Abstract
We examined trophic dynamics of a stream food web where invasive Orconectes neglectus appear to be displacing native O. eupunctus in the Spring River drainage of the Ozark Highlands, Missouri and Arkansas, USA. We collected crayfish species and possible food sources seasonally from a site of sympatry on the South Fork Spring River. We determined diet overlap and potential for competition between O. eupunctus and O. neglectus, and investigated seasonal variation using carbon and nitrogen stable isotope analyses and gut content analyses. Gut content analysis showed both species of crayfish consumed mainly detritus during summer and spring, with other prey categories varying by species and season. Stable isotope analysis showed that O. eupunctus and O. neglectus relied on invertebrates as a major energy and nutrient source throughout summer, autumn, and spring, and the two species showed differences in their stable isotope signatures during spring and summer, but not autumn. Given the trophic overlap between O. eupunctus and O. neglectus, there is a potential for the two species to compete for food and to be ecologically redundant. Ecological redundancy can lead to reduced effects on ecosystem function post-invasion, and therefore examining ecological redundancy of potential invaders should be a conservation priority.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crayfish introductions have elicited drastic changes in aquatic systems, both to native crayfish faunas (Capelli, 1982; Berrill, 1985; Momot, 1996) and to other members of the aquatic communities (Olsen et al., 1991; Gamradt et al., 1997; Nilsson et al., 2012; Ercoli et al. 2015). Invasive crayfish can negatively impact native crayfishes via reproductive interference (Butler & Stein, 1985; Perry et al., 2001), transmission of the crayfish plague (Alderman et al., 1990; Evans & Edgerton, 2002; Westman et al., 2002), interspecific competition (Hill & Lodge, 1994; Usio et al., 2001; Gherardi & Cioni, 2004), and habitat displacement with subsequent selective predation by fish (DiDonato & Lodge, 1993; Garvey et al., 1994).
In the Ozark highlands of Arkansas and Missouri, the crayfish Orconectes neglectus Faxon, 1885 has recently invaded portions of the Spring River drainage, apparently displacing two native crayfish species, Orconectes eupunctus Williams, 1952 and Cambarus hubbsi Creaser, 1931 (Flinders & Magoulick, 2005; Magoulick & DiStefano, 2007). Orconectes neglectus is native to the White River drainage in southern Missouri and northern Arkansas (Pflieger, 1996) and the likely vector of introduction was via bait bucket. Research suggests that; (1) O. neglectus was introduced into the West Fork of the Spring River between 1984 and 1998 (Flinders & Magoulick, 2005; Magoulick & DiStefano, 2007), (2) O. neglectus may be negatively impacting the native crayfish community, especially O. eupunctus (Rabalais & Magoulick, 2006a, b; Larson & Magoulick, 2009; Larson et al., 2009), and (3) O. neglectus has the potential to expand its distribution in the Spring River drainage and may negatively affect other species including the imperiled Orconectes marchandi Hobbs, 1948 (Flinders & Magoulick, 2005; Taylor et al., 2007). Mechanisms driving this apparent displacement have not been determined, although seasonal drought and stream drying likely act as at least a partial mechanism (Larson et al., 2009).
Orconectes eupunctus and O. neglectus show similar habitat use, mainly selecting riffles, suggesting interspecific competition as a potential mechanism involved in the displacement (Magoulick & DiStefano, 2007). In previous studies, habitat use and selection by O. eupunctus and O. neglectus juveniles and adults did not shift in sympatry versus allopatry suggesting the two species did not compete for habitat (Rabalais & Magoulick, 2006a). Additionally, densities of adult and juvenile male O. eupunctus and O. neglectus were manipulated in field experimental enclosures, but no evidence of interspecific competition was found (Rabalais & Magoulick, 2006b; Larson & Magoulick, 2009). However, laboratory experiments showed that O. neglectus aggressively dominated O. eupunctus when food resources were limited (Larson & Magoulick, 2009). Therefore, it is necessary to examine prey resource use between species in situ to determine the potential for interspecific competition.
Most studies that use gut content analysis have described crayfish as omnivores with detritus, periphyton, sediment, and macrophytes making up the majority of the diet (Whitledge & Rabeni, 1997; Whitmore & Huryn, 1999; Helms & Creed, 2005). This diet composition is further substantiated by studies on ecosystem level effects of crayfish, specifically their impact on periphyton, macrophytes, and terrestrial leaf decomposition (Lodge & Lorman, 1987; Olsen et al., 1991; Hart, 1992; Creed, 1994; Charlebois & Lamberti, 1996; Nyström et al., 1999; Ludlam & Magoulick, 2010; Lodge et al., 2012). However, in laboratory experiments crayfish have been shown to have very little or no growth when fed only detritus, periphyton, and macrophytes (Hill et al., 1993). Gut content analysis only provides insight into a very short time frame and may not be indicative of what is being assimilated into crayfish tissue growth. Recent studies have expanded on the use of gut content analysis by using stable isotopes as a method of determining energy and nutrient source (Parkyn et al., 2001). The use of δ13C and δ15N allows for the determination of the assimilated fraction of the diet over an extended period of time as well as identifying carbon and nitrogen sources not obvious in gut content analysis (DeNiro & Epstein, 1978; Deniro & Epstein, 1981; Fry & Sherr, 1984; Peterson & Fry, 1987). The application of stable isotopes in determining crayfish food web structure has suggested that crayfish may be assimilating more protein and energy rich food than has been previously shown with gut content analysis (Whitledge & Rabeni, 1997; Nyström et al., 1999; Parkyn et al., 2001; Rudnick & Resh, 2005; Roth et al., 2006). Therefore, crayfish in many systems may need animal food sources. If these are in limited supply, or if less dominant species are unable to secure these food sources, then it could have a negative effect on crayfish fitness (Hill & Lodge, 1999).
In most streams, hydrologic regimes vary dramatically temporally which might affect food web structure (Closs & Lake, 1994). However, most studies examining food web structure and feeding relationships have ignored temporal variation. In particular, Ozark streams undergo dramatic seasonal shifts from high flows in autumn through spring and low flows in summer and early autumn. This seasonal variation in flows is likely important in the apparent displacement of O. eupunctus by O. neglectus (Larson et al., 2009) and may also effect feeding relationships and food web structure.
Given that O. eupunctus and O. neglectus use similar structural habitats but do not appear to compete for space, while they may potentially compete for food, we examined prey use by O. eupunctus and O. neglectus to determine the potential for interspecific competition. Additionally, we were interested in whether the native and invasive crayfish species may be ecologically redundant in these systems (i.e., species or functional groups that have similar effects on ecosystem structure and function; Walker, 1992). We also sought to determine how food web structure and feeding relationships among O. eupunctus and O. neglectus varied seasonally. Our objectives were to determine whether (1) O. eupunctus and O. neglectus used similar prey, and (2) O. eupunctus and O. neglectus feeding relationships varied temporally. We used gut content and stable isotope analyses to address these questions.
Materials and methods
This study was conducted on the South Fork Spring River in north central Arkansas (36°27′53″N, 91°51′48″W) where the invasive O. neglectus co-occurs with the native O. eupunctus. Because O. eupunctus and O. neglectus select primarily riffle and run habitats (Rabalais & Magoulick, 2006b; Magoulick & DiStefano, 2007) the study site consisted primarily of riffle and run habitats and associated stream margin.
Crayfish were collected during summer (August 2002), autumn (November 2002) and spring (April 2003). Crayfish were collected during daylight hours using a kick net and Smith-Root Model 12 backpack electrofisher. Collected crayfish were identified to species, sexed, and carapace length recorded to the nearest mm before being placed in a sample container. Potential foods were also collected during each sampling event. Fish were collected with a kicknet and electrofisher, and invertebrates were collected with a Hess sampler. Repeat collections were made within the site to ensure enough material was collected for stable isotope analysis. Periphyton were scraped from rocks using scalpels and placed in sample containers. Leaf detritus (hereafter detritus), filamentous green algae, wood, and aquatic vegetation were collected by hand and using forceps. All samples collected were placed in coolers on ice and transported to the laboratory within 5 h.
Crayfish were classified as juveniles (≤12 mm CL) and adults (>12 mm CL) for analysis. Adult crayfish typically ranged 15–27 mm CL. This was based on length-frequency histograms of O. eupunctus and O. neglectus collected in a concurrent study (Rabalais & Magoulick, 2006b). Both species showed similar growth rates and had similar juvenile and adult sizes in this system (Larson & Magoulick, 2008).
At the laboratory, all samples were immediately lyophilized (LabConco Corp.) and frozen. Invertebrates were analyzed whole and all other samples were homogenized using a pestle and mortar. Ground material was passed through a 4 mm mesh screen to reduce sample heterogeneity. Samples were stored in a vacuum desiccator until analyzed at the University of Arkansas Stable Isotope Laboratory. Individual crayfish abdomen muscle tissue was ground and 0.2–0.3 mg/sample used in analysis. Invertebrates were weighed and analyzed as whole organisms with the exception of Chironomidae which were sometimes paired to obtain 0.2–0.3 mg/sample. Filamentous green algae, periphyton, and detritus samples were subsampled to obtain 1.5–2.5 mg/sample of material for analysis. All samples were weighed into tin capsules on a microbalance (Sartorius) prior to analysis.
Elemental and isotopic analyses were performed using an elemental analyzer (Carlos Erba NA 2500) coupled with an isotope ratio mass spectrometer (Thermo Finnigan Delta Plus). Stable isotope ratios are reported in the δ notation where δ13C or δ15N = ([R sample/R standard] − 1) × 1000 where R sample is 13C/12C or 15N/14N of the sample and R standard is 13C/12C of Pee Dee belemnite carbonate or 15N/14N of atmospheric N2. Internal standards of known relation to above-listed international standards were used every six samples. Reproducibility of internal standards at 2 standard deviations was 0.2‰ for δ13C and 0.4‰ for δ15N.
To determine prey ingested, crayfish from stable isotope analyses were dissected and foreguts were removed. Foregut contents were placed in a Petri dish and distributed evenly across the plate following Whitledge & Rabeni (1997). Gut contents were viewed under a dissecting microscope at ×20 magnification. Percent of the total area of the dish of detritus, invertebrates, algae, and inorganic material (i.e., sand and silt) was estimated. Autumn crayfish stomach contents were not determined due to a freezer malfunction.
We used mixing models in IsoSource (Phillips & Gregg, 2003) to determine the percent contribution of various energy and nutrient sources to crayfish. The potential sources were allochthonous detritus (leaves), invertebrates, filamentous green algae, and periphyton. We focused on Trichoptera, Ephemeroptera, and Chironomidae for this analysis because they were the most abundant invertebrate taxa. Sources were determined to be isotopically distinct by examining means and standard errors. We input δ13C and δ15N signatures of each source (prey) and mixture (crayfish) and set the increment size to 1% and the tolerance to 0.1‰. Before running IsoSource, values were adjusted for trophic fractionation from diet items to crayfish (2.3‰ for δ15N and 0.5‰ for δ13C) based on fractionation values determined from literature (France & Peters, 1997; Vanderklift & Ponsard, 2003). We report the ranges of feasible solutions for all possible combinations of source proportions for the population rather than means and standard deviations because they are more informative for mixing model stable isotope analysis (Phillips & Gregg, 2003).
We used ANOVA to determine differences in prey categories ingested by crayfish species. We used MANOVA to determine differences in δ13C and δ15N signatures of crayfish species by season. Significant differences in MANOVA were followed by ANOVA to determine differences in each response variable. We did not correct for differences in basal resource signatures among seasons as only filamentous green algae showed substantial variation in signatures among seasons and primary consumers varied little indicating this variation did not carry up the food web.
Results
Feeding relationships
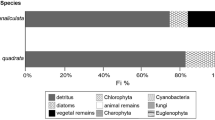
Gut content analysis showed all species-age classes of crayfish consumed mainly detritus, with other prey categories varying by species and season (Fig. 1). Percent of each prey category consumed did not differ significantly among species in either season (ANOVA P > 0.250).
Invertebrates appeared to be the main energy and nutrient source for both species of crayfish (Fig. 2). Mixing models showed that invertebrates were the largest contributing source to all crayfish stable isotope signatures, although there was an overlap among invertebrates, algae, and periphyton for O. eupunctus in summer (Table 1). Periphyton was the second largest crayfish energy and nutrient source in spring. In autumn, invertebrates made up a greater proportion of prey of O. eupunctus than of O. neglectus (Table 1).
Temporal variation
Percent detritus consumed decreased and percent inorganic material consumed increased from summer to spring (Fig. 1). Adult crayfish showed a significant species by season interaction in δ15N and δ13C stable isotope signatures (MANOVA Pillai Trace P = 0.029) driven by differences in δ15N of O. eupunctus among seasons (Fig. 3). Orconectes eupunctus δ15N signatures were significantly greater in autumn than in summer or spring (Tukey’s test P < 0.001), whereas O. eupunctus δ13C signatures did not differ among seasons (ANOVA P = 0.123). Orconectes neglectus δ15N and δ13C signatures did not differ significantly among seasons (MANOVA Pillai’s Trace P = 0.288).
Mean (±SE) δ13C and δ15N signatures (‰) of O. eupunctus and O. neglectus adults and juveniles during summer, autumn and spring at middle site in South Fork Spring River. Sample sizes are as in Fig. 2
When examined on a seasonal basis, adult O. eupunctus and O. neglectus δ15N and δ13C combined signatures differed significantly in summer (Pillai Trace P = 0.013) and spring (Pillai Trace P = 0.011), but not in autumn (Pillai Trace P = 0.063). In summer, O. neglectus adults had significantly greater δ15N (ANOVA P = 0.048) and δ13C (ANOVA P = 0.037) than O. eupunctus adults (Fig. 3), whereas in spring only δ13C (ANOVA P = 0.004) was significantly greater (Fig. 3). Although the combined δ15N and δ13C signatures did not differ in autumn, O. eupunctus adults had significantly greater δ15N (ANOVA P = 0.016) than O. neglectus adults in autumn (Fig. 3). The δ15N difference between O. eupunctus and O. neglectus in autumn was mainly due to increased consumption of invertebrates by O. eupunctus from summer to autumn (Table 1). Stable isotope signatures of O. eupunctus and O. neglectus were significantly different in autumn (Pillai Trace P = 0.002), but not in summer (Pillai Trace P = 0.063; Fig. 3). In autumn, O. eupunctus juveniles had significantly greater δ15N (ANOVA P = 0.001) than O. neglectus juveniles (Fig. 3). We collected no O. eupunctus juveniles in spring.
Discussion
Gut content analysis showed that native O. eupunctus and invasive O. neglectus consumed mainly detritus, whereas stable isotope mixing models showed that both species gained most of their nutrients from invertebrates. Statistical analysis showed that O. eupunctus and O. neglectus adults differed in their stable isotope signatures in some seasons. Therefore, the former two analyses indicate that O. eupunctus and O. neglectus prey resources overlap and show the potential for competition, whereas the latter analysis suggests that prey resources of O. eupunctus and O. neglectus did not overlap during some seasons and may not show potential for competition. The discrepancy between the analyses may be due to subtle seasonal changes in diet between the two species that are not picked up by gut content analysis. It is also possible that the differences in stable isotope concentrations between the species in some seasons may not indicate differences in prey categories, but rather may be due to subtle differences in proportion of prey types. The δ15N difference between O. eupunctus and O. neglectus adults in autumn appears to be an exception with increased consumption of invertebrates by O. eupunctus leading to increased δ15N values from summer to autumn. On the other hand, gut contents analysis and stable isotope mixing models provided strong evidence that native O. eupunctus and invasive O. neglectus were consuming similar diets and gaining the majority of their energy and nutrients from similar prey types.
Others have found strong overlap between native and invasive crayfish diets. In Scandinavia, stable isotope analysis showed that native noble crayfish and introduced signal crayfish did not differ in their niche widths in boreal streams (Olsson et al., 2009) and lakes (Ercoli et al., 2014) and isotopic niches of the two species strongly overlapped in lakes (Ercoli et al., 2014). Using stable isotope analysis in an Australian river, Beatty (2006) found that the native marron and introduced yabbie occupied similar predatory trophic positions in summer, but the two species differed in their trophic positions in winter.
We also found temporal variation in crayfish gut contents and δ15N and δ13C stable isotope signatures. Few studies have examined temporal variation in freshwater food webs based on gut contents and stable isotope analysis, especially related to crayfish trophic dynamics. Stenroth et al. (2006) found that δ15N and δ13C signatures of Pacifastacus leniusculus Dana, 1852 did not differ between August and September or between 2001 and 2003 in lentic systems. Beatty (2006) found that native marron and introduced yabbie showed strong trophic overlap in summer, with most of their energy coming from fish, whereas in winter the yabbie trophic position shifted to herbivory.
Although O. eupunctus and O. neglectus were often using similar prey resources in the South Fork Spring River, competition will not occur unless food is limiting in this system and that has not been established. Field experiments have shown that adult and juvenile male O. eupunctus and O. neglectus did not compete for space and food when enclosed at typical densities in the South Fork Spring River (Rabalais & Magoulick, 2006b; Larson & Magoulick, 2009). Fortino & Creed (2007) also found no evidence for competition between two native crayfish species in Appalachian streams and suggested that predation was more important in structuring these communities. Competition for shelter has also been suggested as an important mechanism in structuring some crayfish communities (Bovbjerg, 1970; Rabeni, 1985; Garvey et al., 1994; Gherardi et al., 2004; Gherardi & Cioni, 2004), but little evidence exists for food competition among crayfish. Food and space may not be limiting crayfish populations in the Spring River.
Our finding that crayfish consume mostly detritus, but gain most of their energy and nutrients from animals or algae has been found in other systems (Whitledge & Rabeni, 1997; Parkyn et al., 2001; Hollows et al., 2002). Momot (1995) suggested that animal nitrogen is important in the diet of all crayfish, but that juvenile crayfish should require more animal protein than adult crayfish. We found that diets and isotopic signatures of juvenile and adult crayfish were similar and that both age classes gained most of their energy and nutrients from consumption of invertebrates. Some studies have found that juvenile crayfish consume more invertebrates and adult crayfish consume more detritus (Whitledge & Rabeni, 1997; Parkyn et al., 2001; Hollows et al., 2002). However, Bondar et al. (2005) found that both juvenile and adult P. leniusculus consumed mainly detritus when presented a choice of detritus and insects even though they grew faster on insects. Stenroth et al. (2006) found that juvenile and adult P. leniusculus were both dependent on animal matter in their diet based on stable isotope analysis. Although crayfish function as omnivores, their high use of animal matter as an energy and nutrient source has led some to suggest that crayfish occupy the trophic role of predators (Parkyn et al., 2001; Roth et al., 2006). Conversely, Stenroth et al. (2006) suggested that carbon and nitrogen may follow different pathways in omnivorous crayfish with plant material contributing to the carbon budget and invertebrates contributing to the nitrogen budget. Therefore, crayfish could be viewed as detritivores with respect to carbon and predators with respect to nitrogen. Our results support the idea that crayfish function as omnivores consuming mainly detritus, but act as trophic predators being relatively enriched in δ15N, placing them as predators in the food web along with omnivorous central stonerollers (a grazing minnow) and below insectivorous rainbow darters.
Additionally, overlap in the food resources of native O. eupunctus and invasive O. neglectus suggests potential for ecological redundancy. This has important implications because if native and invasive species are ecologically redundant then species replacement should have little, if any, effects on ecosystem structure and function. In a stream mesocosm experiment, Magoulick (2014) found that native O. eupunctus and invasive O. neglectus were largely ecologically redundant, although subtle differences in crayfish effects on periphyton and sediment could potentially cascade through the food web. Usio et al. (2006) also found that the functional roles of a native and exotic crayfish were similar in Japan streams. In boreal lakes, Ercoli et al. (2015) found that introduced signal crayfish and native noble crayfish were ecologically equivalent in their effects on littoral macroinvertebrate assemblages. In a study on these two species in Swedish streams, Olsson et al. (2009) suggested the two species are likely to have similar impacts at the stream scale, but the invader is likely to have greater impacts at the regional scale due to their ability to occupy a greater range of stream conditions. Others have found that crayfish that replace native species can have large direct and indirect effects on ecosystem structure and function (Wilson et al., 2004; Lodge et al., 2012). Magoulick (2014) suggested that most studies that have found large effects of invasive species relative to native species have examined extraregional (invaded another continent or crossed major drainage boundaries within a continent) versus extralimital (invaded a drainage or state adjacent to their native range) invasions (Larson & Olden, 2010). Therefore, it is possible that extraregional invasions fundamentally differ from extralimital invasions in terms of ecological redundancy. Further research is needed to address this question.
Identifying crayfish gut contents has associated error and it is possible that this led to part of the discrepancy between gut contents and stable isotope analysis. However, given the large differences in the importance of detritus versus invertebrates it is unlikely that this source of error was responsible for the substantial differences we found using these two approaches. Whitledge & Rabeni (1997) found that Orconectes spp. had high assimilation efficiencies on insects (92%) and low assimilation efficiencies on detritus (14%) and this likely explains the discrepancy between gut content and stable isotope mixing models for crayfish. However, even using higher assimilation efficiencies for animal matter (70%) than leaf detritus (15%), detritus contributed most to production of Orconectes spp. (45%) followed by animal matter (30%) in a Kansas stream (Evans-White et al., 2003).
We found that native O. eupunctus and invasive O. neglectus consumed mainly detritus, but both species gained most of their energy and nutrients from invertebrates and this was the case during three seasons. Our results support the idea that crayfish function as omnivores consuming mainly detritus, but act as trophic predators in the food web. In our study stream and elsewhere, the trophic role of crayfish is not easily generalized, as omnivorous crayfish may function as detritivores or predators depending on techniques used to analyze feeding relationships. Given this trophic overlap, there is a potential for the two species to compete for food in this system as well as display ecological redundancy.
References
Alderman, D. J., D. M. Holdich & I. Reeve, 1990. Signal crayfish as vectors in crayfish plague in Britain. Aquaculture 86: 3–6.
Beatty, S. J., 2006. The diet and trophic positions of translocated, sympatric populations of Cherax destructor and Cherax cainii in the Hutt River, Western Australia: evidence of resource overlap. Marine and Freshwater Research 57: 825–835.
Berrill, M., 1985. Laboratory induced hybridization of two crayfish species, Orconectes rusticus and O. propinquus. Journal of Crustacean Biology 5: 347–349.
Bondar, C. A., K. Bottriell, K. Zeron & J. S. Richardson, 2005. Does trophic position of the omnivorous signal crayfish (Pacifastacus leniusculus) in a stream food web vary with life history stage or density? Canadian Journal of Fisheries and Aquatic Sciences 62: 2632–2639.
Bovbjerg, R. V., 1970. Ecological isolation and competitive exclusion in two crayfish Orconectes virilis and Orconectes immunis. Ecology 51: 227–236.
Butler, M. J. & R. A. Stein, 1985. An analysis of the mechanisms governing species replacements in crayfish. Oecologia 66: 168–177.
Capelli, G. M., 1982. Displacememt of northern Wisconsin crayfish by Orconectes rusticus (Girard). Limnology and Oceanography 27: 741–744.
Charlebois, P. M. & G. A. Lamberti, 1996. Invading crayfish in a Michigan stream: direct and indirect effects on periphyton and macroinvertebrates. Journal of the North American Benthological Society 15: 551–563.
Closs, G. P. & P. S. Lake, 1994. Spatial and temporal variation in the structure of an intermittent-stream food web. Ecological Monographs 64: 1–21.
Creed, R. P., 1994. Direct and indirect effects of crayfish grazing a stream community. Ecology 75: 2091–2103.
DeNiro, M. J. & S. Epstein, 1978. Carbon isotopic evidence for different feeding patterns in two Hyrax species occupying the same habitat. Science 201: 906–908.
Deniro, M. J. & S. Epstein, 1981. Influence of diet on the distribution of nitrogen isotopes in animals. Geochimica et Cosmochimica Acta 45: 341–352.
DiDonato, G. T. & D. M. Lodge, 1993. Species replacements among orconectes crayfishes in wisconsin lakes: the role of predation by fish. Canadian Journal of Fisheries and Aquatic Sciences 50: 1484–1488.
Ercoli, F., T. J. Ruokonen, H. Hamalainen & R. I. Jones, 2014. Does the introduced signal crayfish occupy an equivalent trophic niche to the lost native noble crayfish in boreal lakes? Biological Invasions 16: 2025–2036.
Ercoli, F., T. J. Ruokonen, E. Erkamo, R. I. Jones & H. Hamalainen, 2015. Comparing the effects of introduced signal crayfish and native noble crayfish on the littoral invertebrate assemblages of boreal lakes. Freshwater Science 34: 555–563.
Evans-White, M. A., W. K. Dodds & M. R. Whiles, 2003. Ecosystem significance of crayfishes and stonerollers in a prairie stream: functional differences between co-occurring omnivores. Journal of the North American Benthological Society 22: 423–441.
Evans, L. H. & B. F. Edgerton (eds), 2002. Pathogens, parasites, and commensals. Blackwell Science, Oxford.
Flinders, C. A. & D. D. Magoulick, 2005. Distribution, habitat use and life history of stream-dwelling crayfish in the spring river drainage of Arkansas and Missouri with a focus on the imperiled Mammoth Spring Crayfish (Orconectes marchandi). American Midland Naturalist 154: 358.
Fortino, K. & R. P. Creed, 2007. Abiotic factors, competition or predation: what determines the distribution of young crayfish in a watershed? Hydrobiologia 575: 301–314.
France, R. L. & R. H. Peters, 1997. Ecosystem differences in the trophic enrichment of C-13 in aquatic food webs. Canadian Journal of Fisheries and Aquatic Sciences 54: 1255–1258.
Fry, B. & E. B. Sherr, 1984. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contributions in Marine Science 27: 13–47.
Gamradt, S. C., L. B. Kats & C. B. Anzalone, 1997. Aggression by non-native crayfish deters breeding in California newts. Conservation Biology 11: 793–796.
Garvey, J. E., R. A. Stein & H. M. Thomas, 1994. Assessing how fish predation and interspecific prey competition influence a crayfish assemblage. Ecology 75: 532–547.
Gherardi, F. & A. Cioni, 2004. Agonism and interference competition in freshwater decapods. Behaviour 141: 1297–1324.
Gherardi, F., P. Acquistapace & G. Santini, 2004. Food selection in freshwater omnivores: a case study of crayfish Austropotamobius pallipes. Archiv Fur Hydrobiologie 159: 357–376.
Hart, D. D., 1992. Community organization in streams: the importance of species interactions, physical factors, and chance. Oecologia 91: 220–228.
Helms, B. S. & R. P. Creed, 2005. The effects of 2 coexisting crayfish on an Appalachian river community. Journal of the North American Benthological Society 24: 113–122.
Hill, A. M. & D. M. Lodge, 1994. Diel changes in resource demand: competition and predation in species replacement among crayfishes. Ecology 75: 2118–2126.
Hill, A. M. & D. M. Lodge, 1999. Replacement of resident crayfishes by an exotic crayfish: the roles of competition and predation. Ecological Applications 9: 678–690.
Hill, A. M., D. M. Sinars & D. M. Lodge, 1993. Invasion of an occupied niche by the crayfish Orconectes rusticus: potential importance of growth and mortality. Oecologia 94: 303–306.
Hobbs, H. H., 1948. Two new crayfishes of the same genus Orconectes from Arkansas with a key to the species of the Hylas group (Decapoda, Astacidae). American Midland Naturalist 39: 139–150.
Hollows, J. W., C. R. Townsend & K. J. Collier, 2002. Diet of the crayfish Paranephros zealandicus in bush and pature streams: insights from stable isotopes and stomach analysis. New Zealand Journal of Marine and Freshwater Research 36: 129–142.
Larson, E. R. & D. D. Magoulick, 2008. Comparative life history of native (Orconectes eupunctus) and introduced (Orconectes neglectus) crayfishes in the Spring River drainage of Arkansas and Missouri. American Midland Naturalist 160: 323–341.
Larson, E. R. & D. D. Magoulick, 2009. Does juvenile competition explain displacement of a native crayfish by an introduced crayfish? Biological Invasions 11: 725–735.
Larson, E. R. & J. D. Olden, 2010. Latent extinction and invasion risk of crayfishes in the Southeastern United States. Conservation Biology 24: 1099–1110.
Larson, E. R., D. D. Magoulick, C. Turner & K. H. Laycock, 2009. Disturbance and species displacement: different tolerances to stream drying and desiccation in a native and an invasive crayfish. Freshwater Biology 54: 1899–1908.
Lodge, D. M. & J. G. Lorman, 1987. Reductions in submersed macrophyte biomass and species richness by the crayfish Orconectes rusticus. Canadian Journal of Fisheries and Aquatic Sciences 44: 591–597.
Lodge, D. M., A. Deines, F. Gherardi, D. C. J. Yeo, T. Arcella, A. K. Baldridge, M. A. Barnes, W. L. Chadderton, J. L. Feder, C. A. Gantz, G. W. Howard, C. L. Jerde, B. W. Peters, J. A. Peters, L. W. Sargent, C. R. Turner, M. E. Wittmann & Y. W. Zeng, 2012. Global Introductions of Crayfishes: evaluating the impact of species invasions on ecosystem services. Annual Review of Ecology, Evolution and Systematics 43: 449–472.
Ludlam, J. P. & D. D. Magoulick, 2010. Environmental conditions and biotic interactions influence ecosystem structure and function in a drying stream. Hydrobiologia 644: 127–137.
Magoulick, D. D., 2014. Impacts of drought and crayfish invasion on stream ecosystem structure and function. River Research and Applications 30: 1309–1317.
Magoulick, D. D. & R. J. DiStefano, 2007. Invasive crayfish Orconectes neglectus threatens native crayfishes in the Spring River drainage of Arkansas and Missouri. Southeastern Naturalist 6: 141–150.
Momot, W. T., 1995. Redefining the role of crayfish in aquatic ecosystems. Reviews in Fisheries Science 3: 33–63.
Momot, W. T., 1996. History of the range estension of Orconectes rusticus into northwestern Ontario and Lake Superior. Freshwater Crayfish 11: 61–72.
Nilsson, E., C. T. Solomon, K. A. Wilson, T. V. Willis, B. Larget & M. J. Vander Zanden, 2012. Effects of an invasive crayfish on trophic relationships in north-temperate lake food webs. Freshwater Biology 57: 10–23.
Nyström, P., C. Bronmak & W. Graneli, 1999. Influence of an exotic and a native crayfish species on a littoral benthic community. Oikos 85: 545–553.
Olsen, T. M., D. M. Lodge, G. M. Capelli & R. J. Houlihan, 1991. Mechanisms of impact of an introduced crayfish (Orconectes rusticus) on littoral congeners, snails, and macrophytes. Canadian Journal of Fisheries and Aquatic Sciences 48: 1853–1861.
Olsson, K., P. Stenroth, P. Nystrom & W. Graneli, 2009. Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshwater Biology 54: 1731–1740.
Parkyn, S. M., K. J. Collier & B. J. Hicks, 2001. New Zealand stream crayfish: functional omnivores but trophic predators? Freshwater Biology 46: 641–652.
Perry, W. L., J. L. Feder & D. M. Lodge, 2001. Implications of hybridization between introduced and resident Orconectes crayfishes. Conservation Biology 15: 1656–1666.
Peterson, B. J. & B. Fry, 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18: 293–320.
Pflieger, W. L., 1996. The crayfishes of Missouri. Missouri Department of Conservation, Jefferson City.
Phillips, D. L. & J. W. Gregg, 2003. Source partitioning using stable isotopes: coping with too many sources. Oecologia 136: 261–269.
Rabalais, M. R. & D. D. Magoulick, 2006a. Influence of an invasive crayfish species on diurnal habitat use and selection by a native crayfish species in an Ozark stream. American Midland Naturalist 155: 295–306.
Rabalais, M. R. & D. D. Magoulick, 2006b. Is competition with the invasive crayfish Orconectes neglectus chaenodactylus responsible for the displacement of the native crayfish Orconectes eupunctus? Biological Invasions 8: 1039–1048.
Rabeni, C. F., 1985. Resource partitioning by stream-dwelling crayfish: the influence of body size. American Midland Naturalist 113: 20–29.
Roth, B. M., C. L. Hein & M. J. Vander Zanden, 2006. Using bioenergetics and stable isotopes to assess the trophic role of rusty crayfish (Orconectes rusticus) in lake littoral zones. Canadian Journal of Fisheries and Aquatic Sciences 63: 335–344.
Rudnick, D. & V. Resh, 2005. Stable isotopes, mesocosms and gut content analysis demonstrate trophic differences in two invasive decapod crustacea. Freshwater Biology 50: 1323–1336.
Stenroth, P., N. Holmqvist, P. Nystrom, O. Berglund, P. Larsson & W. Graneli, 2006. Stable isotopes as an indicator of diet in omnivorous crayfish (Pacifastacus leniusculus): the influence of tissue, sample treatment, and season. Canadian Journal of Fisheries and Aquatic Sciences 63: 821–831.
Taylor, C. A., G. A. Schuster, J. E. Cooper, R. J. DiStefano, A. G. Eversole, P. Hamr, H. H. Hobbs III, H. W. Robison, C. E. Skelton & R. E. Thoma, 2007. Feature: endangered species - a reassessment of the conservation status of crayfishes of the united states and Canada after 10 + years of increased awareness. Fisheries 32: 372–389.
Usio, N., M. Konishi & S. Nakano, 2001. Is invertebrate shredding critical for collector invertebrates? A test of the shredder-collector facilitation hypothesis. Ecological Research 16: 319–326.
Usio, N., K. Suzuki, M. Konishi & S. Nakano, 2006. Alien versus endemic crayfish: roles of species identity in ecosystem functioning. Archiv Fur Hydrobiologie 166: 1–21.
Vanderklift, M. A. & S. Ponsard, 2003. Sources of variation in consumer-diet delta15 N enrichment: a meta-analysis. Oecologia 136: 169–182.
Walker, B. H., 1992. Biodiversity and ecological redundancy. Conservation Biology 6: 18–23.
Westman, K., R. Savolainen & M. Julkunen, 2002. Replacement of the native crayfish Astacus astacus by the introduced species Pacifastacus leniusculus in a small, enclosed Finnish lake: a 30-year study. Ecography 25: 53–73.
Whitledge, G. W. & C. F. Rabeni, 1997. Energy sources and ecological role of crayfishes in an Ozark stream: insights from stable isotopes and gut analysis. Canadian Journal of Fisheries and Aquatic Sciences 54: 2555–2563.
Whitmore, N. & A. D. Huryn, 1999. Life history and production of Paranephrops zealandicus in a forest stream, with comments about the sustainable harvest of a freshwater crayfish. Freshwater Biology 42: 467–478.
Wilson, K. A., J. J. Magnuson, D. M. Lodge, A. M. Hill, T. K. Kratz, W. L. Perry & T. V. Willis, 2004. A long-term rusty crayfish (Orconectes rusticus) invasion: dispersal patterns and community change in a north temperate lake. Canadian Journal of Fisheries and Aquatic Sciences 61: 2255–2266.
Acknowledgments
We thank Mike Rabalais for assistance in the field. Michelle Evans-White, Camille Flinders, Eric Larson, Matt Dekar, John Ludlam and Jon Flinders provided helpful comments on the manuscript. This project was supported by the U.S. Fish and Wildlife Service, Missouri Department of Conservation and the U.S. Geological Survey, Arkansas Cooperative Fish and Wildlife Research Unit. This study was performed under the auspices of University of Arkansas protocol #01027. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Nicholas R. Bond
Rights and permissions
About this article
Cite this article
Magoulick, D.D., Piercey, G.L. Trophic overlap between native and invasive stream crayfish. Hydrobiologia 766, 237–246 (2016). https://doi.org/10.1007/s10750-015-2457-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2457-0