Abstract
Impacts of invasive species may manifest most strongly if these organisms are highly distinct functionally from the native species they often replace. Yet, should we expect functional differences between native and invasive species of generalist organisms like freshwater crayfish? Some existing evidence has pointed to native and invasive crayfish species as ecologically equivalent. We contribute to this literature by comparing the trophic niches of the globally invasive crayfishes Pacifastacus leniusculus and Procambarus clarkii, by applying carbon and nitrogen stable isotope analyses to replicated allopatric (alone) and sympatric (together) lake populations in western Washington State, USA, where P. clarkii has been recently introduced and P. leniusculus is presumed native. Our study corrected for potential inherent differences in lake food webs as a consequence of lake abiotic or biotic characteristics using random effects in linear mixed effects models. We found that although overall trophic niche size or area of these species was not significantly different, P. leniusculus was significantly higher in trophic position than P. clarkii when also accounting for the effects of body size, sex, and lakes as random effects. This pattern of increased trophic position of P. leniusculus over P. clarkii was conserved over time in one sympatric lake for which we had data over multiple years. Cumulatively, our findings point to trophic differences between the globally cosmopolitan crayfishes P. leniusculus and P. clarkii, particularly when accounting for the ways that ecosystem context can affect food web structure of communities and the trophic resources available to these consumers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Introduced crayfish have been attributed as among the most detrimental freshwater invasive species globally (Gallardo et al. 2016), negatively impacting a diversity of taxa across lotic and lentic food webs, including aquatic macrophytes, native crayfish species, aquatic invertebrates, and vertebrates including fish and amphibians (Matsuzaki et al. 2009; Lodge et al. 2012; Twardochleb et al. 2013). The breadth of these impacts primarily manifests as a consequence of the omnivorous or polytrophic feeding habits of crayfish (Olsen et al. 1991; Dorn and Wojdak 2004; Reynolds et al. 2013), although the effects of invasive crayfish can vary with habitat or ecosystem context (Peters and Lodge 2013; Magoulick 2014; Ruokonen et al. 2014). For example, the most severe whole-ecosystem impacts of invasive crayfish are often observed from regions that historically lacked native crayfish or analogous decapod crustaceans altogether (Gamradt and Kats 1996; Geiger et al. 2005; Moore et al. 2012; Usio et al. 2013). Alternatively, in those ecosystems with native crayfish species, invasive crayfish have been observed to often achieve higher abundances or densities than native congeners (Hansen et al. 2013; Kreps et al. 2016), and these higher abundances should translate into greater invader impacts or interaction strengths (Kumschick et al. 2015). However, owing to the omnivorous nature of crayfish in general, should we anticipate ecological equivalence (i.e., functional redundancy) between crayfish species when invaders are not more abundant than native congeners?
Researchers have sought to answer this question by applying tools ranging from laboratory behavioral trials (e.g., Renai and Gherardi 2004), to mesocosm experiments (e.g., Usio et al. 2006), to field sampling of crayfish prey communities (e.g., Ercoli et al. 2015), to stable isotope analysis of crayfish trophic function or similarity (e.g., Olsson et al. 2009), to studies that have combined some of the above approaches (e.g., Jackson et al. 2014). To date, several meta-analyses of laboratory and field experiments comparing interactions of native and invasive crayfishes with their food webs have synthesized this literature, and generally found similar effect sizes between crayfish species irrespective of origin (Twardochleb et al. 2013; James et al. 2014). Efforts to allocate limited resources to prevent and manage biological invasions need reliable information on the particular species and habitat combinations where the most severe unwanted effects will manifest (Yokomizo et al. 2009; Hauser and McCarthy 2009). Accordingly, the apparent similarity in ecological function between many native and invasive crayfish species requires ongoing investigation and clarification in order to prioritize management activities.
One reason that the behavioral, enclosure, and mesocosm experiments synthesized in the above meta-analyses have largely failed to find consistent ecological differences between native and invasive crayfishes may be that they occur over too restricted spatial and temporal scales to accurately reflect actual ecological processes and associated subtle, persistent distinctions between these species (Lodge et al. 1998). Stable isotope analysis has emerged over recent decades as one of the primary tools used to infer ecological and trophic interactions between species under natural field conditions (Boecklen et al. 2011), with most tissue-derived stable isotope samples reflecting months to years of foraging behavior for focal organisms (Vander Zanden et al. 2015). For example, stable isotopes of carbon and nitrogen can be used to reflect energy source origins and trophic positions of organisms in freshwater lakes (Vander Zanden et al. 1999), and can be subsequently applied to evaluate and compare the trophic niche of species (Layman et al. 2007). When used to compare trophic function of native and invasive crayfish species, these stable isotope tools have produced inconsistent results, with some studies finding pronounced functional differences (e.g., Olsson et al. 2009; Jackson et al. 2014) and others a high degree of trophic similarity or niche overlap (e.g., Ercoli et al. 2014; Magoulick and Piercey 2016). Yet this emerging literature is small, and clearly needs further inquiry to evaluate whether native and invasive crayfishes are ecologically equivalent.
The two most globally invasive crayfish species are the signal crayfish Pacifastacus leniusculus and the red swamp crayfish Procambarus clarkii (Hobbs et al. 1989; Lodge et al. 2012). Pacifastacus leniusculus is native to the Columbia River drainage and some of the adjacent Pacific Northwest region of North America (Larson et al. 2012), and has been introduced elsewhere in the western United States, as well as to Japan and widely throughout Europe (Usio et al. 2007; Lodge et al. 2012). Impacts of P. leniusculus on recipient communities have included severe population declines and even one likely extinction of native crayfish species (Bouchard 1977; Light et al. 1995; Nakata and Goshima 2003), negative effects on other freshwater invertebrates and fish (Matsuzaki et al. 2012; Machida and Akiyama 2013; Wood et al. 2016), and changes to ecosystem processes owing to the burrowing and foraging behaviors of this species (Harvey et al. 2011). Procambarus clarkii is native to the southern United States and northeastern Mexico, and has been introduced to all continents except Antarctica and Australia, with particularly harmful invasions in the western United States, throughout Europe, and in Asia (Hobbs et al. 1989; Lodge et al. 2012). As a few examples, invasion by P. clarkii has transformed the food webs and ecosystem processes of Mediterranean wetlands in Europe (Geiger et al. 2005), and caused declines of vertebrate species like stream-dwelling newts (Gamradt and Kats 1996). Despite originating from disparate regions in North America, P. leniusculus and P. clarkii share some overlapping climate tolerances (Capinha et al. 2011; Larson and Olden 2012), and have been found occurring in sympatry or close proximity in some regions, like the Pacific Northwest region of North America (Hanshew and Garcia 2012; Pearl et al. 2013). These occurrences provide opportunities to compare the trophic function or niche overlap of the two most globally widespread invasive crayfish species under shared habitat circumstances, and evaluate whether these crayfish species are ecologically equivalent.
We used stable isotopes to compare the trophic niche of P. leniusculus and P. clarkii in a series of replicated allopatric (each species occurring in isolation) and sympatric (both species occurring together) lakes located in the Puget Sound lowlands of Washington State, USA (Larson and Olden 2013; Twardochleb and Olden 2016). Our analysis focused on comparing trophic niche size and similarity between P. leniusculus and P. clarkii using field, laboratory, and statistical approaches similar to a number of recent such studies on native and invasive crayfish species (e.g., Olsson et al. 2009; Ercoli et al. 2014; Jackson et al. 2014). Our comparisons were made between populations of a P. clarkii invasion initially discovered in 2000 (Mueller 2001) and locations in the putative native range of P. leniusculus, although a recent analysis of molecular and historical data suggests that P. leniusculus was introduced to this recently glaciated lake district by humans from a refugia roughly 100 km to the south sometime over the past century (Larson et al. 2012). Together, our study contributes to the developing literature on ecological equivalence between crayfish species, with a focus on the two most globally cosmopolitan invaders from this taxonomic group.
Methods
Study sites
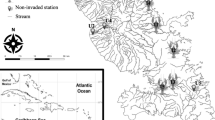
The Puget Sound lowlands of Washington State were glaciated by a lobe of the Cordilleran ice sheet during the last glacial maxima, leaving behind hundreds of natural lakes that have recently experienced varying degrees of land development in association with urban and ex-urban growth (Alberti et al. 2007). These lakes have been invaded by a variety of non-native freshwater species owing to human trade in live organisms and historical ecosystem management practices (i.e., fish stocking), including at least four species of non-native crayfish as documented by Larson and Olden (2013). Larson and Olden (2013) conducted a systematic survey of 100 Puget Sound lowland lakes during the summers of 2007–2009 using a sampling protocol of 15–20 baited traps per lake to estimate crayfish relative abundance, measured as catch-per-unit effort (CPUE) or the average number of crayfish of each species per trap. We used data from the 2007 and 2008 sampling summers to identify eleven lakes for this stable isotope study, stratified as the only two lakes with known sympatric populations of P. leniusculus and P. clarkii at the time, three lakes with allopatric populations of each species, and three lakes with no previously detected crayfish populations (Fig. 1). Inclusion of these presumed no crayfish lakes was intended to allow for examination of how crayfish presence and crayfish species identity might affect trophic function of other members of the community like fish species (Nilsson et al. 2012; Kreps et al. 2016).
Location of study lakes in the vicinity of the city of Seattle, Washington State, USA (a), with the first two axes of a principal component analysis (PCA) of lake physical attributes (Table 1) represented in the inset (b), the relative abundance of the crayfishes Pacifastacus leniusculus and Procambarus clarkii in study lakes from preliminary data as catch per unit effort (CPUE) from baited traps (Electronic Supplementary Material) compared to all other lakes in the region where these crayfish were found (Larson and Olden 2013) (c), and the first two axes of a correspondence analysis (CA) of fish genus abundance (Electronic Supplementary Material) in study lakes from all sampling gears combined (d)
Effort was made to select study lakes according to similarities in physicochemical and watershed characteristics (Table 1). Relative abundance of both crayfish species was low relative to what has been observed for some invasive crayfish species in other systems (e.g., Kreps et al. 2016), ranging from CPUE of 0.05–0.33 for P. leniusculus and 0.15–0.75 for P. clarkii. These CPUE values were on the low end of what was observed in 48 other lakes where P. leniusculus was detected by Larson and Olden (2013), but higher than six other lakes where P. clarkii was collected by the same study (Fig. 1). Study lakes were generally relatively small, with shorelines that had experienced a high degree (30–95 %) of urbanization or human development, as visually estimated by the field sampling crew. We measured Secchi disk depth (m) as a metric of lake clarity during field sampling, used shoreline development index (SDI; ratio of lake perimeter to perimeter of a perfectly circular lake of the same area) values from Bortleson et al. (1976) to reflect littoral zone complexity, and compiled measures of water quality and chemistry (Table 1) from average epilimnetic values measured between 1996 and 2008 at each lake by government management agencies (Larson and Olden 2013).
Field sampling
The eleven study lakes were sampled between July 17 and September 10, 2009. We collected basal resources including benthic algae, aquatic macrophytes, and leaf litter from the littoral zone by hand while snorkeling, or with D-frame nets nearshore and an Ekman grab from a boat offshore that were primarily used to sample the aquatic macroinvertebrate community at multiple locations in each lake. We field sorted these collected organisms to morpho-species or coarse taxonomic categories (e.g., taxonomic level ‘order’), kept them in plastic bags on ice during sampling, and then immediately transported samples back to the laboratory for storage in a freezer in advance of laboratory stable isotope analysis. We collected crayfish and small fish using overnight sets of Gee minnow traps (0.42 m long by 0.21 m diameter) baited with a half cup of dry dog food, using 20–30 traps (dependent on lake size) with 6.0-cm openings to collect primarily crayfish and 9–10 traps (owing to trap theft) with 2.5-cm openings to collect primarily fish. Traps were set at depths between 0.5 and 6.0 m, and distributed around the entire lake perimeter, maintaining a minimum distance of 10 m between any two traps.
We collected larger fish using overnight sets of three hoop nets (7.9 m wing length, five hoops 0.8 m diameter) dispersed around lake perimeters and baited with punctured cans of wet cat food, and overnight sets of an experimental gill net (58.5 m length by 1.8 m height; six panels with 25, 32, 38, 51, 64, and 76 mm mesh). Collected fish were identified to species, measured to total length (mm), and a subset of individuals of each species across the available size range were euthanized and transported to the laboratory, where they were frozen until stable isotope processing. All crayfish of each species were sexed, measured to total carapace length (mm) using vernier calipers, and subsequently euthanized, transported to the laboratory, and frozen as per fish. Collections of crayfish and fish were made under Washington Department of Fish and Wildlife permit 07-323 and 08-344, and University of Washington Institutional Animal Care and Use Committee permit 4172-04.
Stable isotope samples
We dissected fish muscle tissue from the lateral area posterior to the operculum, and muscle tissue from the abdomen of crayfish consistent with Stenroth et al. (2006), for use as stable isotope samples of these larger organisms. We used whole organisms for benthic macroinvertebrates and all collected biomass for basal resources; as an exception, we used a subsample of muscle tissue from the foot of larger snails (see below). Samples were dried at 60 °C for 24 h and then homogenized using a mortar and pestle. We weighed approximately 1 mg of animal tissue and 2–3 mg of plant tissue into tin capsules, and shipped samples to the University of California-Davis Stable Isotope Laboratory for dual carbon and nitrogen analysis using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a 20–20 isotope ratio mass spectrometer. Long-term standard deviations of lab standards used at this facility have been 0.2 ‰ for 13C and 0.3 ‰ for 15N. Stable isotope ratios are reported per convention in δ notation as 13C/12C relative to a Pee Dee belemnite standard and 15N/14N relative to an atmospheric nitrogen standard.
Statistical analyses
We first used multivariate statistics to explore the similarity of our sympatric, allopatric, and no crayfish lakes with respect to both their abiotic attributes (Table 1) and fish communities as observed in our field sampling (above; Electronic Supplementary Materials). Lake attributes like habitat area or productivity can affect trophic position and resource use of consumers (Post et al. 2000; Larson et al. 2011), whereas some predatory fish species may affect the distribution, abundance, and behavior of crayfish (Collins et al. 1983; Edwards et al. 2013), and invasive crayfish may in turn extirpate other fish species from lakes owing to competition or predation (Dorn and Mittelbach 2004; Kreps et al. 2016). We performed a principal component analysis (PCA) on the lake abiotic attributes and a correspondence analysis (CA) on total fish abundance from all sampling gears aggregated to the genus level, with both analyses conducted using the vegan library in R (R Development Core Team 2008; Oksanen et al. 2016). We visually evaluated the dispersion of our sympatric, allopatric, and no crayfish lake categories on the first two axes from each of these ordinations.
Calculations of trophic position using stable isotopes generally require comparison to a baseline organism; in freshwater lakes, this comparison is most often made to pelagic and/or littoral benthic primary consumers (trophic position of two), because the isotopic values of primary producers like phytoplankton or periphyton can be extremely variable in space and time (see Vander Zanden et al. 1999; Post et al. 2000). Long-lived, large bodied primary consumers average out this variability. For our study, we used the non-native Chinese mystery snail Bellamya chinensis to calculate trophic positions for other organisms including P. leniusculus and P. clarkii. We used B. chinensis because this large-bodied (up to 65 mm total length) snail is both a grazer and filter feeder (Olden et al. 2013), and consequently may reflect trophic baselines of both pelagic and littoral benthic food webs simultaneously. Further, both P. leniusculus and P. clarkii had been previously documented to feed on B. chinensis under laboratory conditions (Olden et al. 2009), and we collected B. chinensis from 10 of our 11 study lakes, therefore standardizing trophic position calculations between study sites. In the one lake where we did not collect B. chinensis (Martha), we calculated trophic position using the average of all other collected snails after correcting for their mean − 1.36 ‰ δ15N depletion relative to B. chinensis observed in the other 10 lakes (Electronic Supplementary Material). We calculated trophic position for consumers as:
where 3.4 is an average discrimination or fractionation factor (Δ) of δ15N used between consumers of different trophic levels (Minagawa and Wada 1984).
We compared trophic niche size and overlap between P. leniusculus and P. clarkii by calculating small sample-size corrected standard ellipse areas (SEAc) of trophic position and a standardized δ13C axis (below) using the siar library in R (Jackson et al. 2011; Parnell and Jackson 2013). The SEAc metric of isotopic niche area is analogous to standard deviations for univariate data (Jackson et al. 2011). Our stable isotope sample sizes were relatively low but generally consistent for both crayfish species. Allopatric sample sizes for P. leniusculus were 6 (Killarney), 7 (Wild), and 13 (Martha), sympatric sample sizes for this crayfish were 9 (Steel) and 10 (Pine), and the mean P. leniusculus stable isotope sample size across five lakes was 9. Allopatric sample sizes for P. clarkii were 4 (Five Mile), 8 (North), and 9 (Silver), whereas sympatric sample sizes for this crayfish were 10 (Steel) and 20 (Pine), and the mean P. clarkii stable isotope sample size across lakes was 10. See Discussion for some implications of these levels of replication for our comparison of SEAc between crayfish species.
Consistent with Olsson et al. (2009), we standardized δ13C of crayfish samples relative to the δ13C mean and range of all consumers collected from their lake in order to: (a) place all populations on an equivalent δ13C axis relative to the breadth of available δ13C sources from primary producers in their originating community, and (b) scale axes of trophic position and δ13C over similar ranges with respect to influence in calculating trophic niche area (Larson et al. 2010). We standardized δ13C using the formula:
We compared trophic niche area as SEAc of P. leniusculus and P. clarkii with t-tests across all categories combined (allopatric and sympatric), in order to have enough degrees of freedom for statistical comparisons (i.e., there were only two sympatric lakes). We used the siar library in R to calculate percent overlap of SEAc between (and within; see below) species in some cases where sympatric (Parnell and Jackson 2013).
To further evaluate factors affecting isotope values of our individual crayfish, we performed linear mixed effects models in the nlme library of R (Pinheiro et al. 2016), in which we regressed crayfish trophic position and original (non-standardized) δ13C against factors including whether or not the two crayfish species were occurring in sympatry as defined above (allopatric = 0, sympatric = 1), crayfish species (P. clarkii = 0, P. leniusculus = 1), sex (male = 0, female = 1), and size (continuous as mm carapace length). These models included individual lakes as unordered random effects, to account for ways that factors like ecosystem size, productivity, or disturbance (e.g., degree of urbanization) have been observed to influence the trophic ecology of freshwater consumers (Post et al. 2000; Larson et al. 2011). Given our relatively low level of replication at the lake level and the large number of both abiotic and biotic lake attributes that might affect crayfish trophic ecology, we used random effects to control for this heterogeneity between our study sites in general (Bolker et al. 2009). Finally, organism size can influence trophic function through ontogenetic niche shifts, a phenomenon that has been observed for crayfish (Larson et al. 2010), and sex has similarly been observed to affect crayfish behavior and trophic function under mesocosm conditions (Usio and Townsend 2002).
Isotopic consistency in time
To evaluate whether isotopic niche relationships were consistent between P. leniusculus and P. clarkii over time, we used additional crayfish specimens collected by baited trapping from one of our sympatric lakes (Pine) during summer 2012. Five P. leniusculus and 6 P. clarkii collected during this summer had stable isotope samples processed and analyzed as above, and we calculated SEAc on original δ15N and δ13C (rather than trophic position and standardized δ13C) for these and specimens of both species collected from this lake in summer of 2009. We did not convert to trophic position and standardized δ13C owing to differences in sampling protocols between years. We compared trophic niche area and overlap between these species within years, and within species between years, to evaluate the stability or consistency of our conclusions with respect to P. leniusculus and P. clarkii ecological equivalence.
Results
Lake abiotic characteristics
Lakes supporting crayfish populations of either one or both species demonstrated high environmental similarity. The PCA on lake abiotic attributes (Table 1) resulted in a first ordination axis that explained 46.5 % of the variation in the dataset reflecting a gradient of more productive lakes (negative) to larger and deeper lakes (positive), and 19.7 % of variation on a second axis that captured a gradient of more urban lakes with higher conductivity (positive) to lakes with more complex shorelines per SDI (negative). Lakes with P. leniusculus and P. clarkii both in allopatry and sympatry were well dispersed over these gradients on the PCA, although no crayfish lakes were generally more urban (positive on second axis).
Lake biotic communities
Owing to disparities in fish communities between the study lakes, we chose not to evaluate potential effects of crayfish or particular crayfish species on fish trophic function. The CA on fish abundance per genus collected from field sampling (Electronic Supplementary Material) resulted in a first dimension that explained 41.6 % of the variation in the dataset on a gradient of lakes dominated by rock bass Ambloplites rupestris (negative) relative to all other lakes (more positive), and a second dimension that explained 27.4 % on a gradient of lakes with more Ictalurus, Micropterus, and Pomoxis species (negative) to lakes dominated by sunfishes of the genus Lepomis (Fig. 1). The yellow perch Perca flavescens was common in many lakes (Electronic Supplementary Material). Two of three no crayfish lakes were characterized by Ambloplites, and none of the a priori allopatric P. leniusculus or sympatric lakes contained this species.
In three cases we collected a single (Angle, Silver) or two (Cottage) P. leniusculus from lakes where this crayfish had not been detected in the previous 2007 and 2008 field sampling (Electronic Supplementary Material). Due to the overall rarity of P. leniusculus in these lakes, we retain our a priori sympatric, allopatric, and no crayfish designations in interpreting results. Similarly, as a consequence of the inadequate replication of P. leniusculus samples in these lakes, we excluded these four crayfish from our trophic niche comparisons.
Crayfish trophic niches
There was no significant difference in trophic niche area (as measured by SEAc) between all P. leniusculus and P. clarkii populations (t 4 = −0.728, P = 0.507; Fig. 2). Trophic position and niche width as standardized δ13C varied widely among lake populations. We did observe significant effects of species, size, and sex on crayfish trophic position, and an effect of size on δ13C; no significant effect of whether or not crayfish occurred in sympatry or allopatry was found for either measure of their trophic niche (Table 2). Trophic position was higher for P. leniusculus than P. clarkii, for female rather than male crayfish, and increased with increasing crayfish body size. Crayfish δ13C depleted or decreased with increasing body size, indicating a shift from enriched δ13C associated with littoral benthic primary producers (smaller crayfish) to depleted δ13C associated with pelagic primary producers or terrestrial detritus (larger crayfish). We used sympatric crayfish populations in Pine and Steel lakes to illustrate increased trophic position of P. leniusculus over P. clarkii when accounting for the effect of body size and inherent food web differences between lakes (Fig. 3).
Small sample size standard ellipse areas (SEAc) from the stable isotope analysis in R (siar) package, calculated on standardized δ13C and trophic position for the signal crayfish Pacifastacus leniusculus (a), both crayfishes where sympatric (b), and the red swamp crayfish Procambarus clarkii (c), using only those lakes where more than three individuals were collected for a given species
Crayfish isotopic consistency in time
Higher trophic position or δ15N enrichment of P. leniusculus relative to P. clarkii was consistent over time between 2009 and 2012 in Pine Lake (Fig. 4). Trophic position of P. leniusculus was higher than P. clarkii in 2009, with only 0.8 % overlap of P. clarkii into the P. leniusculus SEAc ellipse. Similarly, trophic position of P. leniusculus was higher than P. clarkii in 2012, with 19.8 % overlap of P. clarkii into the P. leniusculus SEAc ellipse. The 2012 P. leniusculus SEAc overlapped with 9.3 % of the 2009 P. leniusculus SEAc, whereas the 2012 P. clarkii SEAc overlapped with 54.1 % of the 2009 P. clarkii SEAc. Procambarus clarkii trophic niche as SEAc was highly consistent between years, whereas the trophic niche as δ13C seemingly narrowed for P. leniusculus in 2012 (Fig. 4).
Stable isotope biplots for Pacifastacus leniusculus and Procambarus clarkii in Pine Lake for the years 2009 and 2012 with sample sizes in parantheses in legend (a), and small sample size-corrected standard ellipse areas (SEAc) for these data calculated using the stable isotope analysis in R package (b)
Discussion
There are many instances where the introduction of an invasive crayfish species is clearly and unfailingly undesirable. Invasive crayfishes spread diseases including the crayfish plague Aphanomyces astaci that has decimated native European crayfishes (Jussila et al. 2015b), can have strong negative effects on native taxa that are naïve to interactions with crayfish (Gamradt and Kats 1996), may change the phenology of key ecosystem processes like decomposition of detritus (Kobayashi et al. 2011; Alp et al. 2016), and can reach hyper-abundance relative to native crayfish populations (Hansen et al. 2013; Kreps et al. 2016). Yet even outside of these particular examples, our study contributes further evidence that not all crayfish species are ecologically equivalent, despite their categorization as omnivores or polytrophic generalists. Across replicated lake ecosystems and irrespective of occurrence in allopatry or sympatry, P. leniusculus was significantly higher in trophic position than P. clarkii, and this distinction could have important implications for how these organisms interact with and affect freshwater food webs and communities. As one example, such differences in trophic position between P. leniusculus and P. clarkii could result in different roles as vectors of contaminants like mercury in freshwater food webs (Johnson et al. 2014).
We believe that findings of our study are likely transferable over space and time, owing to the general consistency in trophic niche position and size observed between P. leniusculus and P. clarkii from 2009 to 2012 in Pine Lake, as well as the previous results of Larson et al. (2010). That study compared the trophic niche between the native (Pacific Northwest) and invasive (Japan) range of P. leniusculus, and found that although trophic function of this crayfish could vary with ontogeny (size) and habitat context, this range and pattern of trophic niche variability were conserved between native and invasive regions. Similar to our current study, Larson et al. (2010) found increasing trophic position for larger crayfish, and δ13C depletion with increasing crayfish size. This pattern in δ13C may reflect a transition of larger crayfish towards more reliance on terrestrial or detrital food sources in these lakes (Larson et al. 2010, 2011). Furthermore, results of a recent mesocosm experiment also supported a higher trophic position of P. leniusculus relative to P. clarkii. Olden et al. (2009) compared consumption and handling times of the Chinese mystery snail B. chinensis by both P. leniusculus and P. clarkii, using specimens collected from the same lakes as our current study. Olden et al. (2009) found that P. leniusculus consumed more and larger B. chinensis relative to P. clarkii. Accordingly, several lines of evidence — feeding and mesocosm experiments as well as stable isotope analysis — suggest a higher trophic position of P. leniusculus than P. clarkii in lakes of the Puget Sound lowlands of Washington State, USA.
Our findings conflict with those of Jackson et al. (2014), who observed a higher trophic position of P. clarkii than P. leniusculus using stable isotopes on crayfish sampled from field sites in the United Kingdom. Notably, the comparison of Jackson et al. (2014) was made between a single allopatric site for each species, with no controls or corrections for potential ways that site differences may affect trophic resources available to each of these crayfish populations. Such differences may have been particularly severe, given that P. clarkii isotope samples were from a population in a lentic (pond) environment, whereas those for P. leniusculus were from a population in a separate lotic (navigation canal) environment. Our study is somewhat unique in being able to include comparisons of trophic niches of two invasive crayfish species in sympatry, as these studies are most often made only on allopatric populations, owing to the tendency of invasive crayfish to entirely displace native congeners through mechanisms including competition and disease transmission (Olsson et al. 2009; Ercoli et al. 2014).
Our results suggest that — where possible — these types of comparisons should include some sympatric populations, and when only allopatric contrasts are possible, study sites should be as closely matched by abiotic and biotic characteristics as feasible. Further, tools like linear mixed effects models can be deployed to accommodate site differences or heterogeneity. However, a suite of additional factors might contribute to variability in trophic function even between populations of the same crayfish species, ranging from behavioral syndromes associated with newly introduced or spreading populations of invaders (Pintor et al. 2008) to complicated effects of parasites or symbionts on crayfish foraging behaviors and ecological interactions (Reisinger et al. 2015; James et al. 2015). In particular, we propose that some trophic niche similarity (Magoulick and Piercey 2016) or distinctions (Jackson et al. 2014) observed between crayfish species might be attributable to phylogenetic similarity, with closely related crayfish (e.g., within the same genera) being more ecologically equivalent, and more distantly related crayfish (e.g., in different genera or families) being more dissimilar. Specific tests of this phylogenetic niche conservatism hypothesis (Webb et al. 2002) using crayfish stable isotope data might be a fruitful direction for future research (Comte et al. 2016).
Our results are dependent on consistent isotopic discrimination factors (e.g., 3.4 for δ15N; Eq. 1) between species and diets, an assumption that has been called into question over recent years (Caut et al. 2009). Few studies have compared isotopic discrimination or fractionation factors for crayfish species, and those that have generally fail to run experiments on adult crayfish long enough for organisms to reach equilibrium with their diets (Carolan et al. 2012; Jussila et al. 2015a). As one exception, Glon et al. (2016) used fast-growing juvenile crayfish of two congeneric species that were fed both an invertebrate and algal diet. Over experimental durations generally long enough to reach isotopic equilibrium, Glon et al. (2016) found that δ13C and δ15N discrimination factors were largely indistinguishable between these species over both diets and in comparison to literature values (3.4 for δ15N) in the majority of cases. However, more laboratory studies of isotopic discrimination or fractionation factors for crayfish species and diet combinations would be valuable for future applications of this tool. Furthermore, our relatively low sample sizes per crayfish species and lake (four to 20 individuals) could affect our results, particularly as the measure of trophic niche size or area (SEAc) that we used provides less precise estimates of the overall population trophic niche size at lower levels of replication (Syväranta et al. 2013). This may have contributed to our failure to find a significant difference in SEAc between our two crayfish species (i.e., a type II error), although we did have adequate statistical power to detect a suite of other trophic distinctions among these crayfishes (Table 2) and believe that both species do likely share similarly large trophic niches given what we know of their biology in general.
Pacifastacus leniusculus and P. clarkii are globally cosmopolitan freshwater invaders that have spread to multiple continents and may increasingly share sympatric regions or even specific habitats in the future (Capinha et al. 2011; Larson and Olden 2012). Our study provides guidance to researchers and managers in other regions on one type of ecological difference that should be anticipated between these two crayfish species, although there are certainly others (e.g., the superior burrowing ability of P. clarkii; Gherardi 2006). We re-emphasize that both species are highly plastic generalists or omnivores that can feed on a variety of potential diet items, from high reliance on low food-quality terrestrial detritus (Gutiérrez-Yurrita et al. 1998; Bondar et al. 2005) to predation on vertebrates (Gamradt and Kats 1996; Matsuzaki et al. 2012) dependent on community and ecosystem context, as well as crayfish ontogeny or life history (see also Larson et al. 2010). Yet, where all else is equal, our results predict that P. leniusculus will be higher in trophic position than P. clarkii under the same conditions, and we believe this distinction is likely consistent in time and space owing to our past work on niche conservatism for one of these crayfish species (Larson et al. 2010). More work is needed on ecological equivalence between native and invasive crayfish species in light of meta-analysis results (Twardochleb et al. 2013; James et al. 2014) and some stable isotope studies (e.g., Magoulick and Piercey 2016), but we lend support to the expectation that one crayfish species is not necessarily equal to another.
References
Alberti M, Booth D, Hill K, Coburn B, Avolio C, Coe S, Spirandelli D (2007) The impact of urban patterns on aquatic ecosystems: an empirical analysis in Puget lowland sub-basins. Landsc Urban Plan 80:345–361
Alp M, Cucherousset J, Buoro M, Lecerf A (2016) Phenological response of a key ecosystem function to biological invasion. Ecol Lett. doi:10.1111/ele.12585
Boecklen WJ, Yarnes CT, Cook BA, James AC (2011) On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst 42:411–440
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bondar CA, Bottriell K, Zeron K, Richardson JS (2005) Does trophic position of the omnivorous signal crayfish (Pacifastacus leniusculus) in a stream food web vary with life history stage or density? Can J Fish Aquat Sci 62:2632–2639
Bortleson GC, Dion NP, McConnell JB, Nelson LM (1976) Reconnaissance data on lakes in Washington. Washington Department of Ecology, Tacoma
Bouchard RW (1977) Distribution, systematic status and ecological notes on five poorly known species of crayfishes in western North America (Decapoda: Astacidae and Cambaridae). Freshw Crayfish 3:409–423
Capinha C, Leung B, Anastácio P (2011) Predicting worldwide invasiveness for four major problematic decapods: an evaluation of using different calibration sets. Ecography 34:448–459
Carolan JV, Mazumder D, Dimovski C, Diocares R, Twining J (2012) Biokinetics and discrimination factors for δ13C and δ15N in the omnivorous freshwater crustacean, Cherax destructor. Mar Freshw Res 63:878–886
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453
Collins NC, Harvey HH, Tierney AJ, Dunham DW (1983) Influence of predatory fish density on trapability of crayfish in Ontario lakes. Can J Fish Aquat Sci 40:1820–1828
Comte L, Cucherousset J, Olden JD (2016) Global test of Eltonian niche conservatism of nonnative freshwater fish species between their native and introduced ranges. Ecography. doi:10.1111/ecog.02007
Dorn NJ, Mittelbach GG (2004) Effects of a native crayfish (Orconectes virilis) on the reproductive success and nesting behavior of sunfish (Lepomis spp.). Can J Fish Aquat Sci 61:2135–2143
Dorn NJ, Wojdak JM (2004) The role of omnivorous crayfish in littoral communities. Oecologia 140:150–159
Edwards BA, Jackson DA, Somers KM (2013) Linking temporal changes in crayfish communities to environmental changes in boreal Shield lakes in south-central Ontario. Can J Fish Aquat Sci 71:21–30
Ercoli F, Ruokonen TJ, Hämäläinen H, Jones RI (2014) Does the introduced signal crayfish occupy an equivalent trophic niche to the lost native noble crayfish in boreal lakes? Biol Invasions 16:2025–2036
Ercoli F, Ruokonen TJ, Erkamo E, Jones RI, Hämäläinen H (2015) Comparing the effects of introduced signal crayfish and native noble crayfish on the littoral invertebrate assemblages of boreal lakes. Freshw Sci 34:555–563
Gallardo B, Clavero M, Sánchez MI, Vilà M (2016) Global ecological impacts of invasive species in aquatic ecosystems. Glob Chang Biol 22:151–163
Gamradt SC, Kats LB (1996) Effect of introduced crayfish and mosquitofish on California newts. Conserv Biol 10:1155–1162
Geiger W, Alcorlo P, Baltanas A, Montes C (2005) Impact of an introduced crustacean on the trophic webs of Mediterranean wetlands. In: Capdevila-Argüelles L, Zilletti B (eds) Issues in bioinvasion science. Springer, Heidelberg, pp 49–73
Gherardi F (2006) Crayfish invading Europe: the case study of Procambarus clarkii. Mar Freshw Behav Physiol 39:175–191
Glon MG, Larson ER, Pangle KL (2016) Comparison of 13C and 15N discrimination factors and turnover rates between congeneric crayfish Orconectes rusticus and O. virilis (Decapoda, Cambaridae). Hydrobiologia 768:51–61
Gutiérrez-Yurrita PJ, Sancho G, Bravo MA, Baltanás A, Montes C (1998) Diet of the red swamp crayfish Procambarus clarkii in natural ecosystems of the Doñana National Park temporary fresh-water marsh (Spain). J Crustac Biol 18:120–127
Hansen GJ, Vander Zanden MJ, Blum MJ, Clayton MK, Hain EF, Hauxwell J, Izzo M, Kornis MS, McIntyre PB, Mikulyuk A, Nilsson E, Olden JD, Papeş M, Sharma S (2013) Commonly rare and rarely common: comparing population abundance of invasive and native aquatic species. PLoS One 8:e77415
Hanshew BA, Garcia TS (2012) Invasion of the shelter snatchers: behavioural plasticity in invasive red swamp crayfish, Procambarus clarkii. Freshw Biol 57:2285–2296
Harvey GL, Moorhouse TP, Clifford NJ, Henshaw AJ, Johnson MF, Macdonald DW, Reid I, Rice S (2011) Evaluating the role of invasive aquatic species as drivers of fine sediment-related river management problems: the case of the signal crayfish (Pacifastacus leniusculus). Prog Phys Geogr 35:517–533
Hauser CE, McCarthy MA (2009) Streamlining ‘search and destroy’: cost-effective surveillance for invasive species management. Ecol Lett 12:683–692
Hobbs HH, Jass JP, Huner JV (1989) A review of global crayfish introductions with particular emphasis on two North American species (Decapoda, Cambaridae). Crustaceana 56:299–316
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–stable isotope Bayesian ellipses in R. J Anim Ecol 80:595–602
Jackson MC, Jones T, Milligan M, Sheath D, Taylor J, Ellis A, England J, Grey J (2014) Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshw Biol 59:1123–1135
James J, Slater FM, Vaughan IP, Young KA, Cable J (2014) Comparing the ecological impacts of native and invasive crayfish: could native species’ translocation do more harm than good? Oecologia 178:309–316
James J, Davidson KE, Richardson G, Grimstead C, Cable J (2015) Reduced aggression and foraging efficiency of invasive signal crayfish (Pacifastacus leniusculus) infested with non-native branchiobdellidans (Annelida: Clitellata). Parasit Vectors 8:1–9
Johnson BL, Willacker JJ, Eagles-Smith CA, Pearl CA, Adams MJ (2014) Invasive crayfish as vectors of mercury in freshwater food webs of the Pacific Northwest. Environ Toxicol Chem 33:2639–2645
Jussila J, Ruokonen TJ, Syväranta J, Kokko H, Vainikka A, Makkonen J, Korter R (2015a) It takes time to see the menu from the body: an experiment on stable isotope composition in freshwater crayfishes. Knowl Manag Aquat Ecosyst 416:25
Jussila J, Vrezec A, Makkonen J, Kortet R, Kokko H (2015b) Invasive crayfish and their invasive diseases in Europe with the focus on the virulence evolution of the crayfish plague. In: Canning-Clode J (ed) Biological invasions in changing ecosystems: vectors, ecological impacts, management and predictions. De Gruyter Open, Berlin, pp 183–211
Kobayashi R, Maezono Y, Miyashita T (2011) The importance of allochthonous litter input on the biomass of an alien crayfish in farm ponds. Popul Ecol 53:525–534
Kreps TA, Larson ER, Lodge DM (2016) Do invasive rusty crayfish (Orconectes rusticus) decouple littoral and pelagic energy flows in lake food webs? Freshw Sci 35:103–113
Kumschick S, Gaertner M, Vilà M, Essl F, Jeschke JM, Pyšek P, Ricciard A, Bacher S, Blackburn TM, Dick JTA, Evans T, Hulme PE, Kühn I, Mrugata A, Pergl J, Rabitsch W, Richardson DM, Sendek A, Winter M (2015) Ecological impacts of alien species: quantification, scope, caveats, and recommendations. Bioscience 65:55–63
Larson ER, Olden JD (2012) Using avatar species to model the potential distribution of emerging invaders. Glob Ecol Biogeogr 21:1114–1125
Larson ER, Olden JD (2013) Crayfish occupancy and abundance in lakes of the Pacific Northwest, USA. Freshw Sci 32:94–107
Larson ER, Olden JD, Usio N (2010) Decoupled conservatism of Grinnellian and Eltonian niches in an invasive arthropod. Ecosphere 1:1–13 (Article16)
Larson ER, Olden JD, Usio N (2011) Shoreline urbanization interrupts allochthonous subsidies to a benthic consumer over a gradient of lake size. Biol Lett 7:551–554
Larson ER, Abbott CL, Usio N, Azuma N, Wood KA, Herborg LM, Olden JD (2012) The signal crayfish is not a single species: cryptic diversity and invasions in the Pacific Northwest range of Pacifastacus leniusculus. Freshw Biol 57:1823–1838
Layman CA, Quattrochi JP, Peyer CM, Allgeier JE (2007) Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol Lett 10:937–944
Light T, Erman DC, Myrick C, Clarke J (1995) Decline of the Shasta crayfish (Pacifastacus fortis Faxon) of northeastern California. Conserv Biol 9:1567–1577
Lodge DM, Stein RA, Brown KM, Covich AP, Bronmark C, Garvey JE, Klosiewski SP (1998) Predicting impact of freshwater exotic species on native biodiversity: challenges in spatial scaling. Aust J Ecol 23:53–67
Lodge DM, Deines A, Gherardi F, Yeo DC, Arcella T, Baldridge AK, Barnes MA, Chadderton WL, Feder JL, Gantz CA, Howard GW, Jerde CL, Peters BW, Peters JA, Sargent LW, Turner CR, Wittmann ME, Zeng Y (2012) Global introductions of crayfishes: evaluating the impact of species invasions on ecosystem services. Annu Rev Ecol Evol Syst 43:449–472
Machida Y, Akiyama YB (2013) Impacts of invasive crayfish (Pacifastacus leniusculus) on endangered freshwater pearl mussels (Margaritifera laevis and M. togakushiensis) in Japan. Hydrobiologia 720:145–151
Magoulick DD (2014) Impacts of drought and crayfish invasion on stream ecosystem structure and function. River Res Appl 30:1309–1317
Magoulick DD, Piercey GL (2016) Trophic overlap between native and invasive stream crayfish. Hydrobiologia 766:237–246
Matsuzaki SS, Usio N, Takamura N, Washitani I (2009) Contrasting impacts of invasive engineers on freshwater ecosystems: an experiment and meta-analysis. Oecologia 158:673–686
Matsuzaki SS, Sakamoto M, Kawabe K, Takamura N (2012) A laboratory study of the effects of shelter availability and invasive crayfish on the growth of native stream fish. Freshw Biol 57:874–882
Minagawa M, Wada E (1984) Stepwise enrichment of 15 N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Moore JW, Carlson SM, Twardochleb LA, Hwan JL, Fox JM, Hayes SA (2012) Trophic tangles through time? Opposing direct and indirect effects of an invasive omnivore on stream ecosystem processes. PLoS One 7:e50687
Mueller KW (2001) First record of the red swamp crayfish, Procambarus clarkii (Girard, 1852) (Decapoda, Cambaridae), from Washington state, USA. Crustaceana 74:1003–1007
Nakata K, Goshima S (2003) Competition for shelter of preferred sizes between the native crayfish species Cambaroides japonicus and the alien crayfish species Pacifastacus leniusculus in Japan in relation to prior residence, sex difference, and body size. J Crustac Biol 23:897–907
Nilsson E, Solomon CT, Wilson KA, Willis TV, Larget B, Vander Zanden MJ (2012) Effects of an invasive crayfish on trophic relationships in north-temperate lake food webs. Freshw Biol 57:10–23
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2016) vegan: community ecology package. R package version 2.4-0. http://CRAN.R-project.org/package=vegan. Accessed 8 Jan 2016
Olden JD, Larson ER, Mims MC (2009) Home-field advantage: native signal crayfish (Pacifastacus leniusculus) out consume newly introduced crayfishes for invasive Chinese mystery snail (Bellamya chinensis). Aquat Ecol 43:1073–1084
Olden JD, Ray L, Mims MC, Horner-Devine MC (2013) Filtration rates of the non-native Chinese mystery snail (Bellamya chinensis) and potential impacts on microbial commuties. Limnetica 32:107–120
Olsen TM, Lodge DM, Capelli GM, Houlihan RJ (1991) Mechanisms of impact of an introduced crayfish (Orconectes rusticus) on littoral congeners, snails, and macrophytes. Can J Fish Aquat Sci 48:1853–1861
Olsson K, Stenroth P, Nyström P, Granéli W (2009) Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshw Biol 54:1731–1740
Parnell A, Jackson A (2013) siar: stable isotope analysis in R. R package version 4.2. http://CRAN.R-project.org/package=siar. Accessed 8 Jan 2016
Pearl CA, Adams MJ, McCreary B (2013) Habitat and co-occurrence of native and invasive crayfish in the Pacific Northwest, USA. Aquat Invasions 8:171–184
Peters JA, Lodge DM (2013) Habitat, predation, and coexistence between invasive and native crayfishes: prioritizing lakes for invasion prevention. Biol Invasions 15:2489–2502
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) nlme: linear and nonlinear mixed effects models. R package version 3.1-128. http://CRAN.R-project.org/package=nlme. Accessed 8 Jan 2016
Pintor LM, Sih A, Bauer ML (2008) Differences in aggression, activity and boldness between native and introduced populations of an invasive crayfish. Oikos 117:1629–1636
Post DM, Pace ML, Hairston NG (2000) Ecosystem size determines food-chain length in lakes. Nature 405:1047–1049
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org. Accessed 8 Jan 2016
Reisinger LS, Petersen I, Hing JS, Davila RL, Lodge DM (2015) Infection with a trematode parasite differentially alters competitive interactions and antipredator behaviour in native and invasive crayfish. Freshw Biol 60:1581–1595
Renai B, Gherardi F (2004) Predatory efficiency of crayfish: comparison between indigenous and non-indigenous species. Biol Invasions 6:89–99
Reynolds J, Souty-Grosset C, Richardson A (2013) Ecological roles of crayfish in freshwater and terrestrial habitats. Freshw Crayfish 19:197–218
Ruokonen TJ, Karjalainen J, Hämäläinen H (2014) Effects of an invasive crayfish on the littoral macroinvertebrates of large boreal lakes are habitat specific. Freshw Biol 59:12–25
Stenroth P, Holmqvist N, Nyström P, Berglund O, Larsson P, Granéli W (2006) Stable isotopes as an indicator of diet in omnivorous crayfish (Pacifastacus leniusculus): the influence of tissue, sample treatment, and season. Can J Fish Aquat Sci 63:821–831
Syväranta J, Lensu A, Marjomäki TJ, Oksanen S, Jones RI (2013) An empirical evaluation of the utility of convex hull and standard ellipse areas for assessing population niche widths from stable isotope data. PLoS One 8:e56094
Twardochleb LA, Olden JD (2016) Human development modifies the functional composition of lake littoral invertebrate communities. Hydrobiologia. doi:10.1007/s10750-016-2727-5
Twardochleb LA, Olden JD, Larson ER (2013) A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw Sci 32:1367–1382
Usio N, Townsend CR (2002) Functional significance of crayfish in stream food webs: roles of omnivory, substrate heterogeneity and sex. Oikos 98:512–522
Usio N, Suzuki K, Konishi M, Nakano S (2006) Alien vs. endemic crayfish: roles of species identity in ecosystem functioning. Archiv für Hydrobiol 166:1–21
Usio N, Nakata K, Kawai T, Kitano S (2007) Distribution and control status of the invasive signal crayfish (Pacifastacus leniusculus) in Japan. Jpn J Limnol 68:471–482
Usio N, Imada M, Nakagawa M, Akasaka M, Takamura N (2013) Effects of pond draining on biodiversity and water quality of farm ponds. Conserv Biol 27:1429–1438
Vander Zanden MJ, Casselman JM, Rasmussen JB (1999) Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401:464–467
Vander Zanden MJ, Clayton MK, Moody EK, Solomon CT, Weidel BC (2015) Stable isotope turnover and half-life in animal tissues: a literature synthesis. PLoS One 10:e0116182
Webb CO, Ackerly DD, McPeek MA, Donoghue MJ (2002) Phylogenies and community ecology. Annu Rev Ecol Evol Syst 33:475–505
Wood KA, Hayes RB, England J, Grey J (2016) Invasive crayfish impacts on native fish diet and growth vary with fish life stage. Aquat Sci. doi:10.1007/s00027-016-0483-2
Yokomizo H, Possingham HP, Thomas MB, Buckley YM (2009) Managing the impact of invasive species: the value of knowing the density-impact curve. Ecol Appl 19:376–386
Acknowledgments
This research was funded by the University of Washington School of Aquatic and Fishery Sciences and H. Mason Keeler Endowed Professorship to Julian Olden, as well as the Oregon Zoo Future for Wildlife Grants Program. Francis Lin and Kerry Ung provided field assistance through sponsorship by the American Fisheries Society Hutton Junior Fisheries Biology Program. Thomas Pool contributed to additional field sampling, and Mariana Tamayo helped compile lake attribute data. This manuscript was improved by comments from several anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick Fink.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Larson, E.R., Twardochleb, L.A. & Olden, J.D. Comparison of trophic function between the globally invasive crayfishes Pacifastacus leniusculus and Procambarus clarkii . Limnology 18, 275–286 (2017). https://doi.org/10.1007/s10201-016-0505-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-016-0505-8