Abstract
An ultrasound (US) examination is a common noninvasive technique widely applied for diagnosis of a variety of diseases. Based on the rapid development of US equipment, many US images have been accumulated and are now available and ready for the preparation of a database for the development of computer-aided US diagnosis with deep learning technology. On the contrary, because of the unique characteristics of the US image, there could be some issues that need to be resolved for the establishment of computer-aided diagnosis (CAD) system in this field. For example, compared to the other modalities, the quality of a US image is, currently, highly operator dependent; the conditions of examination should also directly affect the quality of US images. So far, these factors have hampered the application of deep learning-based technology in the field of US diagnosis. However, the development of CAD and US technologies will contribute to an increase in diagnostic quality, facilitate the development of remote medicine, and reduce the costs in the national health care through the early diagnosis of diseases. From this point of view, it may have a large enough potential to induce a paradigm shift in the field of US imaging and diagnosis of liver diseases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ultrasonography (US) is a common and widespread modality in the screening and diagnosis of many diseases because of its noninvasiveness and convenience [1,2,3]. However, for operators, extensive experience of US examination should be required for the accurate diagnosis of the lesions because of the necessity of real-time recognition of abnormal lesions during examinations [4, 5].
A lack of specialists for US imagining and diagnosis is an urgent medical matter that needs to be solved. Recently, in addition to conventional machine learning, sophisticated deep learning technology is becoming available in this field. From this point of view, development of computer-aided detection and diagnosis will be an attractive solution for the US diagnosis, especially in underpopulated areas with insufficient medical services. Based on these backgrounds, several reports that applied machine learning technology for the diagnosis of diseases have been published [6,7,8]. However, because of the specific nature of US diagnosis, there are still several issues that need to be resolved for the application of computer-aided diagnosis (CAD) in this field [9]. In this review, we evaluate the current performance of CAD for liver lesions based on the previous publications, and discuss the advantages and problems to be solved for future establishment of deep learning-based CAD system as a screening tool for US examination of liver disease.
Advance in computer-aided diagnosis for medical imaging
Recently, several studies reported the impact of CAD using deep learning algorithm for medical imaging. Gulshan et al. [10] reported the performance of deep convolutional neural networks (CNNs) for automated detection of diabetic retinopathy and diabetic macular edema using a dataset of 128,175 photo images of retinal fundus. The performance of algorithm showed an area under the receiver operating curve (AUC) of 0.991, indicating that the use of this algorithm could show a positive impact on improved care and outcomes. Another study reported the diagnostic assessment of deep learning algorithms for detecting lymph node metastases of breast cancer through the training of whole slide image with and without nodal metastasis [11]. Reportedly, the performance was comparable with that of an expert pathologist without time constraints. In the field of dermatology, deep learning using a dataset of 129,450 clinical images of skin lesions by CNNs achieved performance on par with expert dermatologists for classifying skin cancers [12]. On the other hand, creating large dataset of medical image sometimes requires many skilled people and much time. From this point of view, efficacy of transfer learning algorithm was also reported for identifying the common treatable blinding retinal disease and pediatric pneumonia using smaller dataset of optical coherence tomography and chest X-ray images, respectively [13].
Current status of computer-aided US diagnosis for liver lesions
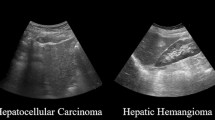
Compared to the image diagnosis using dataset of other photo images, US images are more heterogeneous for the CAD in terms of their image parameters, setting of equipment, and condition of examination [14,15,16,17,18]. To date, several studies have shown the effectiveness of machine learning for the diagnosis of focal liver lesions by a B-mode ultrasound image (Table 1). For example, Hwang et al. [19] tried to extract textural features of focal lesions such as cysts, hemangiomas, and malignant lesions for the diagnosis of a liver tumor; they demonstrated high diagnostic accuracy among all focal liver lesion groups. Contrast-enhanced US (CEUS) was also applied for more accurate diagnosis of liver tumors [20, 21]. Kondo et al. [22] reported the automatic classification of benign tumors, hepatocellular carcinoma, and metastatic tumors using machine learning of the CEUS image. They used a contrast agent, Sonazoid, and found that sensitivity, specificity, and accuracy that distinguish malignant lesions from benign were 94.0%, 87.1%, and 91.8%, respectively. Another report applied a multiple kernel learning-based framework, achieving accuracy of more than 90% for the different diagnosis of benign and malignant liver tumors using CEUS images [23]. Although, some studies applied neural network for CAD of focal liver lesions, their accuracies are similar to those by support vector machine, probably because of the limited size of training cohort. However, it is expected that development of deep neural network-based CAD using larger dataset should help to achieve more accurate and refined US diagnosis of focal liver diseases, such as that reported in CAD for retinopathy and skin lesions [10, 12].
Further, application of CAD for diffuse liver lesions has also been reported (Table 2). It is also known that machine learning is useful for the discrimination of fatty liver disease (FLD) from normal liver [24,25,26]. These studies constructed the computer-aided diagnostic software for detecting FLD using a B-mode US image. More importantly, Acharya et al. [27] reported an algorithm to discriminate automatically the normal, FLD, and cirrhosis US image using a neural network classifier with an accuracy of 97.33%, specificity of 100% and sensitivity of 96%. Because a subset of FLD might progress to liver cirrhosis, such discrimination is informative in the clinical setting.
For the diagnosis of liver cirrhosis, some studies applied liver stiffness by transient elastography for the algorithm of artificial neural networks and showed high diagnostic performance for the diagnosis of cirrhosis, portal hypertension, and esophageal varices [28, 29]. Other studies focused on the parameters of the B-mode image and the Doppler method, such as liver parenchyma, thickness of spleen, hepatic vein waveform and damping index, and hepatic artery pulsatile index, for the grading of liver fibrosis [30]. However, the number of images from previous machine learning trials is still small (< 1000 images); a larger US image database with reliable supervised data should be constructed for the development of a solid CAD algorithm. On the other hand, some reports also showed the efficacy of deep leaning algorithm for diffuse liver disease, such as detecting FLD using B-mode images [31], and staging of liver fibrosis using the share wave elastography images [32]. Interestingly, accuracy for detecting FLD is 100% by deep leaning algorithm; higher than that by conventional machine learning [31]. Deep learning-based CAD also showed detailed classification of liver fibrosis [32]. Using the dataset of US images from dog livers, Banzato et al. [33] developed a deep learning-based US image diagnosis for detecting degenerative hepatic disease. They also used transfer learning, and reported the high sensitivity and specificity for diagnostic accuracy compared to those by cytology and serum biochemical markers. Therefore, it is conceivable that deep learning using larger dataset should be a powerful tool for detecting diffuse liver lesions.
Future perspectives for the development of computer-aided US diagnosis applicable for clinical practice
US is a common and noninvasive imaging modality and, therefore, applicable for the diagnosis of many diseases in a wide range of organs including digestive, reproductive, urogenital, and endocrine organs, and the circulatory system. It is also a useful medical device for screening diseases because of its handiness. In addition, the development of new technology in the field of US diagnosis, such as US elastography, Doppler US, and CEUS, allows us to apply US imaging for detailed examination of abnormal lesions. Therefore, a large number of images with solid supervised data may be collectable for constructing a database essential for deep learning.
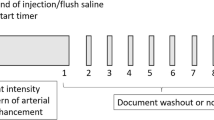
Firstly, for the development of computer-aided or artificial intelligence (AI)-aided US diagnosis, deep learning for disease screening, which can detect the pathological findings and distinguish them from the physiological image, should be required. Secondly, a function for the differential diagnosis of the lesion is also needed (Fig. 1). Because there is a complex normal architecture consisting of vessels and the bile duct in the liver with anatomical anomalies, the pattern of still images in each segment of the liver that are restricted by the position and direction of the probe should be unified among operators to distinguish pathological abnormalities from normal structures [34]. In addition, for the development of deep leaning algorithm for different diagnoses of hepatic lesions, supervised image data, where specific lesions and definitive diagnoses are linked, should be essential. After the learning of still image data and the diagnosis process of the disease, a database of movie images taken in the unified order throughout the entire part of the liver needs to be constructed. Video images of Doppler US and CEUS are also optical materials in the development of a robust AI algorithm for diagnosis.
Workflow for the development of deep learning-based diagnosis system in ultrasonography. The application of deep learning technology for ultrasonography (US) image diagnosis consists of two steps. Firstly, deep learning using supervised images data of pathological lesions is required. At this point, recognition of the lesions is performed by the operators. This step aims at achieving accurate diagnosis through deep learning; both focal (such as liver cancer and benign tumors) and diffuse liver lesions (such as fatty liver and liver cirrhosis) are required using still image (including B-mode and elastography-based images), and movie image (B-mode, contract-enhanced US, and color Doppler images). Secondly, for the development of deep learning-based screening system, a database that includes normal liver images and artifact images needs to be constructed. In this step, computer (or artificial intelligence: AI) is required to detect pathological lesions. The segments and detailed portion, where the lesions exist, also need to be determined. For this purpose, position of normal structure consists of many vessels and ducts should be recognized by AI; still and movie images database should be analyzed. Fusing these technologies will facilitate the next-generation AI-equipped US
Current issues in the development of computer-aided diagnosis on ultrasonography in liver disease
Because of the unique characteristics of the US image, there could be some issues that need to be resolved for the development of CAD system. For example, compared to the other images, the quality of the US image is, currently, highly operator dependent in terms of detection and specification of the lesions. In addition, differences in the software and design of imaging systems among the machines, models, and vendors could also affect the image display. The unique function of US, such as image parameters including gain, sensitivity time control, and mechanical index, could also lead to the difference in image quality. Therefore, the examination condition should directly affect the differences in US images.
On the other hand, a recent report showed the efficacy of deep learning and transfer learning for detecting liver lesion, regardless of the difference of their US image parameters [33]. However, although some studies applied deep neural network algorithm for developing CAD system of US images, the cohort size is still too small to achieve an enough performance of deep learning (Tables 1, 2). Therefore, constructing a big database for deep learning-based CAD system should be essential, which could overcome the heterogeneity of imaging for computer-aided US image diagnosis. In addition, US images may be preferably linked to image parameters unique to each US examination. Further analyses should be conducted to clarify these matters.
In addition, as mentioned, complex normal liver architectures have also made the application of AI-based technology difficult for US diagnosis; the procedure of US examination needs to be unified among the operators for the construction of a well-qualified database of US images. From this point of view, several projects are currently ongoing that overcome the heterogeneity of US images and help to develop the operator-friendly system of computer-aided US diagnosis in the field of liver diseases. As AI-aided US diagnosis should have the potential for the management of liver disease, such as detecting early response to cancer treatment, which is difficult for conventional methods [35,36,37], its development is rapidly expanding in many fields, including the diagnosis of liver diseases [38,39,40].
References
Kudo M. Breakthrough imaging in hepatocellular carcinoma. Liver Cancer 2016;5:47–54
Makino Y, Imai Y, Igura T, Kogita S, Sawai Y, Fukuda K et al. Feasibility of extracted-overlay fusion imaging for intraoperative treatment evaluation of radiofrequency ablation for hepatocellular carcinoma. Liver Cancer 2016;5:269–279
Kudo M. Defect reperfusion rmaging with sonazoid(R): a breakthrough in hepatocellular carcinoma. Liver Cancer 2016;5:1–7
Park HJ, Choi BI, Lee ES, Park SB, Lee JB. How to differentiate borderline hepatic nodules in hepatocarcinogenesis: emphasis on imaging diagnosis. Liver Cancer 2017;6:189–203
Mohammed HA, Yang JD, Giama NH, Choi J, Ali HM, Mara KC et al. Factors influencing surveillance for hepatocellular carcinoma in patients with liver cirrhosis. Liver Cancer 2017;6:126–136
Minhas F, Sabih D, Hussain M. Automated classification of liver disorders using ultrasound images. J Med Syst 2012;36:3163–3172
Esses SJ, Lu X, Zhao T, Shanbhogue K, Dane B, Bruno M et al. Automated image quality evaluation of T2-weighted liver MRI utilizing deep learning architecture. J Magn Reson Imaging 2018;47:723–728
Yasaka K, Akai H, Abe O, Kiryu S. Deep learning with convolutional neural network for differentiation of liver masses at dynamic contrast-enhanced CT: a preliminary study. Radiology 2018;286:887–896
Huang Q, Zhang F, Li X. Machine learning in ultrasound computer-aided diagnostic systems: a survey. Biomed Res Int 2018;2018:5137904
Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016;316:2402–2410
Ehteshami B, Veta M, van Diest PJ, van Ginneken B, Karssemeijer N, Litjens G et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 2017;318:2199–2210
Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017;542:115–118
Kermany DS, Goldbaum M, Cai W, Valentim CCS, Liang H, Baxter SL, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 2018;172(1122–1131):e1129
Huang W, Li N, Lin Z, Huang GB, Zong W, Zhou J et al. Liver tumor detection and segmentation using kernel-based extreme learning machine. Conf Proc IEEE Eng Med Biol Soc 2013;2013:3662–3665
Mittal D, Kumar V, Saxena SC, Khandelwal N, Kalra N. Neural network based focal liver lesion diagnosis using ultrasound images. Comput Med Imaging Graph 2011;35:315–323
Nishida N, Kudo M. Alteration of epigenetic profile in human hepatocellular carcinoma and its clinical implications. Liver Cancer 2014;3:417–427
Virmani J, Kumar V, Kalra N, Khandelwal N. SVM-based characterization of liver ultrasound images using wavelet packet texture descriptors. J Digit Imaging 2013;26:530–543
Virmani J, Kumar V, Kalra N, Khandelwal N. Characterization of primary and secondary malignant liver lesions from B-mode ultrasound. J Digit Imaging 2013;26:1058–1070
Hwang YN, Lee JH, Kim GY, Jiang YY, Kim SM. Classification of focal liver lesions on ultrasound images by extracting hybrid textural features and using an artificial neural network. Biomed Mater Eng 2015;26(Suppl 1):S1599–S1611
Streba CT, Ionescu M, Gheonea DI, Sandulescu L, Ciurea T, Saftoiu A et al. Contrast-enhanced ultrasonography parameters in neural network diagnosis of liver tumors. World J Gastroenterol 2012;18:4427–4434
Gatos I, Tsantis S, Spiliopoulos S, Skouroliakou A, Theotokas I, Zoumpoulis P et al. A new automated quantification algorithm for the detection and evaluation of focal liver lesions with contrast-enhanced ultrasound. Med Phys 2015;42:3948–3959
Kondo S, Takagi K, Nishida M, Iwai T, Kudo Y, Ogawa K et al. Computer-aided diagnosis of focal liver lesions using contrast-enhanced ultrasonography with perflubutane microbubbles. IEEE Trans Med Imaging 2017;36:1427–1437
Guo LH, Wang D, Qian YY, Zheng X, Zhao CK, Li XL et al. A two-stage multi-view learning framework based computer-aided diagnosis of liver tumors with contrast enhanced ultrasound images. Clin Hemorheol Microcirc 2018;69:343–354
Subramanya MB, Kumar V, Mukherjee S, Saini M. A CAD system for B-mode fatty liver ultrasound images using texture features. J Med Eng Technol 2015;39:123–30
Mihailescu DM, Gui V, Toma CI, Popescu A, Sporea I. Computer aided diagnosis method for steatosis rating in ultrasound images using random forests. Med Ultrason 2013;15:184–190
Kim KB, Kim CW. Quantification of hepatorenal index for computer-aided fatty liver classification with self-organizing map and fuzzy stretching from ultrasonography. Biomed Res Int 2015;2015:535894
Acharya UR, Raghavendra U, Fujita H, Hagiwara Y, Koh JE, Hong TJ et al. Automated characterization of fatty liver disease and cirrhosis using curvelet transform and entropy features extracted from ultrasound images. Comput Biol Med 2016;79:250–258
Procopet B, Cristea VM, Robic MA, Grigorescu M, Agachi PS, Metivier S et al. Serum tests, liver stiffness and artificial neural networks for diagnosing cirrhosis and portal hypertension. Dig Liver Dis 2015;47:411–416
Gatos I, Tsantis S, Spiliopoulos S, Karnabatidis D, Theotokas I, Zoumpoulis P et al. A machine-learning algorithm toward color analysis for chronic liver disease classification, employing ultrasound shear wave elastography. Ultrasound Med Biol 2017;43:1797–1810
Zhang L, Li QY, Duan YY, Yan GZ, Yang YL, Yang RJ. Artificial neural network aided non-invasive grading evaluation of hepatic fibrosis by duplex ultrasonography. BMC Med Inform Decis Mak 2012;12:55
Biswas M, Kuppili V, Edla DR, Suri HS, Saba L, Marinhoe RT et al. Symtosis: a liver ultrasound tissue characterization and risk stratification in optimized deep learning paradigm. Comput Methods Programs Biomed 2018;155:165–177
Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, et al. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut 2018. https://doi.org/10.1136/gutjnl-2018-316204.
Banzato T, Bonsembiante F, Aresu L, Gelain ME, Burti S, Zotti A. Use of transfer learning to detect diffuse degenerative hepatic diseases from ultrasound images in dogs: a methodological study. Vet J 2018;233:35–40
Zeng YZ, Zhao YQ, Liao M, Zou BJ, Wang XF, Wang W. Liver vessel segmentation based on extreme learning machine. Phys Med 2016;32:709–716
Nishida N, Kitano M, Sakurai T, Kudo M. Molecular mechanism and prediction of sorafenib chemoresistance in human hepatocellular carcinoma. Dig Dis 2015;33:771–779
Nishida N, Arizumi T, Hagiwara S, Ida H, Sakurai T, Kudo M. MicroRNAs for the prediction of early response to sorafenib treatment in human hepatocellular carcinoma. Liver Cancer 2017;6:113–125
Nishida N, Kudo M. Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol Res 2018;48:622–634
Tarek M, Hassan ME, El-Sayed S. Diagnosis of focal liver diseases based on deep learning technique for ultrasound images. Arab J Sci Eng 2017;42:3127–3140
Meng DZL, Cao G, Cao W, Zhang G, Hu B. Liver fibrosis classification based on trasnfer learning adn FCNet for ultrasound image. IEEE Access 2017;5:5804–5810
Liu X, Song JL, Wang SH, Zhao JW, Chen YQ. Learning to diagnose cirrhosis with liver capsule guided ultrasound image classification. Sensors 2017;17:E149(Basel).
Acknowledgements
This work was supported by Japan Agency for Medical Research and Development under the Grant number 18lk1010030h0001 (M. Kudo, T. Shiina, N. Nishida), and partially supported by Grant-in-Aid for Scientific Research (KAKENHI: 16K09382) from the Japanese Society for the Promotion of Science (N. Nishida) and a grant from the Smoking Research Foundation (N. Nishida).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
This is not a research paper involving human participants and/or animals; informed consent is not required.
Informed consent
Informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishida, N., Yamakawa, M., Shiina, T. et al. Current status and perspectives for computer-aided ultrasonic diagnosis of liver lesions using deep learning technology. Hepatol Int 13, 416–421 (2019). https://doi.org/10.1007/s12072-019-09937-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-019-09937-4