. This disease is the leading cause of death due to cancer for women in France and worldwide [1].
The identification of axillary lymph node involvement has a prognostic impact in breast cancer management, allows the appropriate treatment to be carried out and can influence surgical and adjuvant treatment [2, 3]. But this method is associated with complications like lymphedema or alteration of shoulder mobility [4]. Management of the axilla change and patients with axillary node-negative exploration benefit from sentinel lymph node biopsy (SLNB). Although the sentinel procedure is less invasive and associated with less morbidity than axillary lymph node dissection (ALND), up to 50% of the SLNBs collected are tumoral and require radical axillary surgery [5, 6].
The advantages of pre-operative identification of axillary metastases include allowing direct dissection of the axillary lymph nodes, avoiding unnecessary SLNB and the need for a second surgical procedure involving the axillary lymph nodes.
As physical examination is insufficient (sensitivity of 40% and high false positive rates of more than 50%), axillary ultrasound (AUS) is an economical and non-invasive alternative method [7]. Used alone, it remains limited by its sensitivity and specificity, which in the literature average 63% and 88%, respectively [8]. Its performance is improved when it is combined with fine-needle aspiration cytology (FNAC), especially improving specificity and positive predictive values, up to almost 100% and 95%, respectively [9,10,11,12].
The aim of this study was to evaluate the sensitivity and accuracy of AUS during the initial breast cancer diagnosis and the contribution of ultrasound with guided FNAC (AUS + FNAC) in cases of suspicious node. Other objectives were to identify clinical, ultrasound and pathologic factors that reveal false negatives in AUS results.
Materials and methods
Data were retrieved retrospectively from the Hospital Database of the Department of Surgical Oncology at the Lorraine Cancer Institute, France, between 1 January and 31 December 2015.
Eligibility criteria
The analysis focused on patients who had been diagnosed with breast cancer and who had received an AUS with or without FNAC as part of the initial diagnostic assessment. Patients with newly diagnosed breast cancer during the study period, with tumors classified as T1 or T2, and having pre-operative AUS were included. They also had to have had a surgical evaluation by SLNB and/or ALND. The exclusion criteria were tumors classified as more than T3, ductal carcinoma in situ, men, metastatic lesions, breast or axillary recurrence, and patients treated with neo-adjuvant chemotherapy.
Population characteristics
To determine predictive factors associated with node involvement, demographic and histological data were evaluated for each patient: age, menopausal status, clinical stage T and N, tumor size, multifocality in the breast, type of surgery [breast-conserving surgery (BCS) or total mastectomy, SLNB, ALND], final histological results [tumor size, histological type, Scarff–Bloom–Richardson (SBR) grade, Ki67 rate, estrogen and progesterone receptors, HER2 status], presence of lymphovascular space invasions (LVSIs), number of involved lymph nodes and the presence of extracapsular effraction. Immunohistochemical classification subtypes of breast cancers were defined as Luminal A (estrogen and progesterone receptors > 10%, Ki67 ≤ 20% and negative HER2 immunohistochemical staining) Luminal B (estrogen receptor > 10% and progesterone receptors < 10% or Ki67 > 20%), basal-like (estrogen and progesterone receptors < 10% and negative HER2 immunohistochemical staining) and overexpressed HER2 (HER2 immunohistochemical staining +++ or ++ and amplified in situ hybridization) [13].
AUS, axillary surgery and histological analysis
Each patient had bilateral mammography and breast ultrasound at the time of initial management. An AUS was performed at the same time. These investigations were carried out by four expert radiologists. A lymph node was classified as suspect in case of increased lymph node size, cortical thickening (> 3 mm), cortical hypo echogenicity or loss of fatty hilum and the AUS result was given as positive. All patients with an axillary node suspected of a secondary location had a FNAC using a 21G fine needle. The FNAC result was qualified as positive when lymph node cytology was found to have metastatic involvement. In these cases, ALND was performed; otherwise the patient was eligible for a SLNB. The presence of isolated tumor cells (i+) in the axilla was considered as negative (N0). Except when there were axillary lesions (N+), an ALND was performed without SLNB procedure in cases of multifocality (when 2 tumor foci were more than 5 cm apart), multicentricity (2 foci located in 2 different quadrants or when there were more than 2 foci). Some patients presenting a suspicious node and negative FNAC at the time of the initial evaluation had ALND for a larger or multifocal tumor, after discussion at a multidisciplinary meeting. An ALND was also performed in case of failure of the SLNB procedure.
Statistical analysis
Quantitative parameters were described as mean and standard deviation, qualitative parameters as frequency and percentage. Performance of AUS alone was assessed by considering the results of ALND or SLNB as the gold standard. Sensitivity was measured as the number of patients with a positive AUS result out of the number of patients with positive ALND or SLNB results. Specificity was measured as the number of patients with negative AUS results out of the number of patients with negative ALND and SLNB results. The positive predictive value was the number of patients with positive ALND or SLNB results out of the number of positive AUS results, whereas the negative predictive value was the number of patients with negative ALND and SLNB out of the number of patients with negative AUS results. Accuracy was measured as the true positive plus the true negative out of the total number of patients. The same computations were performed for AUS combined with FNAC by considering patients with positive AUS and positive FNAC results as ‘positive’ patients, and patients with negative AUS or patients with positive AUS and negative FNAC as ‘negative’ patients. The McNemar test was performed to compare sensitivities in the subgroup of patients with positive ALND or SLNB, and to compare specificities in the subgroup of patients with negative ALND and SLNB. Prognostic factors of false negative (or true positive) for the combined method (AUS + FNAC) were investigated in the subgroup of the 131 patients with axillary involvement. Bivariate logistic regression was performed for each prognostic factor and parameters with a p value of less than 0.1 were introduced in a multivariate logistic regression with backward selection. Results were expressed as the odds ratio and 95% confidence interval.
All p values of less than 0.05 were considered statistically significant. All data were analyzed using SAS software, version 9.4 (Institute, Inc., Cary, NC, USA).
Results
A total of 292 patients were included retrospectively from the study period. Table 1 details their demographic, clinical and surgical characteristics.
The average age at diagnosis was 61.7 ± 12.2 years. 219 patients had a cT1 tumor (75%) and 73 patients had a cT2 tumor (25%). Clinical axillary lymph node evaluation was negative in 91.8% of cases (n = 268) and cN1 in the remaining cases. 58 patients had multifocal involvement in the breast (19.9%). The majority of the lesions were classified as ACR4 (34.8%, n = 100) or ACR5 (59.9%, n = 172) and more than half had associated microcalcifications (54.9%, n = 104). The average size of the breast lesions on ultrasound was 14.4 ± 16 mm. 88 patients (30.1%) had a suspicious lymph node on ultrasound and had FNAC.
197 patients received BCS (67.5%), while the others underwent total mastectomy (32.5%). A SLNB procedure was performed in 67.8% of cases (n = 198). The average number of lymph nodes collected during this procedure was 3.1 ± 1.8 nodes. 94 patients (32.2%) benefited from ALND, including 53 patients with positive FNAC results and 41 who had multifocality or additional ALND after positive SLNB results. Among the 24 patients who had additional ALNDs out of the 56 who had positive SLNB results, 3 had complementary metastatic lymph nodes.
Final histological results
Tumor histological characteristics are presented in Table 2. The majority of lesions were of an invasive ductal carcinoma (82.9%), and the average size of the lesions was 19.3 + 15.7 mm. Histopronostic criteria were distributed as follows: 32.9% of SBR 3 grade tumors, 25.5% had a Ki67 greater than 30%, 11.7% overexpressed HER2. The biomolecular classification showed 51.9% of luminal A subtype, 27.1% of luminal B subtype, 9.3% basal-like and 11.7% overexpressing HER2. Concerning axillary data (Table 3), 40.7% had axillary lymph node positivity, 22.2% (n = 44) of which had positive SLNB results out of the 198 patients who benefit from SLNB. Among the 94 patients who benefit from ALND, 79.8% of ALND were positive, with an average of 5.9 ± 6.8 positive nodes; 19.3% were micrometastases. The average size of the tumor site was 8.9 ± 10.0 mm. More than half of axillary involvement had extracapsular extension. When cytology was positive, the mean number of positive nodes was approximately 4.
AUS + FNAC performance
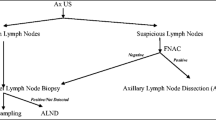
Axillary lymph node examination is summarized in Fig. 1. We selected all patients who received AUS. Of the 204 negative AUSs, 51 procedures had positive lymph nodes on pathologic assessment of the surgical specimen. The 88 patients with positive AUSs had FNAC. The main characteristic that led to FNAC was cortical thickness greater than 3 mm (71.6%). All cytologies were performed with fine-needle aspiration. 60.2% were positive (n = 53). Among the 35 negative FNACs, 15 of the next procedures (SLNB/ALND) were positive with metastatic axillary metastatic involvement. For the combined method (AUS + FNAC), the detection rate was 60.2% (53/88) and the false negative rate was 42.8% (15/35). The sensitivity, specificity, positive predictive and negative predictive values (Table 4) of the combined method were 44.5%, 100%, 100% and 72.4%, respectively, with an accuracy of 77.4%. Sensitivity of the combined method was better for AUS alone (p < 0.001), whereas specificity was better for AUS + FNAC (p < 0.001).
Predictive factors of false negatives of AUS + FNAC
The data are presented in Table 5. The data considered were from a group of 119 patients with axillary involvement and included 66 false negative cases (15 negatives cases after FNAC and 51 negative cases with only AUS). In bivariate analyses, an ultrasound tumor size of less than 20 mm, grade 1, with a low Ki67 rate or luminal A was significantly associated with a risk of false negative evaluation. At the axillary level, two or fewer affected lymph nodes, a tumor site of less than 7 mm and no capsular rupture were associated with a risk of false negative evaluation. In multivariate analysis, luminal A biomolecular status, minimal axillary involvement with less than two affected lymph nodes or tumor site of less than 7 mm were independent factors of false negative of AUS + FNAC evaluation procedure.
Discussion
Axillary lymph node involvement is a major prognostic factor in the management of breast cancer [14]. A pre-operative axillary evaluation will allow the axillary surgical procedure to be adapted from the outset during initial management, which will consist of ALND in the case of positivity or a SLNB in other cases.
AUS is now performed systematically [15]. Several ultrasound criteria can be used to characterize suspect lymph nodes. Its detection rate is good, but alone it remains insufficient [16]. When AUS is combined with FNAC, a greater number of pathological axillary lymph nodes are detected during pre-operative assessment [2, 10]. In our study, AUS–FNAC had low sensitivity (44.5%) but high specificity (100%), whereas AUS alone had a sensitivity of 57.1% and specificity of 88.4%. Identification of a positive lymph node had a positive predictive value of 100% and negative predictive value of 72.4%. These data are consistent with the literature [15, 17]. The sensitivity of AUS + FNAC was lower than AUS alone with similar accuracy. This difference could be explained by the fact that FNAC was performed as soon as an axillary node was appeared suspicious and not as soon as it was visualized on ultrasound. These data are linked to the performance of radiologists, ultrasound being a dynamic ‘operator-dependent’ examination. Ultrasound by experienced radiologists could be used to rule out significant lymph node involvement in most early breast cancer patients [18].
Ultrasound criteria were not exhaustively collected because they were not always available in the files, but 2/3 of the suspect lymph nodes in this study had a cortical thickening of more than 3 mm. The other signs indicating FNAC were loss of fatty hilum, lymph node size or nodular thickening. These data improved the performance of the test. Cortical thickness is the most sensitive and specific ultrasound characteristic for identifying a metastatic axillary lymph node according to the literature [19]. Another criterion is loss of lymph node hilum. It had good sensitivity but worse specificity [20, 21].
It may also be possible to perform core needle biopsy (CNB). This technique is more sensitive than FNAC but carries a higher risk of hematoma or vascular wound [22]. No CNBs were performed in the study. The other factor that could influence the accuracy is the tumor size. An increased tumor size was an increased risk factor of lymph node involvement [23]. Clinical palpability could also be a factor influencing sensitivity and accuracy, making it easier to guide ultrasound sampling [10]. The presence of several suspicious nodes could be another factor influencing the tests.
The false negative rate in this study was approximately 26%, which is similar to data in the literature (average of 25%) [24,25,26,27]. In bivariate analysis, factors significantly associated with higher false negative rates were tumor sizes of less than 20 mm (OR = 2.7, p = 0.009), lower histological grades (OR = 5.5, p = 0.003), Ki67 rates less than 10% (OR = 2.31, p = 0.001), luminal A histological subtypes (OR = 4.10, p < 0.001), 2 or fewer axillary pathological nodes (OR = 7.43, p < 0.001), lymph node tumor site of less than 8 mm (OR = 8.59, p < 0.001) and absence of extracapsular extension (OR = 7.52, p < 0.001). The independent predictive clinical pathological criteria for false negatives in multivariate analysis in our study were luminal A histological subtype (OR = 3.32, p < 0.001), a maximum of 2 metastatic axillary lymph nodes (OR = 5.11, p < 0.001) and an axillary tumor node of less than 8 mm (OR = 5.11, p < 0.001). Some of these factors were consistent with the literature [24, 28].
Not all micrometastatic diseases were detected by axillary FNAC, owing to the maximum focus size of 2 mm. These data correspond to those in the literature [24, 29]. Lobular histological type and LVSI were not predictive criteria for false negatives in our study, in contrast to previous reports [8, 30,31,32]. This is most likely due to the low percentage of lobular diseases (9.9%) or LVSI (24.6%) in our study. Similarly, BMI had no impact on AUS analysis. Most patients with false negative AUS have significantly fewer than 3 metastatic axillary lymph nodes (OR = 5.11). Reyna et al. studied axillary disease in patients with false negative pre-operative axillary assessment and concluded that the rates of post-operative axillary damage found were similar in most patients with 2 or fewer positive nodes [30]. This is all the more important as the ACOSOG Z0011 study reported that patients with limited axillary metastatic disease do not gain additional benefit from ALND in terms of survival [33, 34]. The detection rate of axillary involvement was lower for the luminal A subtype compared to other histological subtypes (OR = 3.63). A recent study by Helfgott et al. reported a lower sensitivity of AUS in luminal A (25%) and luminal B (40%) cancers than in non-luminal cancers (70%), exposing these patients to a higher risk of inappropriate treatment [28]. The design of this retrospective study does not allow us to determine whether the negative cytology of the suspected node was the same as the positive node on the final histology, it could have been another node and could also explain a part of the false negative rate.
The false positive rate of AUS–FNAC was 0%. This rate is similar to the low rates ranging from 0 to 2% reported in other studies [24, 35].
There were some limitations to our study. First, this was a retrospective study, which limits the validity of the results. The sample size is small and hence there are low numbers for certain variables, limiting some interpretations. Second, we did not have all of the data collected by the radiologists that allowed them to determine the indication for FNAC; for example, other than the ultrasound aspect, it is possible that they were suspicious but near to a vascular pedicle or deep and inaccessible. Third, another potential bias is the interpretation of ultrasound pathological nodes by radiologists. In the center, all external imaging examinations are reviewed systematically. Nevertheless, 20.5% of axillary involvement was found among the negative ultrasound analyses. This bias could be minimized by establishing specific and reproducible imaging criterion to limit inter-observer variability, and to systematize the reviewing of external examinations with the aim to reduce the false negative rates.
Conclusion and perspectives
AUS–FNAC has many advantages. It allows the pre-operative detection of axillary involvement, indicating from the outset the need for surgical ALND in case of positivity, which avoids an unnecessary SLNB procedure.
Low numbers of involved lymph nodes and small metastatic nodes are the strongest independent predictors of false negatives in axillary exploration, followed by histological subtype luminal A.
AUS and FNAC do not seem to detect axillary micrometastases. Further progress is needed to better detect axillary disease.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Houssami N, Diepstraten SCE, Cody HS et al (2014) Clinical utility of ultrasound-needle biopsy for preoperative staging of the axilla in invasive breast cancer. Anticancer Res 34:1087–1097
Yoshihara E, Smeets A, Laenen A et al (2013) Predictors of axillary lymph node metastases in early breast cancer and their applicability in clinical practice. Breast 22:357–361. https://doi.org/10.1016/j.breast.2012.09.003
McLaughlin SA, Wright MJ, Morris KT et al (2008) Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol Off J Am Soc Clin Oncol 26:5213–5219. https://doi.org/10.1200/JCO.2008.16.3725
Purushotham AD, Upponi S, Klevesath MB et al (2005) Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol 23:4312–4321. https://doi.org/10.1200/JCO.2005.03.228
Tanis PJ, Nieweg OE, Valdés Olmos RA, Hoefnagel CA (2002) An original approach in the diagnosis of early breast cancer: use of the same radiopharmaceutical for both non-palpable lesions and sentinel node localisation. Eur J Nucl Med Mol Imaging 29:436–437; author reply 437–438
Valente SA, Levine GM, Silverstein MJ et al (2012) Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol 19:1825–1830. https://doi.org/10.1245/s10434-011-2200-7
Johnson S, Brown S, Porter G et al (2011) Staging primary breast cancer. Are there tumour pathological features that correlate with a false-negative axillary ultrasound? Clin Radiol 66:497–499. https://doi.org/10.1016/j.crad.2010.11.015
Lee MC, Kilbride KE (2009) Timing of axillary staging. Ann Surg Oncol 16:1065. https://doi.org/10.1245/s10434-009-0352-5
Jankowski C, Hudry D, Vaillant D et al (2015) Evaluation of axillary involvement by ultrasound-guided lymph node biopsy: a prospective study. Gynecol Obstet Fertil 43:431–436. https://doi.org/10.1016/j.gyobfe.2015.04.007
Park SH, Kim MJ, Park B-W et al (2011) Impact of preoperative ultrasonography and fine-needle aspiration of axillary lymph nodes on surgical management of primary breast cancer. Ann Surg Oncol 18:738–744. https://doi.org/10.1245/s10434-010-1347-y
O’Leary DP, O’Brien O, Relihan N et al (2012) Rapid on-site evaluation of axillary fine-needle aspiration cytology in breast cancer. Br J Surg 99:807–812. https://doi.org/10.1002/bjs.8738
Goldhirsch A, Winer EP, Coates AS et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–2223. https://doi.org/10.1093/annonc/mdt303
Khout H, Richardson C, Toghyan H, Fasih T (2013) The role of combined assessment in preoperative axillary staging. Ochsner J 13:489–494
Alvarez S, Añorbe E, Alcorta P et al (2006) Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. Am J Roentgenol 186:1342–1348. https://doi.org/10.2214/AJR.05.0936
Moore A, Hester M, Nam M-W et al (2008) Distinct lymph nodal sonographic characteristics in breast cancer patients at high risk for axillary metastases correlate with the final axillary stage. Br J Radiol 81:630–636. https://doi.org/10.1259/bjr/21933846
Houssami N, Ciatto S, Turner RM et al (2011) Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg 254:243–251. https://doi.org/10.1097/SLA.0b013e31821f1564
Nwaogu IY, Yan Y, Appleton CM et al (2015) Predictors of false negative axillary ultrasound in breast cancer. J Surg Res 198:351–354. https://doi.org/10.1016/j.jss.2015.03.057
Choi JS, Kim MJ, Moon HJ et al (2012) False negative results of preoperative axillary ultrasound in patients with invasive breast cancer: correlations with clinicopathologic findings. Ultrasound Med Biol 38:1881–1886. https://doi.org/10.1016/j.ultrasmedbio.2012.07.011
Abe H, Schmidt RA, Sennett CA et al (2007) US-guided core needle biopsy of axillary lymph nodes in patients with breast cancer why and how to do it. Radiogr Rev Publ Radiol Soc N Am Inc 27(Suppl 1):S91–S99. https://doi.org/10.1148/rg.27si075502
Ewing DE, Layfield LJ, Joshi CL, Travis MD (2015) Determinants of false-negative fine-needle aspirates of axillary lymph nodes in women with breast cancer: lymph node size, cortical thickness and Hilar fat retention. Acta Cytol 59:311–314. https://doi.org/10.1159/000440797
Rao R, Lilley L, Andrews V et al (2009) Axillary staging by percutaneous biopsy: sensitivity of fine-needle aspiration versus core needle biopsy. Ann Surg Oncol 16:1170–1175. https://doi.org/10.1245/s10434-009-0421-9
Gilissen F, Oostenbroek R, Storm R et al (2008) Prevention of futile sentinel node procedures in breast cancer: ultrasonography of the axilla and fine-needle aspiration cytology are obligatory. Eur J Surg Oncol 34:497–500. https://doi.org/10.1016/j.ejso.2007.07.198
Kane G, Fleming C, Heneghan H et al (2019) False-negative rate of ultrasound-guided fine-needle aspiration cytology for identifying axillary lymph node metastasis in breast cancer patients. Breast J. https://doi.org/10.1111/tbj.13402
Diepstraten SCE, Sever AR, Buckens CFM et al (2014) Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol 21:51–59. https://doi.org/10.1245/s10434-013-3229-6
Cools-Lartigue J, Meterissian S (2012) Accuracy of axillary ultrasound in the diagnosis of nodal metastasis in invasive breast cancer: a review. World J Surg 36:46–54. https://doi.org/10.1007/s00268-011-1319-9
Neal CH, Daly CP, Nees AV, Helvie MA (2010) Can preoperative axillary US help exclude N2 and N3 metastatic breast cancer? Radiology 257:335–341. https://doi.org/10.1148/radiol.10100296
Helfgott R, Mittlböck M, Miesbauer M et al (2019) The influence of breast cancer subtypes on axillary ultrasound accuracy: a retrospective single center analysis of 583 women. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 45:538–543. https://doi.org/10.1016/j.ejso.2018.10.001
Chadha M, Chabon AB, Friedmann P, Vikram B (1994) Predictors of axillary lymph node metastases in patients with T1 breast cancer. a multivariate analysis. Cancer 73:350–353. https://doi.org/10.1002/1097-0142(19940115)73:2<350:aid-cncr2820730219>3.0.co;2-5
Reyna C, Lee MC, Frelick A et al (2014) Axillary burden of disease following false-negative preoperative axillary evaluation. Am J Surg 208:577–581. https://doi.org/10.1016/j.amjsurg.2014.05.015
Pestalozzi BC, Zahrieh D, Mallon E et al (2008) Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol Off J Am Soc Clin Oncol 26:3006–3014. https://doi.org/10.1200/JCO.2007.14.9336
Malter W, Hellmich M, Badian M et al (2018) Factors predictive of sentinel lymph node involvement in primary breast cancer. Anticancer Res 38:3657–3662. https://doi.org/10.21873/anticanres.12642
Giuliano AE, Hunt KK, Ballman KV et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575. https://doi.org/10.1001/jama.2011.90
Giuliano AE, Ballman K, McCall L et al (2016) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg 264:413–420. https://doi.org/10.1097/SLA.0000000000001863
Gilani SM, Fathallah L, Al-Khafaji BM (2014) Preoperative fine needle aspiration of axillary lymph nodes in breast cancer: clinical utility, diagnostic accuracy and potential pitfalls. Acta Cytol 58:248–254. https://doi.org/10.1159/000362682
Acknowledgements
The authors thank all of the Teams of the Department of Surgical Oncology of the Lorraine Cancer Institute (Vandoeuvre-lès-Nancy) for their technical support. The authors also thank AngloScribe Society for English proofreading.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
JH designed the study, developed the methodology, collected data and wrote the manuscript. FM designed the study, developed the methodology, analyzed data and wrote the manuscript. JS performed the statistical analysis, helped to write the manuscript and to interpret data. PH analyzed radiological data. PR collected data and helped to interpret data. JB and LL helped to interpret data. All the authors have reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to declare.
Ethical approval
According to French regulation, patients were informed of the researches performed and did not express opposition. The study was authorized by the Internal Scientific Committee and Ethical Board of the Institut de Cancérologie de Lorraine (05-2019).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hotton, J., Salleron, J., Henrot, P. et al. Pre-operative axillary ultrasound with fine-needle aspiration cytology performance and predictive factors of false negatives in axillary lymph node involvement in early breast cancer. Breast Cancer Res Treat 183, 639–647 (2020). https://doi.org/10.1007/s10549-020-05830-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05830-z