Abstract
Ecological applications of stable isotope analysis are dependent on knowing consumer-diet discrimination factors (Δ) and consumer metabolic turnover rates (m). We used an 80-day laboratory experiment to test for differences in the δ13C, δ15N and m of two species of crayfish (Orconectes rusticus and O. virilis) fed one of two diets (algae wafers and bloodworms). Over the course of the experiment, the δ13C and δ15N signatures of the crayfish approached equilibrium with those of their diets. We fit our data to a growth-based model and found δ13C, δ15N, and m to be largely indistinguishable between species, except in the case of δ15N and m of crayfish on the algae diet. We thus pooled parameters to calculate Δ13C (algae diet: 1.57‰ [95% confidence interval: 0.86–2.35]; bloodworm diet: 0.8‰ [0.14–1.55]) and Δ15N (bloodworm diet: 1.2‰ [0.32–2.11]), and used species-specific data to calculate Δ15N for the algae diet (O. rusticus: 2.54‰ [2.06–3.08]; O. virilis: 3.35‰ [2.53–4.51]). Our results provide values of stable isotope Δ and m for applications to crayfish, and offer a rare comparison of these values between two closely related species and to commonly used literature values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stable isotope analysis is a valuable tool that ecologists use to study food webs (Vander Zanden et al., 1999; Post, 2002). This technique is based on the principle that the ratios of heavy to light isotopes in the tissues of a consumer reflect those of their diets in a predictable way (DeNiro & Epstein, 1978, 1981). Carbon and nitrogen isotopes (denoted δ13C and δ15N, respectively) are the most commonly used in freshwater biology as they allow researchers to make inferences about sources of primary productivity and trophic level, respectively. Stable isotope analysis has broad applications across all levels of ecological organization, from individual and population levels [e.g., diet composition (Hilderbrand et al., 1996; Whitledge & Rabeni, 1997; Newsome et al., 2009), trophic position (Vander Zanden et al., 1997; Roth et al., 2006; Cherel et al., 2008), and migration patterns (Cherel et al., 2007; McClellan et al., 2010)] to community and ecosystem levels [e.g., impacts of invasive species (Vander Zanden & Rasmussen, 1999; Olsson et al., 2009; Nilsson et al., 2012) and the contribution of allochthonous versus autochthonous food resources in aquatic ecosystems (Dekar et al., 2009; Solomon et al., 2011; Venarsky et al., 2014)].
A commonality among different applications of stable isotope analysis is their dependence on consumer-diet discrimination factors (i.e., absolute difference between the δ13C or δ15N of a consumer and its diet, written as Δ13C and Δ15N, respectively; also known as fractionation). A frequent assumption is that mean Δ of 0–1‰ for δ13C and 3.4‰ for δ15N is generally applicable (Fry & Sherr, 1984; Minagawa & Wada, 1984). However, Δ13C and Δ15N have been shown to vary across taxa, diets, and tissues (e.g., Vander Zanden & Rasmussen, 2001; Stenroth et al., 2006; Caut et al., 2009). Further, differences in the rate at which different tissues in an organism equilibrate with the isotopic signature of their diet (metabolic turnover rate, written as m) can confound results of stable isotope experiments if these rates are not known and accounted for (Hesslein et al., 1993; Buchheister & Latour, 2010; Thomas & Crowther, 2014). Tissues that are more metabolically active turnover more quickly and therefore have isotopic signatures that reflect a more recent diet than those that are less metabolically active (Bosley et al., 2002; del Rio et al. 2009, Boecklen et al. 2011). In light of these possible sources of uncertainty, it is prudent to determine the generality of Δ13C, Δ15N, and m across taxa, diets, and tissues through laboratory experiments prior to using this technique to make inferences about field data (Caut et al., 2008; Bond & Diamond, 2011; Rosenblatt & Heithaus, 2013). However, despite a wealth of studies suggesting the potential need for specific Δ13C, Δ15N, and m, these values have rarely been compared between closely related species under the same conditions.
In this study, we conducted a laboratory experiment to test the hypothesis that two closely related species of crayfish, the rusty crayfish Orconectes rusticus (Girard, 1852) and the virile crayfish O. virilis (Hagen, 1870), have the same Δ13C, Δ15N, and m. Crayfish are important components of ecosystems whose polytrophic interactions often influence the presence and abundance of other organisms (Momot et al., 1978; Lodge et al., 2000; Reynolds et al., 2013). Numerous studies have investigated the trophic roles and interactions of crayfish using stable isotope analysis (Roth et al., 2006; Stenroth et al., 2006; Olsson et al., 2008), several of which have explored differences between native and invasive crayfish species (Olsson et al., 2009; Ercoli et al., 2014; Jackson et al., 2014). Despite this, our study is the first to directly evaluate and compare Δ13C, Δ15N, and m between two crayfish species under the same laboratory conditions. Further, only two previous studies have evaluated Δ13C, Δ15N, and m for any crayfish species under controlled laboratory conditions (Rudnick & Resh, 2005; Carolan et al., 2012). Differences in Δ13C, Δ15N, and m between species of crayfish could inform past and future isotope-based studies in which the same Δ13C, Δ15N, and m have been used for closely related crayfish or other organisms.
We chose to conduct this experiment on O. rusticus and O. virilis because O. rusticus, native to the Ohio River drainage, is invasive in the Great Lakes basin (part of the native range of O. virilis) where it was introduced circa 1960 (Peters et al, 2014). Since its introduction, O. rusticus has negatively affected macrophytes, benthic invertebrates, and fish (Charlebois & Lamberti, 1996; McCarthy et al., 2006) and has displaced native crayfishes such as O. virilis (Peters et al., 2014). Although this invasion has been relatively well studied, there is ongoing ambiguity with respect to potential differences in trophic function and ecological impacts between O. rusticus and O. virilis (Peters & Lodge, 2013; Twardochleb et al., 2013; Kreps et al., in press). Stable isotope analyses could be applied to evaluate trophic differences and whole-ecosystem effects of O. rusticus and O. virilis, but such studies would benefit from knowing if the same Δ13C, Δ15N, and m can be used for the two species.
Materials and methods
We carried out an 80-day laboratory experiment to test for differences in the δ13C, δ15N, and m of O. rusticus and O. virilis. The crayfish were fed one of two diets (Appendix 1—Supplementary material), algae wafers (Aquaristik and SR Enterprises, Inc., St. Charles, Illinois, USA) or bloodworms (Chironomidae; Kyorin Food Industries, Ltd, Japan). Algae and macroinvertebrates represent two important components of the omnivorous diets of crayfish in nature and therefore allowed us to test the generality of our findings across diet types (Reynolds et al., 2013). We used juvenile crayfish for the experiment because they have higher rates of food consumption and growth than adult crayfish (Hill et al., 1993; Momot, 1995; Whitledge & Rabeni, 1997). Isotopic turnover in tissues is driven primarily by growth (Buchheister & Latour, 2010), and we expected that fast-growing juvenile crayfish would be more likely than adults to reach equilibrium with their new diets during the span of the experiment. Juvenile crayfish are also ecologically important as the numerically dominant component of crayfish populations and the primary prey item for many fish species that forage on crayfish, but are gape-limited by the size of larger adult crayfish (Fortino & Creed 2006).
Crayfish used in the experiment were collected using dip nets on 7 September 2014 from a pond in the Fabiano Botanical Garden (43°35′21.4″N 84°46′33.3″W) on Central Michigan University’s campus, where the two species occur in sympatry. We dried the crayfish with a paper towel for 10 s then weighed them to the nearest thousandth of a gram (g) using a digital balance and measured carapace length (CL) to the nearest hundredth of a millimeter (mm) using digital calipers. The initial size of O. rusticus (mean ± standard deviation; CL: 16.64 ± 1.59 mm; weight: 1.17 ± .34 g) and O. virilis (CL: 17.37 ± 1.98 mm, weight: 1.26 ± .41 g) did not significantly differ (Two sample t test: CL, t 68 = 1.7055, P = 0.09267; weight, t 68 = 0.9589, P = 0.341). We used crayfish of both sexes (15 females, 20 males for O. rusticus; 16 females, 19 males for O. virilis), balancing sex ratios as closely as possible within treatments and blocks (below).
Over the duration of the experiment, crayfish were maintained in a recirculating system (Pentair Ltd, Schaffhausen, Switzerland) with multiple stages of biological and mechanical filtration containing five rows of twelve, 3–l aquaria (60 aquaria, total). Each aquarium was outfitted with a segment of PVC pipe that served as refuge for the crayfish. We filled the system with deionized water; therefore, chemicals (Seachem Replenish, Seachem Laboratories, Inc., Madison, Georgia, USA) were added prior to the experiment and as needed throughout the experiment to replenish physiologically relevant minerals that were removed by deionizing filtration (Hammond et al., 2006). We maintained the water at 21°C (a temperature within the natural temperature ranges of both of these crayfish [Mundahl, 1989; Bergman & Moore, 2003]), with a pH of 7.5, carbonate hardness of 180 ppm, and a constant photoperiod of 13 h light: 11 h dark.
At the start of experiment, 30 crayfish of each species were transferred to aquaria (one per aquarium) and allowed to acclimate for a 24-h period prior to first feeding. Each crayfish was assigned to a diet and to a predetermined sampling date using a random block design in which the five rows of aquaria served as blocks. In addition to the 60 crayfish used in the experiment, we randomly chose and sacrificed five individuals of each species on day 0 and froze them at −17.8°C in order to obtain initial δ13C and δ15N values for our analysis.
Each day of the experiment, crayfish received a food ration that surpassed the mass-specific daily consumption rate outlined in Roth et al. (2006):
where C is consumption in grams dry weight of food per gram wet weight of crayfish per day and W is the wet weight of the crayfish in grams. To verify that crayfish were satiated, we looked for the presence of uneaten food in the crayfish tanks, which we always found. Uneaten food, as well as feces, was removed on a daily basis to keep the tanks clean. Water parameters were monitored daily to ensure that they were at levels conducive to crayfish growth and survival.
We sacrificed five crayfish per species and treatment on days 15, 30, and 80 in order to trace the tissue turnover of our crayfish over time and to estimate when equilibrium was reached relative to the diets. We based the duration of our experiment on a previous study in which it was found that the crayfish Cherax destructor took approximately 80 days to equilibrate with a new diet (Carolan et al., 2012). On each sampling day, we weighed the sacrificed crayfish and used their mean weight increase to adjust the food ration for the remaining crayfish. In order to calculate Δ13C and Δ15N between the crayfish and their diets, we also sampled both diets (n = 12 per diet) to determine their respective δ13C and δ15N signatures.
Sample processing
On each sampling day, the selected crayfish were sacrificed and frozen at −17.8°C. At the conclusion of the experiment (day 80), all remaining crayfish (five per treatment) were sacrificed. Crayfish were thawed and dissected to obtain samples of abdominal muscle. Stenroth et al. (2006) recommended untreated abdominal tissue for isotopic studies of crayfish, and this has been widely adopted by the majority of subsequent crayfish isotope studies (e.g., Olsson et al., 2008; Ruokonen et al., 2011; Ercoli et al., 2014). We dried the dissected crayfish muscle as well as diet samples in a drying oven at 60°C for 48 h. The dried samples were then ground to powder using an ethanol-rinsed mortar and pestle. Aliquots weighing 0.65 ± 0.05 mg of each sample were weighed and encapsulated in tin capsules, and then sent to the Stable Isotope Mass Spectrometry Lab in the Department of Geological Sciences at the University of Florida, where they were analyzed for 13C and 15N isotope composition using a Micromass PRISM II isotope ratio mass spectrometer coupled with an elemental analyzer. The stable isotope values were calculated as
where δx is δ13C or δ15N (expressed in per mille ‰) and R is 13C/12C or 15N/14N, respectively. The standard 13C/12C and 15N/14N values to which our samples were compared to were V-PDB (Vienna PeeDee Belemnite) and air for carbon and nitrogen, respectively. Two internationally recognized standards (both l-glutamic acids), USGS40 (mean ± standard deviation; δ13C, −4.51‰ ± 0.12; δ15N, −26.38‰ ± 0.1149; used for calibration) and USGS41 (δ13C, 47.57‰; δ15N, 37.36‰; used as a check standard), were repeatedly measured during the analysis to ensure precision. This same analysis yielded measurements of the amount of carbon and nitrogen in our samples, which we used to calculate carbon to nitrogen ratios (C:N), useful in determining protein and lipid content of tissues (Post et al., 2007). The mean C:N ratio of our crayfish was 3.4, a value below the threshold of 3.5 beyond which Post et al. (2007) recommended lipid correction for aquatic animals. Therefore, we did not lipid correct our data.
Data analysis
We compared the initial δ15N signatures of O. rusticus and O. virilis using a two-sample t test. We used a non-parametric Mann–Whitney U test to compare the initial δ13C of O. rusticus and O. virilis because our data did not meet normality assumptions and could not be transformed.
We estimated δ13C and δ15N of crayfish at equilibrium (δf) with their diets as well as m by fitting our data to the growth-based model of Hesslein et al. (1993):
where δ t is the δ13C or δ15N value of a crayfish at time t, δ f is the estimated δ13C or δ15N of crayfish when in equilibrium with their new diet, δ i is the initial δ13C or δ15N of the crayfish, m is the metabolic turnover rate, and k is the growth rate of our crayfish calculated as
where W t is the weight of our crayfish at time of sampling (t), and W 0 is the initial weight of our crayfish. We used this equation as it allowed us to separate the relative contributions of crayfish growth and metabolic tissue replacement to our isotope values. We calculated the discrimination factors (Δ) that reflected the difference between the equilibrium δ values of our crayfish (δ f ) and the δ of their diets (δ d ) as
We ran several different iterations of the model in order to evaluate differences in δf and m between species. Specifically, we looked for differences in δf and m between species, and pooled values for species if we did not find differences. To evaluate these models, we calculated log-likelihood ratio statistics and computed p-values between models by running analyses of variance (Ritz & Streibig, 2008). We used Q–Q plots to confirm normality of our data, and Bartlett’s tests to confirm homoscedasticity. Our alpha value was set at 0.05, and all statistical analyses were conducted using the statistical program R (R Core Team, 2014).
Finally, we also estimated stable isotope half-lives (i.e., the point at which tissues of our crayfish reached 50% equilibrium with their new diets [Fry & Arnold, 1982]). We derived an equation for half-life from the growth-based model of Hesslein et al. (1993; above) by setting δ t equal to the midpoint between the measured δ i and the model-estimated δf and then solving for t
where δ f , δ i , k, and m are the same parameters outlined above.
Results
The initial (day 0) δ13C values of O. rusticus and O. virilis were (mean ± standard deviation) −30.84‰ ± 0.91 and −31.84‰ ± 0.58, respectively, and were not significantly different (Mann–Whitney U Test, U = 20, P = 0.1508). The initial δ15N values of O. rusticus and O. virilis were 4.47‰ ± 0.29 and 4.31‰ ± 0.23, respectively, and were not significantly different (t 8 = −0.97, P = 0.3604). The δ13C signatures for algae wafers and bloodworms were −25.70‰ ± 0.18 and −29.97‰ ± 0.13, respectively, and the δ15N signatures for algae wafers and bloodworms were 4.91‰ ± 0.57 and 7.36‰ ± 0.61, respectively. We did not find a significant effect of block (i.e., row of aquaria) on final crayfish δ13C (F 4,52 = 0.487, P = 0.745) or δ15N (F 4,52 = 2.306, P = 0.071), so this factor was not included in further analyses.
Carbon
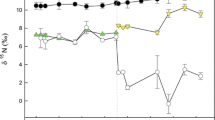
Over the course of the experiment, abdominal muscle δ13C signatures of both crayfish species became enriched relative to mean δ13C values of their respective diets (Fig. 1a, b). For the algae wafer treatment, δ13C values for O. virilis and O. rusticus at equilibrium were estimated as −24.20 and −23.93‰ (Table 1), respectively, and these values did not differ significantly between species (\({\mathcal{L}}\) ratio = 0.00, F 1, 25 = 0, P = 0.99). The metabolic turnover rate (m) for O. virilis and O. rusticus was estimated as 0.015 day−1 (CI 0.006–0.027) and 0.008 day−1 (0.001–0.019), respectively, and these values also did not differ significantly between species (\({\mathcal{L}}\) ratio = 0.03, F 1, 25 = 0.03, P = 0.87). Because we found no significant difference between species in either of these parameters, we reran the analysis using a pooled dataset. The parameters of this analysis were −24.13‰ (CI −24.84 to −23.35) and 0.012 day−1 (CI 0.006–0.019) for δ13C at equilibrium and m, respectively (overall model fit; r 2 = 0.94). Using this pooled δ13C value, we calculated the carbon discrimination factor (Δ13C) between our experimental crayfish and their diet to be 1.57‰ (CI 0.86–2.35).
For the bloodworm treatment, the δ13C values for O. virilis and O. rusticus at equilibrium were estimated as −28.69 and −29.10‰, respectively, and these values did not differ significantly between species (\({\mathcal{L}}\) ratio = 1.89, F 1, 23 = 1.73, P = 0.201). The metabolic turnover rate (m) for O. virilis and O. rusticus was estimated as -0.001 day−1 (CI −0.006 to 0.011) and 0.000 day−1 (CI −0.005 to 0.034), respectively, and these values also did not differ significantly (\({\mathcal{L}}\) ratio = 3.39, F 1, 23 = 3.20, P = 0.09). As in the analysis of the algae wafer treatment, we reran the analysis using a pooled dataset. The parameters of this analysis were −29.17‰ (CI −29.83 to −28.42) and 0.003 day−1 (CI −0.002 to 0.014) for δ13C value at equilibrium and m, respectively (r 2 = 0.77). Using this pooled δ13C value at equilibrium, we calculated Δ13C as 0.8‰ (CI 0.14–1.55).
Nitrogen
During the course of the experiment, abdominal muscle δ15N of our crayfish became enriched relative to the mean δ15N values of their respective diets (Fig. 1c & 1d). For the algae wafer diet, the δ15N values for O. virilis and O. rusticus at equilibrium were estimated as 8.26 and 7.45‰, respectively; and these values differed significantly between species (\({\mathcal{L}}\) ratio = 8.26, F 1, 25 = 8.57, P = 0.01). The metabolic turnover rate (m) for O. virilis and O. rusticus was estimated as 0.013 day−1 (CI 0.000–0.037) and 0.009 day−1 (CI 0.002–0.020), respectively, and these values also differed significantly between species (\({\mathcal{L}}\) ratio = 4.84, F 1, 25 = 4.71, P = 0.04). The final model, with species-specific δ15N at equilibrium and m values, had an r 2 of 0.89. Because of the significant difference in these parameters between species, we calculated individual Δ15N for each crayfish species and their diet as 3.35‰ (CI 2.53–4.51) for O. virilis and 2.54‰ (CI 2.06–3.08) for O. rusticus.
For the bloodworm treatment, the δ15N values for O. virilis and O. rusticus at equilibrium were estimated as 8.20 and 8.90‰, respectively, and these values did not differ significantly between species (\({\mathcal{L}}\) ratio = 0.28, F 1, 23 = 0.25, P = 0.62). The metabolic turnover rate (m) for O. virilis and O. rusticus was estimated as 0.007 day−1 (CI −0.003 to 0.025) and 0.003 day−1 (CI −0.009 to 0.017), respectively, and these values also did not differ significantly (\({\mathcal{L}}\) ratio = 0.04, F 1, 23 = 0.04, P = 0.84). Because we found no significant difference between species in either of these parameters, we reran the analysis using a pooled dataset. The parameters of this analysis were 8.56‰ (CI 7.68–9.47) and 0.004 day−1 (CI −0.002 to 0.014) for δ15N value at equilibrium and m, respectively (r 2 = 0.86). Using this pooled δ15N value at equilibrium, we calculated Δ15N between our experimental crayfish and their diet to be 1.2‰ (CI 0.32–2.11).
Half-lives
Mean δ15N and δ13C half-lives of O. rusticus were 20.84 and 19.67 days, respectively, for crayfish on the algae wafer diet, and 30.38 and 36.71 days, respectively, for crayfish on the bloodworm diet (Table 1). Mean δ15N and δ13C half-lives of O. virilis were 21.80 and 21.56 days, respectively, for crayfish on the algae wafer diet and 27.96 and 33.20 days, respectively, for crayfish on the bloodworm diet (Table 1). Consumers generally attain equilibrium with their diets after four (93.75% turnover) or five (96.875% turnover) half-lives (Hobson & Clark 1992); our experimental duration was approximately four half-lives for crayfish on the algae wafer diet and two (75% turnover) to three (87.5% turnover) half-lives for crayfish on the bloodworm diet. Despite not reaching equilibrium in all cases, the change in isotopic composition decelerated over the duration of our experiment for all treatments (Fig 1), allowing us to estimate equilibrium with a high degree of certainty (see δf 95% CI, Table 1).
Discussion
We found that Δ13C, Δ15N, and m of Orconectes virilis and O. rusticus were generally similar to each other and to the average values typically used across taxa (3.4 ± 1.1‰ for Δ15N, Minagawa & Wada, 1984; 0–1‰ for Δ13C Fry & Sherr, 1984), but differed significantly in some cases. Specifically, we found significant differences between O. rusticus and O. virilis in Δ15N and m when fed the algae wafer diet. The magnitude of these differences was not small, as mean Δ15N between species differed by 0.8‰. Further, we found a significant difference in Δ15N between diets when comparing the pooled Δ15N of both species on the bloodworm diet and the individual Δ15N of O. virilis on the algae wafer diet.
An important assumption of most applications of stable isotopes in ecology is that the same Δ and m values can be used across species; for example, in calculations of trophic positions or estimates of diet contributions from mixing models. Yet, despite a number of laboratory experiments that have investigated Δ and m across a breadth of taxa, few studies to date have compared these parameters between closely related species like O. rusticus and O. virilis for which these types of isotopic comparisons are common. In one of the few previous examples, Deniro & Epstein (1981) fed two species of fly (Calliphora vicina [Robineau-Desvoidy, 1830] and Musca domestica [L., 1758]) and two species of weevil (Sitophilus granarius [Linnaeus, 1758] and S. oryzae [Linnaeus, 1763]) the same diets and found Δ15N differences of 3.2 and 1.6‰ between species in the respective pairs. No such comparisons have previously been made between species of crayfish, where laboratory studies of isotopic Δ and m have been rare (Rudnick & Resh, 2005; Carolan et al., 2012). This knowledge gap is somewhat surprising given the popularity of stable isotope analysis as a technique to investigate and compare crayfish ecology between species under field conditions (e.g., Olsson et al., 2009; Ercoli et al., 2014; Jackson et al., 2014). Inferences from such studies that compare trophic function and ecological interactions of sympatric populations of crayfish species using stable isotopes are dependent on the heretofore untested assumption that these species share the same Δ and m. Our results suggest that this assumption may not be valid in all cases.
A possible explanation for the different Δ15N values observed in our study is that the two species differentially assimilated the different ingredients (appendix 1—Supplementary material) of the algae wafers, and that these ingredients had different δ15N signatures. Isotopic signatures of consumers reflect what has been assimilated out of the mix of heterogeneous diet items, rather than simply what has been ingested (Hecky & Hesslein, 1995). Furthermore, different foods may be assimilated more or less efficiently, which can also lead to differences in Δ (Stenroth et al., 2006). Therefore, although our crayfish species were fed the same diet, their tissues could have become enriched relative to different ingredients of that diet, leading to differences in Δ15N. Our estimated m values also differed significantly between the two species on the algae wafer diet, with O. virilis having a higher m than O. rusticus. Metabolic turnover rate (m) is a fitted parameter that quantifies the rate at which body mass is replaced independently of growth (Hesslein et al., 1993). Accordingly, our results suggest that muscle tissue of O. virilis was more metabolically active than for O. rusticus, and consequently, this species equilibrated more quickly with the algae wafer diet. For field applications, such differences between sympatric species would mean that observed δ15N signatures reflect different time scales of food consumption, and consequently, the interval of trophic comparison in an analysis might be mismatched.
Another common assumption in stable isotope analysis is that the same Δ can be used across diet items or prey resources. However, we found that pooled Δ15N of both crayfish species on the bloodworm diet (1.2‰, CI 0.32–2.11) was distinct from Δ15N of O. virilis on the algae wafer diet (3.34‰, CI 2.53–4.51), as evaluated from comparison of 95% confidence intervals. A number of previous studies have similarly found discrimination factors to differ between diet items (e.g., Webb et al., 1998; Fantle et al., 1999; Caut et al., 2009). One possible explanation for our observed difference in Δ15N between diets is a relationship between diet C:N (carbon to nitrogen ratio; i.e., a surrogate for protein content) and Δ15N. Fantle et al. (1999) argued that organisms consuming foods high in protein are able to grow and meet basic metabolic demands by obtaining nitrogen solely from their diet. The resulting δ15N of these organisms is slightly enriched as a result of deamination and transamination of amino acids, but generally low and relatively close to that of their diet (Minagawa & Wada, 1984). Conversely, organisms eating foods low in protein catabolize their own tissues to obtain enough nitrogen to meet basic metabolic demands. This nitrogen has already been processed and enriched through deamination and transamination and furthermore undergoes additional enrichment, thus resulting in a higher Δ15N (Hobson et al., 1993; Vander Zanden et al., 1997). Consistent with these predictions, we observed low Δ15N of crayfish on the bloodworm diet with high protein content (65%) and a low C:N ratio (4.27) and high Δ15N of crayfish on the algae wafer diet with a low protein content (36%) and a high C:N ratio (6.72).
Stable isotope studies are often based on the assumption that generic discrimination factors of 3.4 ± 1.1 ‰ for Δ15N (Minagawa & Wada, 1984) and 0–1‰ for Δ13C (Fry & Sherr, 1984) are widely applicable across taxa. The pooled Δ13C values of our crayfish fed algae wafers and bloodworms (Table 1) overlapped with these literature values, as did the Δ15N value of O. virilis fed the algae wafer diet; however, the pooled Δ15N values for crayfish fed bloodworms and the Δ15N value of O. rusticus fed algae wafers did not overlap with the literature values. The only other studies that we could find that determined Δ for crayfish under laboratory conditions found equally ambiguous results relative to the literature values. Rudnick & Resh (2005) fed a diet of carrots and a mixed diet of carrots and oligochaetes to red swamp crayfish (Procambarus clarkii [Girard, 1852]) and found Δ15N and Δ13C values of 6.0 and 1.9‰ (carrot diet), and 1.0 and 2.0‰ (mixed diet), respectively. None of these Δ overlapped with the literature values. In a separate experiment, Carolan et al. (2012) fed commercial fish food to common yabbies (Cherax destructor [Clark, 1936]) and obtained Δ15N values of 1.5 ± 1.0‰ (Δ13C values not included because crayfish did not reach equilibrium), which overlapped with the literature values. Combined, these results suggest that use of the mean literature values for Δ15N and Δ13C may not always be appropriate, as these values can vary widely.
To demonstrate the potential implications of ignoring variability in Δ, we present two hypothetical situations based on our experimental results. First, if we were to compare two species of crayfish living in sympatry and found that they had significantly different δ15N signatures, we would likely conclude that they were consuming different diets. Results of our study suggest that this conclusion could be wrong in many cases if these crayfish are eating the same diet but instead assimilating it differently. Similarly, if we were to collect crayfish from a population in the field that were enriched relative to their food by only 1.2‰, but we applied the commonly used Δ15N value of 3.4‰ to estimate trophic positions, we would underestimate trophic position by two levels. Such errors could have major implications for applications like comparing niche size and overlap between sympatric crayfishes (Olsson et al., 2008) or even calculating total food chain length in freshwater habitats (Post, 2002). The implications of such a result could lead to a misunderstanding of the ecosystem in study, especially if low Δ were a phenomenon for several organisms in the food web in question.
Conclusion
In conclusion, our study is one of the first to evaluate Δ13C, Δ15N, and m for crayfish, despite the popularity of stable isotope analysis as a technique to examine the role of crayfish in ecosystems (Roth et al., 2006; Stenroth et al., 2006; Olsson et al., 2008). Furthermore, our study is one of the few, regardless of taxa, that has examined Δ13C, Δ15N, and m of closely related species fed the same diets under controlled laboratory conditions. We found that Δ13C, Δ15N, and m are generally similar across species and diets, but that some exceptions exist. These exceptions provide evidence that, when studying sympatric species of organisms through stable isotope analysis, differences in discrimination factors between species may not reflect real differences in diet or trophic position. Further, we found that the widely used Δ13C and Δ15N values may not always be appropriate. We therefore recommend continued exploration of these parameters through laboratory experiments, using a C:N gradient to explore the relationship that protein content has on Δ, and a greater range of closely related species to determine how specifically Δ must be resolved in order to be accurately applied. However, given that laboratory studies focused on Δ and m are unlikely to be available for all taxa and diet contributions of interest under field conditions, we also recommend the use of new statistical methods to analyze stable isotope data that can incorporate uncertainty in discrimination factors (e.g., Bayesian isotope mixing models, Moore & Semmens, 2008). Accounting for this uncertainty improves accuracy of statistical tools in stable isotope analysis, and our laboratory study lends further evidence that this variation is common and can considerably confound interpretation of stable isotope data for ecological applications.
References
Bergman, D. A. & P. A. Moore, 2003. Field observations of intraspecific agonistic behavior of two crayfish species. Biological Bulletin 205: 26–35.
Boecklen, W. J., C. T. Yarnes, B. A. Cook & A. C. James, 2011. On the use of stable isotopes in trophic ecology. Annual Review of Ecology, Evolution, and Systematics 42: 411–440.
Bond, A. L. & A. W. Diamond, 2011. Recent Bayesian stable-isotope mixing models are highly sensitive to variation in discrimination factors. Ecological Application 21: 1017–1023.
Bosley, K. L., D. A. Witting, R. C. Chambers & S. C. Wainright, 2002. Estimating turnover rates of carbon and nitrogen in recently metamorphosed winter flounder Pseudopleuronectes americanus with stable isotopes. Marine Ecology Progress Series 236: 233–240.
Buchheister, A. & R. J. Latour, 2010. Turnover and fractionation of carbon and nitrogen stable isotopes in tissues of a migratory coastal predator, summer flounder (Paralichthys dentatus). Canadian Journal of Fisheries and Aquatic Sciences 67: 445–461.
Carolan, J. V., D. Mazumder, C. Dimovski, R. Diocares & J. Twining, 2012. Biokinetics and discrimination factors for 13C and δ15N in the omnivorous freshwater crustacean, Cherax destructor. Marine Freshwater Research 63: 878–886.
Caut, S., E. Angulo & F. Courchamp, 2008. Discrimination factors (Δ15N and Δ13C) in an omnivorous consumer: effect of diet isotopic ratio. Functional Ecology 22: 255–263.
Caut, S., E. Angulo & F. Courchamp, 2009. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46: 443–453.
Charlebois, P. M. & G. A. Lamberti, 1996. Invading crayfish in a Michigan stream: direct and indirect effects on periphyton and macroinvertebrates. Journal of the North American Benthological Society 15: 551–563.
Cherel, Y., K. A. Hobson, C. Guinet & C. Vanpe, 2007. Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialization in diving predators from the Southern Ocean. Journal of Animal Ecology 76: 826–836.
Cherel, Y., S. Ducatez, C. Fontaine, P. Richard & C. Guinet, 2008. Stable isotopes reveal the trophic position and mesopelagic fish diet of female southern elephant seals breeding on the Kerguelen Islands. Marine Ecology Progress Series 370: 239–247.
Dekar, M. P., D. D. Magoulick & G. R. Huxel, 2009. Shifts in the trophic base of intermittent stream food webs. Hydrobiologia 635: 263–277.
del Rio, C. M., N. Wolf, S. A. Carleton & L. Z. Gannes, 2009. Isotopic ecology ten years after a call for more laboratory experiments. Biological Reviews of the Cambridge Philosophical Society 84: 91–111.
DeNiro, M. J. & S. Epstein, 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica Et Cosmochimica Acta 45: 341–351.
DeNiro, M. J. & S. Epstein, 1978. Influence of diet on the distribution of carbon isotopes in animals. Geochimica Et Cosmochimica Acta 42: 495–506.
Ercoli, F., T. J. Ruokonen, H. Hämäläinen & R. I. Jones, 2014. Does the introduced signal crayfish occupy an equivalent trophic niche to the lost native noble crayfish in boreal lakes? Biological Invasions 16: 2025–2036.
Fantle, M. S., A. I. Dittel, S. M. Schwalm, C. E. Epifanio & M. L. Fogel, 1999. A food web analysis of the juvenile blue crab, Callinectes sapidus, using stable isotopes in whole animals and individual amino acids. Oecologia 120: 416–426.
Fortino, K. & R. P. Creed Jr, 2006. Abiotic factors, competition or predation: what determines the distribution of young crayfish in a watershed? Hydrobiologia 575: 301–314.
Fry, B. & C. Arnold, 1982. Rapid 13C/12C turnover during growth of brown shrimp (Penaeus aztecus). Oecologia 54: 200–204.
Fry, B. & E. B. Sherr, 1984. Δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contributions in Marine Sciences 27: 13–47.
Hammond, K. S., J. W. Hollows, C. R. Townsend & P. M. Lokman, 2006. Effects of temperature and water calcium concentration on growth, survival and moulting of freshwater crayfish, Paranephrops zealandicus. Aquaculture 251: 271–279.
Hecky, R. E. & R. H. Hesslein, 1995. Contributions of benthic algae to lake food webs as revealed by stable isotope analysis. Journal of the North American Benthological Society 14: 631–653.
Hesslein, R. H., K. A. Hallard & P. Ramlal, 1993. Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Canadian Journal of Fisheries and Aquatic Sciences 50: 2071–2076.
Hilderbrand, G. V., S. D. Farley, C. T. Robbins, T. A. Hanley, K. Titus & C. Servheen, 1996. Use of stable isotopes to determine diets of living and extinct bears. Canadian Journal of Zoology 74: 2080–2088.
Hill, A. M., D. M. Sinars & D. M. Lodge, 1993. Invasion of an occupied niche by the crayfish Orconectes rusticus: potential importance of growth and mortality. Oecologia 94: 303–306.
Hobson, K. A. & R. G. Clark, 1992. Assessing avian diets using stable isotopes I: turnover of 13C in tissues. The Condor 94: 181–188.
Hobson, K. A., R. T. Alisauskas & R. G. Clark, 1993. Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: implications for isotopic analyses of diet. The Condor 95: 388–394.
Jackson, M. C., T. Jones, M. Milligan, D. Sheath, J. Taylor, A. Ellis, J. England & J. Grey, 2014. Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshwater Biology 59: 1123–1135.
Kreps, T.A., E. R. Larson & D. M. Lodge, 2016. Do invasive rusty crayfish (Orconectes rusticus) decouple benthic and pelagic energy flows in lake food webs? Freshwater Science (in press).
Lodge, D. M., C. A. Taylor, D. M. Holdich & J. Skurdal, 2000. Reducing impacts of exotic crayfish introductions. Fisheries 25: 21–23.
McCarthy, J. M., C. L. Hein, J. D. Olden & M. J. Vander Zanden, 2006. Coupling long-term studies with meta-analysis to investigate impacts of non-native crayfish on zoobenthic communities. Freshwater Biology 51: 224–235.
McClellan, C. M., J. Braun-McNeill, L. Avens, B. P. Wallace & A. J. Read, 2010. Stable isotopes confirm a foraging dichotomy in juvenile loggerhead sea turtles. Journal of Experimental Marine Biology and Ecology 387: 44–51.
Minagawa, M. & E. Wada, 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta 48: 1135–1140.
Momot, W. T., 1995. Redefining the role of crayfish in aquatic ecosystems. Reviews in Fisheries Science 3: 33–63.
Momot, W. T., H. Gowing & P. D. Jones, 1978. The dynamics of crayfish and their role in ecosystems. American Midland Naturalist 99: 10–35.
Moore, J. W. & B. X. Semmens, 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters 11: 470–480.
Mundahl, N. D., 1989. Seasonal and diel changes in thermal tolerance of the crayfish Orconectes rusticus, with evidence for behavioral thermoregulation. Journal of the North American Benthological Society 8: 173–179.
Newsome, S. D., M. T. Tinker, D. H. Monson, O. T. Oftedal, K. Ralls, M. M. Staedler, M. L. Fogel & J. A. Estes, 2009. Using stable isotopes to investigate individual diet specialization in California sea otters (Enhydra lutris nereis). Ecology 90: 961–974.
Nilsson, E., C. T. Solomon, K. A. Wilson & T. V. Willis, 2012. Effects of an invasive crayfish on trophic relationships in north-temperate lake food webs. Freshwater Biology 57: 10–23.
Olsson, K., P. Nyström, P. Stenroth, E. Nilsson, M. Svensson & W. Granéli, 2008. The influence of food quality and availability on trophic position, carbon signature, and growth rate of an omnivorous crayfish. Canadian Journal of Fisheries and Aquatic Sciences 65: 2293–2304.
Olsson, K., P. Stenroth, P. Nyström & W. Granéli, 2009. Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshwater Biology 54: 1731–1740.
Peters, J. A. & D. M. Lodge, 2013. Habitat, predation, and coexistence between invasive and native crayfishes: prioritizing lakes for invasion prevention. Biological Invasions 15: 2489–2502.
Peters, J. A., M. J. Cooper, S. M. Creque, M. S. Kornis, J. T. Maxted, W. L. Perry, F. W. Schueler, T. P. Simon, C. A. Taylor, R. F. Thoma, D. G. Uzarski & D. M. Lodge, 2014. Historical changes and current status of crayfish diversity and distribution in the Laurentian Great Lakes. Journal of Great Lakes Research 40: 35–46.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montaña, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152: 179–189.
R Core Team, 2014. R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria
Reynolds, J., C. Souty-Grosset & A. Richardson, 2013. Ecological roles of crayfish in freshwater and terrestrial habitats. Freshwater Crayfish 19: 197–218.
Ritz, C. & J. C. Streibig, 2008. Model reduction and parameter models. In Gentleman, R., K. Hornik & G. Parmigiani (eds), Nonlinear Regression with R. Springer Science, New York: 114–118.
Rosenblatt, A. E. & M. R. Heithaus, 2013. Slow isotope turnover rates and low discrimination values in the American alligator: implications for interpretation of ectotherm stable isotope data. Physiological and Biochemical Zoology 86: 137–148.
Roth, B. M., C. L. Hein & M. J. Vander Zanden, 2006. Using bioenergetics and stable isotopes to assess the trophic role of rusty crayfish (Orconectes rusticus) in lake littoral zones. Canadian Journal of Fisheries and Aquatic Sciences 63: 335–344.
Rudnick, D. & V. Resh, 2005. Stable isotopes, mesocosms and gut content analysis demonstrate trophic differences in two invasive decapod crustacea. Freshwater Biology 50: 1323–1336.
Ruokonen, T. J., J. Karjalainen, M. Kiljunen, M. Pursiainen & H. Hämäläinen, 2011. Do introduced crayfish affect benthic fish in stony littoral habitats of large boreal lakes? Biological Invasions 14: 813–825.
Solomon, C. T., S. R. Carpenter, M. K. Clayton, J. J. Cole, J. J. Coloso, M. L. Pace, M. J. Vander Zanden & B. C. Weidel, 2011. Terrestrial, benthic, and pelagic resource use in lakes: results from a three-isotope Bayesian mixing model. Ecology 92: 1115–1125.
Stenroth, P., N. Holmqvist, P. Nyström, O. Berglund, P. Larsson & W. Granéli, 2006. Stable isotopes as an indicator of diet in omnivorous crayfish (Pacifastacus leniusculus): the influence of tissue, sample treatment, and season. Canadian Journal of Fisheries and Aquatic Sciences 63: 821–831.
Thomas, S. M. & T. W. Crowther, 2014. Predicting rates of isotopic turnover across the animal kingdom: a synthesis of existing data. Journal of Animal Ecology 84: 861–870.
Twardochleb, L. A., J. D. Olden & E. R. Larson, 2013. A global meta-analysis of the ecological impacts of nonnative crayfish. Freshwater Science 32: 1367–1382.
Vander Zanden, M. J. & J. B. Rasmussen, 1999. Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80: 1395–1404.
Vander Zanden, M. J. & J. B. Rasmussen, 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography 46: 2061–2066.
Vander Zanden, M. J., G. Cabana & J. B. Rasmussen, 1997. Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (δ15N) and literature dietary data. Canadian Journal of Fisheries and Aquatic Sciences 54: 1142–1158.
Vander Zanden, M. J., J. M. Casselman & J. B. Rasmussen, 1999. Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401: 464–467.
Venarsky, M. P., B. M. Huntsman, A. D. Huryn, J. P. Benstead & B. R. Kuhajda, 2014. Quantitative food web analysis supports the energy-limitation hypothesis in cave stream ecosystems. Oecologia 176: 859–869.
Webb, S. C., R. E. M. Hedges & S. J. Simpson, 1998. Diet quality influences the δ13C and δ15N of locusts and their biochemical components. The Journal of Experimental Biology 201: 2903–2911.
Whitledge, G. W. & C. F. Rabeni, 1997. Energy sources and ecological role of crayfishes in an Ozark stream: insights from stable isotopes and gut analysis. Canadian Journal of Fisheries Aquatic Sciences 54: 2555–2563.
Acknowledgments
We thank Central Michigan University and Shedd Aquarium for providing funding. We also thank Matthew Cooper, James Student, and Daelyn Woolnough for allowing us to conduct the experiment using their lab space and equipment, Jonathan Benstead for early conversations on crayfish stable isotope discrimination factors, and two anonymous reviewers for their insightful comments on the manuscript. Mael Glon thanks Stanley and Barbara Adams for early inspiration in the study of crayfish.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: M. Power
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Glon, M.G., Larson, E.R. & Pangle, K.L. Comparison of 13C and 15N discrimination factors and turnover rates between congeneric crayfish Orconectes rusticus and O. virilis (Decapoda, Cambaridae). Hydrobiologia 768, 51–61 (2016). https://doi.org/10.1007/s10750-015-2527-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2527-3