Abstract
The spread of non-native species results in novel and often unexpected assemblages. Using stable isotopes, we disentangled the trophic relationships between three invasive crayfish species at two sites of a small thermal tributary of the Barát stream, Hungary. We studied Procambarus virginalis and Faxonius limosus living in sympatry in the upper section of this thermal tributary, and then an assemblage in a lower section also containing P. clarkii. The two species in the upper section largely shared trophic niches, although P. virginalis was more carnivorous than F. limosus, which fed more on detritus and aquatic plants. In the lower section, P. clarkii had a distinctive trophic niche, being more carnivorous than the other species and also preying on other crayfish and fish. The trophic niches of the other two species shifted slightly, being narrower and more overlapping in the presence of P. clarkii. It seems that the presence of P. clarkii affects the feeding habits and trophic niches of the other two crayfish. Our results also indicate that the species have somewhat distinctive feeding niches, which suggests that the ecosystem effects of these species are likely to be at least partially additive in the shared localities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing human-mediated species translocations have led to the establishment of various non-indigenous taxa worldwide (Hulme, 2009). Although the majority of translocated species pose no apparent environmental or socioeconomic threat, some can have a notable negative effect on native biota (Simberloff, 2011). Freshwater ecosystems are particularly endangered in this regard, since the early stages of invasions typically go unnoticed, and the ecosystems themselves are put under pressure by multiple stressors (Ricciardi & Rasmussen, 1999; Strayer & Dudgeon, 2010; Gebauer et al., 2018). This often leads to irreversible changes in native biodiversity and ecosystem functioning (Strayer, 2010).
Crayfish (Decapoda: Astacidea) are a highly diverse taxonomic group of freshwater taxa containing almost 700 described species with biodiversity hotspots in North America, Australia and New Guinea (Bláha et al., 2016; Crandall & De Grave, 2017). In Europe, biodiversity of indigenous crayfish species (ICS) is relatively low (Holdich et al., 2009) and newly discovered species only appear very rarely (Pârvulescu, 2019). A certain degree of species diversity is expected to occur in Eastern Europe (Crandall & De Grave, 2017). However, this remains largely unsupported by molecular data (Maguire et al., 2014; Bláha et al., 2017; Bláha et al., 2020). In contrast, the number of non-indigenous crayfish species (NICS) increases rapidly (Kouba et al., 2014; Weiperth et al., 2017, 2020). Many translocated crayfish species have proven to be invasive in their new environments and frequently affect local crayfish stocks and/or other taxa (Gherardi et al., 2011; Twardochleb et al., 2013). As large-bodied omnivorous macroinvertebrates, crayfish often represent an important proportion of the biomass of the benthos and serve as a prey for a range of predators (Hein et al., 2007; Tablado et al., 2010). As a result, these species mediate nutrient and energy flows within (Ruokonen et al., 2012; Lipták et al., 2019) and even between ecosystems as they also feed in riparian and terrestrial habitats (Grey & Jackson, 2012).
Innumerable populations of ICS have been lost and many more have declined substantially, largely due to the direct or indirect effects of NICS (Holdich et al., 2009; Kozak et al., 2011). Several life history traits mainly associated with growth and reproduction (Kozák et al., 2007; Buřič et al., 2011), spread of diseases e.g. crayfish plague (Schrimpf et al., 2012; Svoboda et al., 2017), dominance in direct interactions, and competition for resources including food and shelter (Vorburger & Ribi, 1999; Lele & Pârvulescu, 2017), as well as environmental tolerance, have been identified as causes of these species replacements (Lodge et al., 2012). The impact of NICS on ICS as well as on entire ecosystems in Europe has been extensively studied and verified in recent years (Correia & Anastacio, 2008; Cruz et al., 2008; Jackson et al., 2014; Pacioglu et al., 2020). Albeit to date still rather rare, localities where more than one NICS occur in sympatry are continuously being detected (e.g. Chucholl et al., 2012; Herrmann et al., 2018; Garzoli et al., 2020; Weiperth et al., 2020). This has stimulated comparative research on the factors that could determine the success of particular NICS when living in sympatry (Jackson et al., 2014; James et al., 2016; Linzmaier et al., 2018; Linzmaier & Jeschke, 2020; Linzmaier et al., 2020). However, studies that provide simultaneous comparisons of multiple NICS in natural settings are still few and far between (Jackson, 2015). Nevertheless, given that the number of NICS keeps rising (Weiperth et al., 2017, 2020) and their ranges expand (Kouba et al., 2014; Lipták et al., 2017), such places gradually appear and are worth closer investigation.
Understanding the trophic relationships between co-occurring invasive species is pivotal as these interactions affect species competition, possible replacements and finally the ecological effects of the species overall. Examining the energy flow through a complex ecological food web (Post, 2002) using stable isotope analysis has often been used for characterizing trophic niches in crayfish; likewise, a range of studies has focused on comparing two sympatric crayfish species, either an ICS and a NICS or, less frequently, two NICS (Olsson et al., 2009; Ercoli et al., 2014; Ercoli et al., 2015; Magoulick & Piercey, 2016). However, these multiple-species comparisons are based on mesocosm studies or allopatric populations, and have therefore profound limitations (Hudina et al., 2011; Jackson et al., 2014). In this study, we aimed to investigate the trophic web of a small thermal tributary of the Barát stream near Budapest, Hungary. The stream has been invaded by three NICS of EU concern (EU, 2014, 2016), namely, the spiny-cheek crayfish Faxonius limosus (Rafinesque, 1817), the red swamp crayfish Procambarus clarkii (Girard, 1852) and the marbled crayfish Procambarus virginalis Lyko, 2017. This provides us with a unique opportunity to examine the trophic niche of each NICS and the overlap between them, which is important for our understanding of ongoing processes and predicting the future development of crayfish communities dominated by NICS.
Methods
Locality description
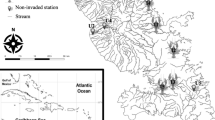
The Barát is a small stream of the River Danube located between Budapest and Budakalász, Hungary. The sampled locality (47.6091 N, 19.0618 E) consists of two sites of a small thermal tributary of the Barát, 400 m long and approximately one metre wide (Fig. 1). This tributary can be divided into three sections: the upper and lower sections are very similar and have a sandy-muddy bottom and few aquatic plants, while the middle section has a soft muddy bottom completely overgrown by the aquatic macrophyte branched bur-reed Sparganium erectum L., which presumably reduces migrations between sections. In all sections, the tributary banks are paved with concrete panels. Sympatric populations of marbled crayfish and spiny-cheek crayfish occur in the upper section, while the red swamp crayfish is additionally present in the lower section. In this study we thus compared the upper and lower sections of this watercourse, both being represented by a transect of 100 m (Fig. 1). These sections are involved in a long-term monitoring programme targeting primarily on crayfish, which are assessed by means of electrofishing, trapping and hand search four times a year (Weiperth et al., unpublished data). The most recent observations in Autumn 2018 revealed a dominance of spiny-cheek crayfish (38 and 19 individuals) and marbled crayfish (49 and 11 individuals) in the upper and lower section, respectively. Red swamp crayfish were rare (1 individual) and present only in the lower section, in concordance with our sampling campaign. Three-spined sticklebacks Gasterosteus aculeatus (L., 1758) and mosquitofish Gambusia holbrooki Girard, 1859 are regularly seen at both sections, but we failed to capture the latter in the upper section. Zoobenthos taxa were similar at both sites, only chironomid larvae, which were rare in the upper section, were absent from the lower one. The amount of detritus, algae and macrophytes was well comparable.
Field sampling
All potential food sources at each trophic level were sampled from both tributary sections in September 2018. Fish and crayfish were collected by electrofishing (DEKA 3000 Lord) or caught manually with nets. Zoobenthos was collected using a hand net (mesh size 500 µm). Macrophytes, periphyton and detritus were collected manually from the shoreline and streambed. All samples were placed on dry ice immediately after collection and then transferred to a laboratory freezer (− 30 °C) until further processing for stable carbon (δ13C) and nitrogen (δ15N) isotope analysis (SIA). A piece of white dorsal muscle tissue of fish and a piece of abdominal muscle tissue of crayfish were used for SIA, as recommended by Stenroth et al. (2006). All fish and crayfish were measured and weighed to the nearest 0.1 mm and 0.1 g (Tables S1 and S2), respectively. Samples of zoobenthos, terrestrial detritus and macrophytes were identified to species or genus level (Tables S3, S4 and S5). The number of analysed samples is summarized in Table 1.
Stable isotope analyses
All analysed samples were dried at 50 °C for 48 h to constant weight and ground to a fine homogenous powder. Approximately 0.6 mg of animal samples and 1.5 mg of plant and detritus samples were weighed in tin cups. Stable isotope analyses were performed at the University of Jyväskylä using a Carlo Erba Flash EA 1112 elemental analyser connected to Thermo Finnigan DELTAplus and Advantage continuous-flow isotope ratio mass spectrometer (Thermo Electron Corporation, Waltham, MA, USA).
Vienna Pee Dee belemnite and atmospheric N2 were used as reference standards for carbon and nitrogen. To control for instrument stability, after every six samples northern pike Esox lucius L. 1758 muscle tissue and European white birch Betula pendula Roth leaves of known isotopic compositions were run as internal working standards for animal and plant samples. Results were expressed using the conventional δ notation in parts per thousand as per international standards. The analytical precision was < 0.1‰ for δ13C and < 0.3‰ for δ15N.
The trophic position of each species/functional group was calculated using the formula devised by Anderson & Cabana (2007):
where Tp is the trophic position of an organism, δ15Nsample represents the nitrogen isotope value of a given organism, δ15Nbaseline is the isotopic ratio from several individual scrapers, 3.23 is the nitrogen isotope fractionation between trophic levels (Vander Zanden & Rasmussen, 2001) and Ep = 2 is the trophic position of the organism selected as a baseline. We used a scraper of periphyton (the dominant primary producers), the New Zealand mud snail Potamopyrgus antipodarum Smith, 1889 occurring at the locality, as a baseline organism, following Anderson & Cabana (2007). This species is a long-lived first-order consumer, which integrates stable isotope signals from primary producers over a season in a given ecosystem.
Trophic web of the Barát
To assess the contribution of the different food sources to the isotopic signature of each target crayfish, separate Bayesian stable isotope mixing models (Moore & Semmens, 2008) with a specified number of putative sources were run for the upper and lower sections of the tributary using the R package MixSiar (R Core Team, 2016; Stock & Semmens, 2016). Prior to the final Bayesian modelling, we followed the approach of Smith et al. (2013) and ran models based on large numbers of possible mixing polygons with a Monte Carlo simulation. We considered our model valid if all specimens of a given consumer fell inside the 95% mixing region (the outermost contour), which means that an alternative model is not needed (Fig. S1). For marbled and spiny-cheek crayfish from the upper section, a six-source mixing model including three-spined sticklebacks, crayfish (to calculate the degree of cannibalism), zoobenthos, macrophytes, algae and detritus was employed. However, sticklebacks and crayfish were omitted from the final model due to their marginal contribution as putative food sources. For red swamp crayfish, a seven-source model was used (including three-spined stickleback, mosquitofish, crayfish, zoobenthos, macrophytes, algae and detritus).
For marbled and spiny-cheek crayfish from the lower locality, we initially ran the same seven-source mixing model. However, as for the upper section, three-spined stickleback, mosquitofish and crayfish were omitted from the final model due to their very low estimated contribution to the diet of the two crayfish species. Thus, a four-source mixing model (zoobenthos, macrophytes, algae and detritus) was employed for both marbled and spiny-cheek crayfish. As recommended by Vander Zanden & Rasmussen (2001), our models assumed the fractionation factors 3.23 ± 0.41‰ for δ15N and 0.47 ± 1.23‰ for δ13C for animals, and 2.4 ± 0.42‰ for δ15N and 0.40 ± 0.28‰ for δ13C for detritus and macrophytes (McCutchan et al., 2003).
Trophic niche width
We described the trophic niche of crayfish in both sections using the standard ellipse area SEA and the R package SIBER. Specifically, we estimated SEAb, which is the corresponding 95% convex hull area that encompasses a sampled population in a δ15N/δ15C bi‐plot space and serves as a measure of the total isotopic niche-space occupied. We then compared the species’ trophic niche widths between the two sections. The overlap between the ellipses of the given species was calculated as a proportion of the non-overlapping area of ellipses (Jackson et al., 2011).
Results
The food webs of both sections of the Barát tributary roughly consist of three trophic levels (Fig. 2). Primary producers and detritus formed the bottom level of the food web and in both sections had very similar carbon and nitrogen isotope values. In both sections, three-spined stickleback occupied the top-most position within the food web. In the upper section, spiny-cheek crayfish and marbled crayfish were in a central position along with zoobenthos (Fig. 2), while in the lower section three-spined sticklebacks were followed by red swamp crayfish and mosquitofish (Fig.1). Marbled crayfish, spiny-cheek crayfish and zoobenthos occupied the trophic level below the red swamp crayfish.
The mixing model suggested that in the upper section marbled crayfish rely on detritus (estimated average contribution of 31%) and algae (30%) as their most important food sources, with zoobenthos (24%) and macrophytes (15%) contributing slightly less (Table 2). Spiny-cheek crayfish rely on detritus (37%), algae (27%), and macrophytes (22%) as their most important food sources, with zoobenthos (13%) as the least preferred option (Table 2).
In the lower section, red swamp crayfish seemed to feed mostly on algae (33%), mosquitofish (20%) and three-spined stickleback (17%) (Table 2), and only to a lesser extent on other crayfish (9%), zoobenthos (8%) and detritus (7%). Marbled crayfish apparently mainly fed on detritus (36%) and algae (28%), but also on zoobenthos (18%), and macrophytes (17%) (Table 2). Spiny-cheek crayfish relied more on detritus (47%) and algae (25%) than macrophytes (15%) and zoobenthos (13%) (Table 2).
Spiny-cheek and marbled crayfish had wider trophic niches in the upper section (Figs. 2 and 3). In the lower section in sympatry with the red swamp crayfish both species occupied smaller niches. In addition, marbled and spiny-cheek crayfish shared a significant part of their trophic niches in both the upper (43% overlap) and lower sections (50% overlap) (Fig. 3), whereas the trophic niche of the red swamp crayfish was clearly distinct in the lower section (Fig. 4).
Standard ellipse area (SEAb) representing the trophic niche width of crayfish captured from the upper and lower sections of the Barát thermal tributary. Dark grey, light grey and outer light grey boxes represent 50%, 75% and 95% confidence intervals, respectively. The red cross marks the SEAc for each species
Discussion
The spread of non-native species accelerates with no sign of any slowing down (Seebens et al., 2017) and in doing so creates novel interactions between native and non-native species. The number of studies of sympatric invasive species is steadily increasing but only a few have ever been conducted in natural freshwater settings (Hudina et al., 2011; Jackson et al., 2014; Larson et al., 2017). Our study of three sympatric non-native crayfish species in the Barát contributes to filling this knowledge gap and provides insights into trophic interactions between invasive species in the wild.
Our results show that the diets of the spiny-cheek and marbled crayfish overlapped when in sympatry and also when a third species, the red swamp crayfish, was present. The two former species fed mostly on detritus, algae and macrophytes in both studied sections, as suggested previously (Vojkovská et al., 2014; Lipták et al., 2019). These results are similar to those of Linzmaier et al. (2020) who studied sympatric populations of marbled crayfish and spiny-cheek crayfish in German lakes and suggest that spiny-cheek crayfish rely more on plant-based food, while marbled crayfish rely more on invertebrates. As was mentioned, spiny-cheek and marbled crayfish can occur in sympatry (Chucholl & Pfeiffer, 2010; Chucholl et al., 2012; Weiperth et al., 2020), which could indicate a degree of niche partitioning (Jackson et al., 2014). However, the results of Linzmaier et al. (2020) suggest overlap of the trophic niche in two lakes, but extensive niche partitioning in one lake. These differences in trophic niche portioning among the lakes can be caused by lake size, trophic state or existing habitats (Post et al., 2000; Ruokonen et al., 2014). We found that the trophic niche of spiny-cheek and marbled crayfish clearly overlapped in both sections. The much larger trophic niche (SEAb) in the upper section of the investigated tributary suggests that the spiny-cheek crayfish has a wider range of food resources than the marbled crayfish. However, the trophic niche widths of both the marbled and spiny-cheek crayfish were smaller and decreased in their utilized carbon range when in sympatry with the red swamp crayfish. This indicates increased competition or a niche shift when the third species is present in the same area (Jackson et al., 2014). The trophic niche width of the spiny-cheek crayfish decreased substantially in the presence of the third crayfish species and its position in the stable isotope space changed. Jackson et al. (2014) found no evidence for a reduction in trophic niche width in the presence of other crayfish species. Red swamp crayfish were clearly more carnivorous than the other two species, and fish, crayfish and other zoobenthos comprised over 50% of its diet. These results are similar to those of Mao et al. (2016), who found that the diet of red swamp crayfish was 43% of animal origin. Nevertheless, the realised trophic niche of the red swamp crayfish did not clearly overlap with the niches of the other crayfish in the lower section, even when compared to the trophic niches of the two species from the upper section. Their fundamental niches in the absence of other species might be even more similar. Hence, the constrained trophic niches of the marbled and spiny-cheek crayfish might at least partly be due to direct food competition with the red swamp crayfish. However, we presume that this is to some extent due to interactions not related to direct competition for food sources but possibly associated with behavioural avoidance mechanisms. The spiny-cheek crayfish is not a very effective competitor against other invasive crayfish as it is subordinate in any encounters (Chucholl et al., 2008; Hudina et al., 2011; Linzmaier et al., 2018).
Our results suggest that the red swamp crayfish predates to a certain extent on other crayfish. Hence, this predation risk could lead to evasion and changes in habitat use, which provoke alterations in food sources. However, the marbled crayfish is probably able to establish populations even in the presence of other NICS (Chucholl & Pfeiffer, 2010; Weiperth et al., 2020) and can use food sources from different trophic levels (Lipták et al., 2019; Linzmaier et al., 2020), thereby indicating its adaptability. We did not observe any dramatic changes in food source use or in trophic niche width in the presence of red swamp crayfish, possibly because the food supply was not limited. This finding highlights the need for species-specific evaluations of roles and impacts in individual ecosystems.
Conclusion
Our results indicate that the sympatry of two or three NICS could cause cumulative/additive effects on other biota, and that their combined net effect is likely to be higher than the effect of a single NICS in this tributary of the Danube. We detected two different groups feeding at different trophic levels (marbled and spiny-crayfish, and carnivorous red swamp crayfish), which creates an additive net effect on other ecosystem members. Our results support the view that no single location or population, or any laboratory experiment, can provide a comprehensive and representative picture of species interactions. Therefore, more research on interactions and the impact of sympatric invasive species is needed at ecologically relevant scales.
References
Anderson, C. & G. Cabana, 2007. Estimating the trophic position of aquatic consumers in river food webs using stable nitrogen isotopes. Journal of the North American Benthological Society 26(2): 273–285.
Bláha, M., J. Patoka, P. Kozák & A. Kouba, 2016. Unrecognized diversity in New Guinean crayfish species (Decapoda, Parastacidae): the evidence from molecular data. Integrative Zoology 11: 447–458.
Bláha, M., M. Uzhytchak, V. Bondarenko & T. Policar, 2017. The least known European native crayfish Astacus pachypus (Rathke, 1837) revealed its phylogenetic position. Zoologischer Anzeiger 267: 151–154.
Bláha, M., J. Patoka, B. Japoshvili, A. Kouba, M. Buřič & L. Mumladze, 2020. Genetic diversity, phylogenetic position and morphometric analysis of Astacus colchicus (Decapoda, Astacidae): a new insight into Eastern European crayfish fauna. Integrative Zoology. https://doi.org/10.1111/1749-4877.12493.
Buřič, M., M. Hulák, A. Kouba, A. Petrusek & P. Kozák, 2011. A successful crayfish invader is capable of facultative parthenogenesis: a novel reproductive mode in decapod crustaceans. PLoS ONE 6(5): e20281.
Chucholl, C. & M. Pfeiffer, 2010. First evidence for an established Marmorkrebs (Decapoda, Astacida, Cambaridae) population in Southwestern Germany, in syntopic occurrence with Orconectes limosus (Rafinesque, 1817). Aquatic Invasions 5(4): 405–412.
Chucholl, C., H. B. Stich & G. Maier, 2008. Aggressive interactions and competition for shelter between a recently introduced and an established invasive crayfish: Orconectes immunis vs. O. limosus. Fundamental and Applied Limnology 172(1): 27–36.
Chucholl, C., K. Morawetz & H. Gross, 2012. The clones are coming - strong increase in Marmorkrebs Procambarus fallax (Hagen, 1870) f. virginalis records from Europe. Aquatic Invasions 7(4): 511–519.
Correia, A. M. & P. M. Anastacio, 2008. Shifts in aquatic macroinvertebrate biodiversity associated with the presence and size of an alien crayfish. Ecological Research 23(4): 729–734.
Crandall, K. A. & S. De Grave, 2017. An updated classification of the freshwater crayfishes (Decapoda: Astacidea) of the world, with a complete species list. Journal of Crustacean Biology 37(5): 615–653.
Cruz, M. J., P. Segurado, M. Sousa & R. Rebelo, 2008. Collapse of the amphibian community of the Paul do Boquilobo Natural Reserve (central Portugal) after the arrival of the exotic American crayfish Procambarus clarkii. Herpetological Journal 18(4): 197–204.
Ercoli, F., T. J. Ruokonen, H. Hämäläinen & R. I. Jones, 2014. Does the introduced signal crayfish occupy an equivalent trophic niche to the lost native noble crayfish in boreal lakes? Biological Invasions 16(10): 2025–2036.
Ercoli, F., T. J. Ruokonen, S. Koistinen, R. I. Jones & H. Hämäläinen, 2015. The introduced signal crayfish and native noble crayfish have different effects on sublittoral macroinvertebrate assemblages in boreal lakes. Freshwater Biology 60(8): 1688–1698.
EU, 2014. Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. Official Journal of the European Union 57(317): 35.
EU, 2016. Commission Implementing Regulation (EU) 2016/1141 of 13 July 2016 adopting a list of invasive alien species of Union concern pursuant to Regulation (EU) No 1143/2014 of the European Parliament and of the Council. Official Journal of the European Union 189(4): 4–8.
Garzoli, L., S. Mammola, M. Ciampittiello & A. Boggero, 2020. Alien crayfish species in the deep subalpine Lake Maggiore (NW-Italy), with a focus on the biometry and habitat preferences of the spiny-cheek crayfish. Water 12(5): 1391.
Gebauer, R., J. Divíšek, M. Buřič, M. Večeřa, A. Kouba & B. Drozd, 2018. Distribution of alien animal species richness in the Czech Republic. Ecology and Evolution 8: 4455–4464.
Gherardi, F., L. Aquiloni, J. Dieguez-Uribeondo & E. Tricarico, 2011. Managing invasive crayfish: is there a hope? Aquatic Sciences 73(2): 185–200.
Grey, J. & M. C. Jackson, 2012. ‘Leaves and eats shoots’: Direct terrestrial feeding can supplement invasive red swamp crayfish in times of need. PLoS ONE 7(8): e42575.
Hein, C. L., M. J. Vander Zanden & J. J. Magnuson, 2007. Intensive trapping and increased fish predation cause massive population decline of an invasive crayfish. Freshwater Biology 52(6): 1134–1146.
Herrmann, A., A. Schnabler & A. Martens, 2018. Phenology of overland dispersal in the invasive crayfish Faxonius immunis (Hagen) at the Upper Rhine River area. Knowledge and Management of Aquatic Ecosystems 419: 30.
Holdich, D. M., J. D. Reynolds, C. Souty-Grosset & P. J. Sibley, 2009. A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowledge and Management of Aquatic Ecosystems 394–395: 11.
Hudina, S., N. Galić, I. Roessink & K. Hock, 2011. Competitive interactions between co-occurring invaders: identifying asymmetries between two invasive crayfish species. Biological Invasions 13(8): 1791–1803.
Hulme, P. E., 2009. Trade, transport and trouble: managing invasive species pathways in an era of globalization. Journal of Applied Ecology 46(1): 10–18.
Jackson, M. C., 2015. Interactions among multiple invasive animals. Ecology 96(8): 2035–2041.
Jackson, A. L., R. Inger, A. C. Parnell & S. Bearhop, 2011. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80(3): 595–602.
Jackson, M. C., T. Jones, M. Milligan, D. Sheath, J. Taylor, A. Ellis, J. England & J. Grey, 2014. Niche differentiation among invasive crayfish and their impacts on ecosystem structure and functioning. Freshwater Biology 59(6): 1123–1135.
James, J., J. Thomas, A. Ellis, K. Young, J. England & J. Cable, 2016. Over-invasion in a freshwater ecosystem: newly introduced virile crayfish (Orconectes virilis) outcompete established invasive signal crayfish (Pacifastacus leniusculus). Marine and Freshwater Behaviour and Physiology 49(1): 9–18.
Kouba, A., A. Petrusek & P. Kozák, 2014. Continental-wide distribution of crayfish species in Europe: update and maps. Knowledge and Management of Aquatic Ecosystems 413: 5.
Kozák, P., M. Buřič, T. Policar, J. Hamáčková & A. Lepičová, 2007. The effect of inter-and intra-specific competition on survival and growth rate of native juvenile noble crayfish Astacus astacus and alien spiny-cheek crayfish Orconectes limosus. Hydrobiologia 590(1): 85–94.
Kozák, P., L. Füereder, A. Kouba, J. Reynolds & C. Souty-Grosset, 2011. Current conservation strategies for European crayfish. Knowledge and Management of Aquatic Ecosystems 401: 1.
Larson, E. R., L. A. Twardochleb & J. D. Olden, 2017. Comparison of trophic function between the globally invasive crayfishes Pacifastacus leniusculus and Procambarus clarkii. Limnology 18(3): 275–286.
Lele, S.-F. & L. Pârvulescu, 2017. Experimental evidence of the successful invader Orconectes limosus outcompeting the native Astacus leptodactylus in acquiring shelter and food. Biologia 72(8): 877–885.
Linzmaier, S. M. & J. M. Jeschke, 2020. Towards a mechanistic understanding of individual-level functional responses: Invasive crayfish as model organisms. Freshwater Biology 65(4): 657–673.
Linzmaier, S. M., L. S. Goebel, F. Ruland & J. M. Jeschke, 2018. Behavioral differences in an over-invasion scenario: marbled vs. spiny-cheek crayfish. Ecosphere 9(9): e02385.
Linzmaier, S. M., C. Musseau, S. Matern & J. M. Jeschke, 2020. Trophic ecology of invasive marbled and spiny-cheek crayfish populations. Biological Invasions. https://doi.org/10.1007/s10530-020-02328-z.
Lipták, B., M. Mojžišová, D. Gruľa, J. Christophoryová, D. Jablonski, M. Bláha, A. Petrusek & A. Kouba, 2017. Slovak section of the Danube has its well-established breeding ground of marbled crayfish Procambarus fallax f. virginalis. Knowledge and Management of Aquatic Ecosystems 418: 40.
Lipták, B., L. Veselý, F. Ercoli, M. Bláha, M. Buřič, T. Ruokonen & A. Kouba, 2019. Trophic role of marbled crayfish in a lentic freshwater ecosystem. Aquatic Invasions 14(2): 299–309.
Lodge, D. M., A. Deines, F. Gherardi, D. C. J. Yeo, T. Arcella, A. K. Baldridge, M. A. Barnes, W. L. Chadderton, J. L. Feder, C. A. Gantz, G. W. Howard, C. L. Jerde, B. W. Peters, J. A. Peters, L. W. Sargent, C. R. Turner, M. E. Wittmann & Y. Zeng, 2012. Global introductions of crayfishes: evaluating the impact of species invasions on ecosystem services. Annual Review of Ecology, Evolution and Systematics 43: 449–472.
Magoulick, D. D. & G. L. Piercey, 2016. Trophic overlap between native and invasive stream crayfish. Hydrobiologia 766(1): 237–246.
Maguire, I., M. Podnar, M. Jelic, A. Stambuk, A. Schrimpf, H. Schulz & G. Klobucar, 2014. Two distinct evolutionary lineages of the Astacus leptodactylus species-complex (Decapoda: Astacidae) inferred by phylogenetic analyses. Invertebrate Systematics 28(2): 117–123.
Mao, Z., X. Gu & Q. Zeng, 2016. Food sources and trophic relationships of three decapod crustaceans: insights from gut contents and stable isotope analyses. Aquaculture Research 47(9): 2888–2898.
McCutchan, J. H., W. M. Lewis, C. Kendall & C. C. McGrath, 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102(2): 378–390.
Moore, J. W. & B. X. Semmens, 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecology Letters 11(5): 470–480.
Olsson, K., P. Stenroth, P. Nyström & W. Graneli, 2009. Invasions and niche width: does niche width of an introduced crayfish differ from a native crayfish? Freshwater Biology 54(8): 1731–1740.
Pacioglu, O., K. Theissinger, A. Alexa, C. Samoilă, O.-I. Sîrbu, A. Schrimpf, J. P. Zubrod, R. Schulz, M. Pîrvu & S.-F. Lele, 2020. Multifaceted implications of the competition between native and invasive crayfish: a glimmer of hope for the native’s long-term survival. Biological Invasions 22(2): 827–842.
Pârvulescu, L., 2019. Introducing a new Austropotamobius crayfish species (Crustacea, Decapoda, Astacidae): a miocene endemism of the Apuseni Mountains, Romania. Zoologischer Anzeiger 279: 94–102.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83(3): 703–718.
Post, D. M., M. L. Pace & N. G. Hairston, 2000. Ecosystem size determines food-chain length in lakes. Nature 405(6790): 1047–1049.
R Core Team, 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria. http://www.R-project.org.
Ricciardi, A. & J. B. Rasmussen, 1999. Extinction rates of North American freshwater fauna. Conservation Biology 13(5): 1220–1222.
Ruokonen, T., M. Kiljunen, J. Karjalainen & H. Hämäläinen, 2012. Invasive crayfish increase habitat connectivity: a case study in a large boreal lake. Knowledge and Management of Aquatic Ecosystems 407: 8.
Ruokonen, T. J., J. Karjalainen & H. Hämäläinen, 2014. Effects of an invasive crayfish on the littoral macroinvertebrates of large boreal lakes are habitat specific. Freshwater Biology 59(1): 12–25.
Seebens, H., T. M. Blackburn, E. E. Dyer, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Pagad, P. Pyšek, M. Winter & M. Arianoutsou, 2017. No saturation in the accumulation of alien species worldwide. Nature Communications 8: 14435.
Schrimpf, A., L. Parvulescu, D. Copilas-Ciocianu, A. Petrusek & R. Schulz, 2012. Crayfish plague pathogen detected in the Danube Delta - a potential threat to freshwater biodiversity in southeastern Europe. Aquatic Invasions 7(4): 503–510.
Simberloff, D., 2011. How common are invasion-induced ecosystem impacts? Biological Invasions 13(5): 1255–1268.
Smith, J. A., D. Mazumder, I. M. Suthers & M. D. Taylor, 2013. To fit or not to fit: evaluating stable isotope mixing models using simulated mixing polygons. Methods in Ecology and Evolution 4(7): 612–618.
Stenroth, P., N. Holmqvist, P. Nyström, O. Berglund, P. Larsson & W. Granéli, 2006. Stable isotopes as an indicator of diet in omnivorous crayfish (Pacifastacus leniusculus): the influence of tissue, sample treatment, and season. Canadian Journal of Fisheries and Aquatic Sciences 63(4): 821–831.
Stock, B. C. & B. X. Semmens, 2016. Unifying error structures in commonly used biotracer mixing models. Ecology 97(10): 2562–2569.
Strayer, D. L., 2010. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshwater Biology 55: 152–174.
Strayer, D. L. & D. Dudgeon, 2010. Freshwater biodiversity conservation: recent progress and future challenges. Journal of the North American Benthological Society 29(1): 344–358.
Svoboda, J., A. Mrugała, E. Kozubíková-Balcarová & A. Petrusek, 2017. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: a review. Journal of Fish Diseases 40(1): 127–140.
Tablado, Z., J. L. Tella, J. A. Sánchez-Zapata & F. Hiraldo, 2010. The paradox of the long-term positive effects of a North American crayfish on a European community of predators. Conservation Biology 24(5): 1230–1238.
Twardochleb, L. A., J. D. Olden & E. R. Larson, 2013. A global meta-analysis of the ecological impacts of nonnative crayfish. Freshwater Science 32(4): 1367–1382.
Vander Zanden, M. & J. B. Rasmussen, 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography 46(8): 2061–2066.
Vojkovská, R., I. Horká, E. Tricarico & Z. Ďuriš, 2014. New record of the parthenogenetic marbled crayfish Procambarus fallax f. virginalis from Italy. Crustaceana 87(11–12): 1386–1392.
Vorburger, C. & G. Ribi, 1999. Aggression and competition for shelter between a native and an introduced crayfish in Europe. Freshwater Biology 42(1): 111–119.
Weiperth, A., B. Gál, P. Kuříková, M. Bláha, A. Kouba & J. Patoka, 2017. Cambarellus patzcuarensis in Hungary: the first dwarf crayfish established outside of North America. Biologia 72(12): 1529–1532.
Weiperth, A., M. Bláha, B. Szajbert, R. Seprős, Z. Bányai, J. Patoka, & Kouba, A., 2020. Hungary: a European hotspot of non-native crayfish biodiversity. Knowledge and Management of Aquatic Ecosystems 421: 43.
Acknowledgements
This study was supported by the Czech Science Foundation (No. 19-04431S), the Ministry of Education, Youth and Sports of the Czech Republic (CENAKVA, LM2018099), the National Research, Development and Innovation Office (No. NVKP 16-1-2016-0003) and the Estonian University of Life Sciences (No. P190254PKKH). The authors would like to thank Edit Répás, Veronika Gábris, Richárd Seprős and Zsombor Bányai for help with the fieldwork. We acknowledge Julian Reynolds for English editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Lee B. Kats
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Veselý, L., Ruokonen, T.J., Weiperth, A. et al. Trophic niches of three sympatric invasive crayfish of EU concern. Hydrobiologia 848, 727–737 (2021). https://doi.org/10.1007/s10750-020-04479-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04479-5