Abstract
Marine heterotrophic microalgal species which are potentially rich in docosahexaenoic acid (DHA, C22:6n−3) have been found in Taiwan; however, there was a lack of detailed analysis and characterization of these indigenous algae which is needed for the development of commercial applications. Hence, the objective of this study was to screen DHA-rich heterotrophic microalgae species indigenous to Taiwan for commercial purposes. Heterotrophic microalgae from a variety of marine habitats were isolated, cultivated, and then identified according to their 18S rRNA gene sequences and morphological characteristics. A comparison was made of their fatty acid profiles, fatty acid content, and amount of biomass. For the strain with highest DHA yield, the optimal growth conditions were determined in order to establish the best fermentation conditions for scale-up. In this study, 25 heterotrophic microalgal strains were successfully isolated from marine habitats around Taiwan. All of the isolated strains showed a close phylogenic relationship with the Thraustochytriaceae family according to their 18S rRNA gene sequences. GC/MS analysis discerned seven distinctive fatty acid profiles of these strains, with the production of eicosapentaenoic acid (C20:5n−3) ranging from 0.02 to 2.61 mg L−1, and DHA ranging from 0.8 to 18.0 mg L−1. An Aurantiochytrium strain BL10 with high DHA production was subsequently chosen for further manipulation. Under optimal growth conditions it could produce up to 59.0 g of dry biomass per liter of culture, with dry biomass containing 73% total fatty acid and 29% DHA, revealing BL10 as an excellent source of microbial DHA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Docosahexaenoic acid (DHA) is an essential fatty acid for human beings as well as for other vertebrates (Muskiet et al. 2004). It is the major fatty acid in the gray matter of the brain and in the retina of the eye. The importance of DHA for the development of the brain and retina during fetal life and infancy and in the maintenance of brain function during adulthood had been well confirmed (Muskiet et al. 2004; Singh 2005). In addition, DHA shows positive effects on cardiovascular diseases such as coronary heart disease, stroke, hypertension (Kris-Etherton et al. 2002), and also neural disorders such as dementia, Alzheimer’s, and depression (Das 2008). Up to date, fish oil is the primary source of DHA commercially available; however it comes from a limited source with variable composition and quality (De Swaaf et al. 2003). In addition, safety issues about fish oil have been raised because of possible contamination by significant levels of toxins such as heavy metals, polychlorinated biphenyls and dioxins, which are especially hazardous to pregnant women and young children (Kris-Etherton et al. 2002). Moreover, the application of fish oil as a food additive is restricted due to problems associated with its characteristic smell, unpleasant taste, and poor oxidative stability (Spolaore et al. 2006). Hence, many efforts were focused to find other alternative high-quality DHA sources which are sustainable and also free of toxins (De Swaaf et al. 2003; Spolaore et al. 2006).
Some marine microalgae such as dinoflagellates and species belonging to the Phylum Heterokonta are rich in DHA (Barclay et al. 1994; Apt and Behrens 1999; De Swaaf et al. 2003; Wu and Lin 2003; Wu et al. 2005). Most of them are autotrophic microalgae which rely strictly on light as the energy source. Alternatively, others are heterotrophic microalgae which can derive metabolic energy from simple organic compounds and grow to fairly high densities in fermenter. It was suggested that the production of high levels of biomass by using fermenter could make the cost of cultivation an order of magnitude less than that of photobioreactors for cultivating autotrophic microalgae (Apt and Behrens 1999). Two main categories of heterotrophic microalgae have been used for commercial production of DHA: Cryptothecodinium cohnii belonging to the Phylum Dinophyta (De Swaaf et al. 2003; Spolaore et al. 2006), and Schizochytrium spp. belonging to the Phylum Heterokonta of the Kingdom Straminipila (Nakahara et al. 1996; Wu and Lin 2003; Spolaore et al. 2006; Ganuza et al. 2008). The uses of other species belonging to Heterokonta such as Thraustochytrium (Bajpai et al. 1991; Burja et al. 2006) and Ulkenia (Spolaore et al. 2006) for the same purposes are currently under investigation.
Taiwan is an island with diverse marine habitats including coral reefs, sandy beaches, rocky shores, salt marshes, and estuaries with plentiful mangroves, in which many unique microorganisms have been found. Heterotrophic microalgae belonging to Heterokonta such as Thraustochytrium, Schizochytrium, and Ulkenia have been isolated from these areas and were well identified in previous reports (Volz et al. 1976; Chen and Chien 2002). However, none of these species have been explored for their DHA production or evaluated for their commercial values.
Hence, our study was focused on the screening of some indigenous heterotrophic microalgae (especially Heterokonta algae) for DHA production. These strains were classified according to their ribosomal rRNA gene sequence, morphological characteristics, and fatty acid profiles. The growth conditions for DHA production using the candidate strains were optimized in order to establish the best fermentation conditions for scale-up.
Materials and Methods
Collection of Samples, Establishment and Identification of Algal Strains
Samples including macroalgae, fallen leaves of mangrove trees, sand, and water were collected from intertidal or subtidal zones of different marine habitats including rocky shores, coral reefs, estuaries, and salt marshes in Taiwan. The samples were stored in sterile plastic bags and sent to the laboratory within 1 day for algal cell isolation. Microalgal cells attached to samples were washed down using sterile seawater, passed through a 60-μm plankton net to remove zooplankton, then collected using a 10-μm plankton net. The cells were rinsed several times over a filter using sterile seawater to remove as many bacteria as possible, then transferred by loop, and streaked onto agar plates. The 0.8% agar medium was prepared using full-strength seawater, containing 1 g L−1 peptone, 2 g L−1 yeast extract, 4 g L−1 glucose, and antibiotics including ampicillin (sodium form), streptomycin sulfate, and kanamycin sulfate (100 mg L−1 each). After inoculation, the plates were wrapped with Parafilm® and stored at 26°C for 2–5 days. Single colonies composed of spherical cells atypical of either yeast, fungi, or bacteria were picked and carefully transferred to a new plate.
After becoming established, these algal strains were identified according to their 18S rRNA gene sequences, as well as some morphological characteristics such as development of ectoplasmic nets, formation of zoospores, aplanospores, and amoeboid cells, and et cetera (Yokoyama et al. 2007). Three commercial available algal strains: ATCC 30336 (C. cohnii), ATCC 26185 (Thraustochytrium sp.), and ATCC 28209 (Schizochytrium aggregatum) purchased from American Type Culture Collection (Manassas, VA, USA) were used as reference strains. For morphological observation, cells from each strain were transferred into a 24-well plate with H medium (Takao et al. 2005), and observed using a Leica DMIL inverted phase contrasting microscope (Welzlar, Germany).
For obtaining DNA sequences of 18S rRNA gene from one strain, a single colony of the strain grown on an agar plate was carefully transferred to a 50 mL tube with 10 mL liquid medium prepared with seawater and containing 1 g L−1 peptone, 2 g L−1 yeast extract, and 4 g L−1 glucose. The culture was then cultivated at 26°C for 1 week with continuous shaking (150 rpm). The algal cells were collected by centrifugation (3,000 rpm × 5 min), rinsed with 5 mL deionized water, and lyophilized before DNA sequencing.
The genomic DNA of algal cells was extracted by using DNeasy Plant Mini Kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s guideline. Two primers, 16S1N (forward, 5′-TCCTGCCAGTAGTCATATGC-3′) and 16S2N (reverse, 5′-TGATCCTCT/CGCAGGTTCAC-3′; Grzebyk et al. 1998) were used to amplify 18S rRNA gene in the genomic DNA. Genomic DNA template, 50 ∼ 100 ng, was mixed with the polymerase chain reaction (PCR) mixture containing 50 μL 1× PCR buffer (TaKaRa, Otsu, Japan), 0.2 mM dNTP, 0.2 μM of 16S1N, and 16S2N primers respectively, and 2.5 U Taq polymerase (TaKaRa, Otsu, Japan), then denatured at 94°C for 5 min. The PCR program was run for 30 s at 94°C, 30 s at 50°C, 2 min at 72°C for 40 cycles, and a 7-min final extension at 72°C. The PCR products were purified by using QiAquick gel extraction kit (Qiagen, Germantown, MD, USA), transformed into Escherichia coli by using TA cloning kit (Yeastern, Taipei, Taiwan), then sent to Mission Biotech (Taipei, Taiwan) for DNA sequencing.
The resulting 18S rRNA gene sequences were aligned and compared to the nucleotide sequences of some known microorganisms in GenBank database of the National Center for Biotechnology Information by using Basic Local Alignment Search Tool (BLAST), and also analyzed by using MEGA4 software (Tamura et al. 2007) with the multiple alignment program CLUSTAL W to construct a neighbor-jointing (NJ) tree. The bootstrap values were obtained from 1,000 replications of NJ analyses (Kuo et al. 2005; Burja et al. 2006).
Comparison of Biomass, Fatty Acid Profile, and Fatty Acid Content
Strains cultivated under the same conditions were compared for the amounts of dry biomass, fatty acid profiles, and fatty acid content. Approximately 5 mg algae of each strain was inoculated in a 1 L serum bottle with 400 mL medium containing 1 g L−1 peptone, 2 g L−1 yeast extract, and 4 g L−1 glucose prepared using full-strength natural seawater, then statically cultivated at 26°C for 2 weeks. The cells were harvested by centrifugation (4,000×g, 5 min), rinsed with 5 mL deionized water, centrifuged again, and then lyophilized. For each sample, 30 mg cells were weighed, mixed with 75 μL of a chloroform solution containing 1 mg C19:0 fatty acid as an internal standard (Sigma-Aldrich, St. Louis, MO, USA) and exhaustively extracted with 5 mL chloroform/methanol (2:1, v/v). The extracts were dried by rotary evaporator (40°C), re-dissolved in pure chloroform, transferred into a 10 mL reaction vial, and then dried again using a nitrogen stream.
For the saponification/esterification reactions, the samples were each mixed with 2 mL 0.5 N NaOH methanol solution, heated in a 90°C water bath for 15 min, cooled to room temperature, then mixed with 2 mL 0.7 N HCl in methanol, and 1 mL 14% boron trifluoride methanol solution (Sigma-Aldrich, St. Louis, MO, USA), heated again in 90°C water bath for 15 min, then cooled again. Next, 3 mL saturated NaCl aqueous solution and 2 mL n-hexane were added and the sample was mixed well. The upper liquid layer was transferred to a 4 mL amber vial, dried with a nitrogen stream, sealed with Parafilm®, then stored at −20°C until analysis could be performed.
The fatty methyl ester (FAME) samples were analyzed using a CP-380 gas chromatography machine equipped with a 320 single-quadrupole mass spectrometer (Varian, Palo Alto, CA, USA) and a Supelco SP-2380 capillary column (30 m × 0.25 mm i.d.; Sigma-Aldrich, St. Louis, MO, USA). The injector and interface temperature were set at 250°C and 270°C, respectively, and the column temperature was raised from 50°C to 150°C in increments of 15°C/min, and then 150°C to 250°C in increments of 3°C/min. The linear velocity of the carrier gas was 38.0 cm/min. Methyl esters prepared from fatty acids including C20:2n−6, C20:4n−6, C22:2n−6, C22:3n−3, and C22:5n−6, and an FAME standard mixture (Supelco 18919-1AMP) were all purchased from Sigma-Aldrich and used as standards for identifying fatty acids in the samples. These fatty acids were quantified according to their peak area relative to the C19:0 fatty acid internal standard, and expressed as a percentage of total fatty acid content.
Culture Optimization for Strain BL10
BL10, the strain with the highest DHA production among our isolates, was chosen for optimization of growth conditions. All of the research in this section was performed using 1-L serum bottles containing 100 mL of various media shaken at 150 rpm, and inoculated with 5 × 10−4 g biomass (dry weight). The media composition and other experimental conditions employed are described in the relevant results. For glucose feeding during cultivation, a glucose solution (1 g/mL) was prepared by dissolving glucose in hot water, which was then filtered through a 0.22-μm Millex-GS syringe filter (Millipore, Billerica, MA, USA) before use. Glucose concentration of the medium was measured using a glucose (HK) assay kit purchased from Sigma-Aldrich (product code GAHK-20). Sampling was done every 1–2 days after inoculation. Two milliliters medium samples were collected from the culture and centrifuged at 4,000×g for 2 min. The supernatant was transferred to an Eppendorf tube for analysis of glucose concentrations and pH values. The cells were rinsed with 5 mL distilled water, re-centrifuged, lyophilized, weighed for calculating dry biomass per liter, and stored at −20°C before preparation for fatty acid analysis.
Results
Collection of Samples, Establishment and Identification of Algal Strains
Twenty-five strains of algae-like microorganisms capable of heterotrophic growth were isolated from four types of marine habitats: mangroves in estuaries, rocky shores, coral reefs, and salt marshes located on the western side of Taiwan. The sampling dates and sites of these strains are listed in Table 1.
By using alignment and comparison of 18S rRNA gene sequences with other known microorganisms in GenBank database with BLAST software, these strains are found to show a close phylogenic relationship with Thraustochytriaceae family, which are heterotrophic microalgae well known for their high DHA content (Fig. 1). The GenBank accession numbers of 18S rRNA genes from our strains and all of the reference strains used for the phylogenetic analysis are listed in Table 2. According to the NJ phylogenetic tree, these strains could be grouped into eight monophylogenetic clusters based on their 18S rRNA gene homologies (Fig. 1). The BL-series strains including BL2, BL3, BL4, BL5, BL7, BL8, and BL13 show close phylogenic relationship with ATCC26185 (Thraustochytrium sp.). Another three BL-strains including BL1, BL10, and BL11 form a monophylogenic group with Aurantiochytrium spp. Strain KL1 is close related to Thraustochytrium kinnei KMBP 1694d (Cavalier-Smith et al. 1994). Strain S1a showed close phylogenic relationship with Aplanochytrium minutum (Leipe et al. 1994) and Aplanochytrium stocchinoi (Moro et al. 2003), and strain HK9 and TN6 formed a monophylogenic group with Oblongichytrium strains including Oblongichytrium multirudimentale KMBP N-BA-113 (Honda et al. 1999), Oblongichytrium sp. minutum (Honda et al. 1999), and O. sp. SEK 347 (Yokoyama and Honda 2007).
According to the phylogenetic analysis, it is reasonable to identify BL2, 3, 4, 5, 7, 8, and 13 as Thraustochytrium sp. (T. sp 1); BL1, BL10, and BL11 as Aurantiochytrium sp. (Aurantiochytrium sp 1); KL1 as Thraustochytrium sp. (T. sp. 2); S1a as Aplanochytrium sp.; and HK9 and TN6 as Oblongichytrium sp. These Aplanochytrium, Aurantiochytrium, or Oblongichytrium species could be newly recorded species for Taiwan. The observation of some morphological characteristics, i.e. gliding of vegetative cells for extended periods, formation of zoospores or amoeboid cells, development of ectoplasmic nets, and occurrence of successive binary division of vegetative cells of these strains also supports the identifications (Table 3).
The other strains form four distinctive monophylogenic groups. The first group includes strains BL6, BL9, and BL14; the second one includes TN3 only; the third one includes HK1, HK8, HK8a, KL2, and KL2a; and the fourth one includes HK5 and HK10 (Fig. 1). The first group shows a close phylogenic relationship with T. sp. 1, and since their morphological characteristics also fit the features of Thraustochytrium (Table 3), they are regarded as Thraustochytrium sp. (T. sp. 3). The thallus of strain TN3 undergoes successive binary division of vegetative cells, which fits the features of Aurantiochytrium, Schizochytrium, and Oblongichytrium. Since the strain shows a closer phylogenic relationship with Aurantiochytrium, rather than Schizochytrium and Oblongichytrium (Fig. 1), it is reasonable to identify this strain as Aurantiochytrium sp. (Aurantiochytrium sp. 2). The strains of the third group form a monophylogenic group with an unidentified Thraustochytriidae species strain #32 (Tsui et al. 2009). Since their thallus do not undergo successive binary division, and does develop into a single zoosporangium rather than amoeboid cells, it is reasonable to identify these strains accordingly as Thraustochytrium sp. (T. sp. 4). HK5 and HK10 also have the same morphological features as T. sp. 4, and they show a closer phylogenic relationship with Thraustochytrium striatum ATCC 24473 (Honda et al. 1999), so they are also identified as Thraustochytrium sp. (T. sp. 5).
Comparison of Biomass, Fatty Acid Profile, and Fatty Acid Content
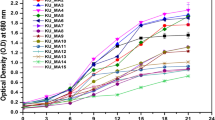
The biomass, production of total fatty acid, EPA, DHA, and fatty acid profiles of these strains are shown in Figs. 2 and 3, respectively. The production of EPA of these strains range from 0.02 to 2.61 mg L−1. In contrast, higher DHA production which range from 0.8 to 18.0 mg L−1 is found (Fig. 2).
Higher homologies of DHA production and fatty acid profiles are found within the strains that have closer phylogenic relationships. Strains belonging to T. sp. 3 and Aurantiochytrium spp. have higher DHA yields compared to strains in other groups. Among them the strain BL10 showed the highest DHA production. In the strain BL10, the DHA content compared to total fatty acid is near 47%, which is slightly lower than the highest strain BL8 at 51%. But since the biomass and total fatty acid in BL10 are much higher than that of BL8, BL10 rather than BL8 was chosen for further manipulation. The growing conditions for BL10 were optimized for maximizing the DHA production to evaluate if it was suitable for commercial application.
Fatty acid profiles could be a nice chemotaxonomical characteristic since comparatively higher homologies of fatty acid profiles, especially the profiles of highly unsaturated fatty acids (HUFAs, the fatty acids with three or more double bonds), were found within the strains with closer phylogenic relationships (Fig. 3). Seven HUFA profiles are classified among these strains (Fig. 3) according to their total HUFA content compared to their total fatty acid content, the presence of C20:3n-6 and C20:4n-6, and the relative amount of C22:5n-6 (n-6 DPA) and C22:5n-3 (n-3 DPA). The methods we used to define the fatty acid profiles and the results of grouping these strains according to their profiles are showed in Fig. 4.
The types of fatty acid profiles observed in these strains are quite different from that of C. cohnii (ATCC30336) (Fig. 3), in which C18:1n-9 and DHA are the only two detectable unsaturated fatty acids.
Culture Optimization for Strain BL10
In this following study we focused on how to increase DHA production of BL10 by optimizing growth conditions. In the first experiment a medium was prepared using seawater with 30 ppt salinity containing 3 g L−1 peptone, 6 g L−1 yeast extract, and 90 g L−1 glucose (n = 3). After inoculation the cultures were shaken without aeration. The pH and glucose concentration of the medium, along with the amount of biomass produced, and the percentage of total fatty acid and DHA in the dry biomass were analyzed.
As the results shown in Fig. 5, all of the glucose was exhausted after 7.5 days, which resulted in terminating growth of this strain. During the first 7.5 days the biomass increased 6000 times from 0.005 g L−1 to 30.0 g L−1. The supplement of glucose to medium to a final concentration of 25 g L−1 at day 7.5 and 10.5 restarted the growth, which resulted in the biomass reaching 42.5 g L−1 at day 17.5, with total fatty acid production at 17.1 g L−1, and DHA production at 8.2 g L−1.
Change of biomass, total fatty acid content (TFA), DHA yield, and glucose concentration ([Glc]) over a 17.5-day period of cultivation of BL10. Cultivation was carried out at 27°C in a 1 L serum bottle containing 100 mL media with 9% glucose, 0.3% peptone, and 0.6% yeast extract. The culture was fed 2.5% glucose on days 9.5 and 13.5, and was shaken at 150 rpm with no aeration
Based on these results, we continued modification of the growth conditions to further maximize the DHA production. The initial glucose concentration in the basal medium was adjusted from 90 to 140 g L−1, the salinity of the seawater decreased from 30 to 10 ppt , and aeration was added to satisfy the oxygen demand of BL10 at the higher cell density, n = 3. For aeration, air from a pump was filtered through a Millex-FG50 filter unit (Millipore, Billerica, MA, USA) and was provided at 200 mL/min.
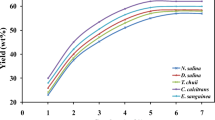
As the results shown in Fig. 6, the high glucose concentration in the basal medium did not inhibit the growth of BL10 at all. Instead, the BL10 grew faster compared to the previous experiment. It took only 3.5 days for the biomass to increase 8000 times from 0.005 g L−1 to 40.0 g L−1, and the feeding of 50 g L−1 glucose at day 3.5 further increased the biomass production to 59 g L−1 at day 6.
Growth curves of BL10 under optimal growth conditions. Cultivations were performed at 27°C in a 1 L serum bottle containing 100 mL basal media with 14% glucose, 0.3% peptone, and 0.6% yeast extract, and fed with 5% glucose at day 3.5, shaken at 150 rpm with aeration (200 ml/min), n = 3. Two salinities, 10 and 30 ppt were compared
It is obvious that the change of salinity from 30 to 10 ppt has little influence on the growth rate (Fig. 6), biomass (Fig. 6), or fatty acid profile (Fig. 7); however, it showed dramatic influence on the total fatty acid content and DHA production. The lower salinity is no doubt more suitable for BL10 to accumulate fatty acid.
Fatty acid profiles of BL10 under various growth conditions. Cultivations were performed under 27°C in a 1 L serum bottle with 100 mL media, with or without shaking (150 rpm) or aeration. a Basal medium containing 0.4% glucose, 0.1% peptone, and 0.2% yeast extract, salinity 30 ppt, without shaking and aeration. b Basal medium containing 9% glucose, 0.3% peptone, and 0.6% yeast extract, 2.5% glucose was fed twice during cultivation; with shaking (150 rpm) but without aeration. c Compared with b, the concentration of glucose in the basal medium was increased to 14%, and 5% glucose was fed once during cultivation; with aeration (200 mL/min). d Compared with c, adjusted salinity from 30 to 10 ppt. Ten fatty acids including 1 C14:0, 2 C15:0, 3 C16:0, 4 C17:0, 5 C18:0, 6 C20:4n−6, 7 C20:5n−3, 8 C22:5n−6, 9 C22:5n−3, 10 C22:6n−3 are found in BL10
After 6 days of cultivation, the total fatty acid and DHA content of BL10 cultivated at 10-ppt were 73% and 29% of dry biomass respectively, which are higher than those of BL10 cultivated at 30-ppt (58% and 21%, respectively).
The fatty acid profile of BL10 was influenced by cultivation conditions. As shown in Fig. 7, when BL10 was cultivated by using the medium with lower C/N ratio (peptone/yeast extract/glucose = 1/2/4 g L−1), the fatty acid profile is more complex that at least 10 fatty acids were detected (Fig. 7a). In contrast, the use of medium with much higher carbon sources which support mass growth resulted in a very simple fatty acid profile (Figs. 7b–d). DHA and palmitic acid (C16:0) are the two predominant fatty acids, with only 2 or 3 other fatty acids present.
Discussion
Twenty-five algal-like microorganisms were isolated from local marine habitats and identified as heterotrophic microalgae belonging to 4 genera of Thraustochytriaceae family – Aplanochytrium, Thraustochytrium, Oblongichytrium, and Aurantiochytrium. The Aurantiochytrium strain BL10 with the highest DHA production was chosen for further manipulations. Under optimal conditions, this strain can grow up to 59 g L−1, with 73% of the dry biomass as fatty acid and 29% as DHA. The production of this strain (59.0 g L−1 cells, 16.8 g L−1 DHA) is higher than most strains reported to date (Bajpai et al. 1991; Yokochi et al. 1998; De Swaaf et al. 2003; Kamlangdee and Fan 2003; Wu et al. 2005; Burja et al. 2006; Ganuza et al. 2008). It is no doubt that BL10, as well as the other strains belonging to Aurantiochytrium are the best candidates for DHA production due to their extremely high DHA production (Yokochi et al. 1998; Jakobsen et al. 2008). The fatty acid profile of this strain is very simple. Compared with fish oil and most microalgae which contain various polyunsaturated fatty acids, the oil from this strain has an advantage in the DHA purification process as it has passed an oral acute toxicity test. Under the dosage of 8 g dry algae per kilogram SD rats, it showed no acute toxicity symptoms (data not shown) for male or female rats. All of these characteristics make BL10 the best candidate for DHA production.
The reasons why we performed the isolation program targeting local algal species are: (1) to understand biodiversity of our local marine habitats, (2) to avoid invasion of foreign species imported for commercial purposes, and (3) to evade patent issues, the greatest obstacle for commercialization in some opinions. As the results show, we successfully found some strains, especially those belonging to Aurantiochytrium and Thraustochytrium sp. 3 which could be readily used for commercial purposes. We also found that compared to the strains isolated from marine habitats including coral reefs and rocky shores which are permanently covered by full-strength seawater, strains isolated from estuaries are generally more suitable for applications due to the following reasons. Firstly, they can grow well in medium of lower salinity due to their euryhalinity. Secondly, they can grow in medium with an extremely high concentration of carbon source without growth inhibition, perhaps due to their acclimation to a highly polluted environment. Thirdly, they are more tolerant to desiccation, making them easier to preserve on an agar surface, which is sometimes too dry to reserve species from subtidal zones of coral reefs and rocky shores.
Strain BL10, one of the strains belonging to Aurantiochytrium, is superior to other strains not only for high DHA production, but also for ease of growth. This strain is regarded as an excellent osmoregulator or osmoconformer since it can grow well under a glucose concentration as high as 140 g L−1, which inhibits growth of many DHA-producing strains such as C. cohnii ATCC30772 (De Swaaf et al. 2003), Aurantiochytrium limacinum SR21 (Yokochi et al. 1998; De Swaaf et al. 2003) and Thraustochytrium ONC-T18 (Burja et al. 2006). In addition, BL10 also grows well from 2 ppt (in our preliminary study) to full-strength seawater. Hence, this strain could be a nice model organism for research in osmoregulation among microorganisms.
Since the biomass of BL10 can increase rapidly from 0.005 g L−1 to 40 g L−1 in only 3.5 days when the glucose concentration of 140 g L−1 was applied, it can be simply cultivated by using batch-culture methods. The feeding of glucose at day 3.5 further increased the biomass to 59 g L−1. Since media with high carbon/nitrogen ratio can stimulate algal cells to accumulate fatty acids (De Swaaf et al. 2003; Burja et al. 2006), and carbon source is generally much cheaper than nitrogen source, the fed-batch method of feeding a carbon source during cultivation can enhance growth rates as well as the DHA production of BL10. According to the growth curves we obtained, we can enhance the growth rate by advancing the glucose feeding time from day 3.5 to day 2.5. The only concern was that under high-density cultivation, there was an increase in n-6 DPA content, which made the preparation of high purity DHA more difficult.
As described previously, this strain can grow well from full-strength seawater to 2 ppt salinity. This is important when considering mass-production on an industrial scale since salinity higher than 5 ppt exerts a strong corrosive effect on stainless steel fermenters (Zu, personal communication). Obviously this strain can accumulate more fatty acid in media with lower salinity compared to that in full-strength seawater. Hence, in the near future we will investigate further to see if the fatty acid content of BL10 can be increased by simply lowering the salinity to less than 10 ppt, and also why the lower salinity is more suitable for fatty acid accumulation.
Fatty acid profiles have been thought to be a possible chemotaxonomical characteristic since a comparatively higher homology of fatty acid profile always found within the strains with closer phylogenic relationships (Huang et al. 2003; Yokoyama et al. 2007; Raghukumar 2008). However, the rules that given how to group and identify algal strains belonging to Thraustochytriaceae family according to their fatty acid compositions are not well established. This research provides a simple method which uses three criteria for grouping and identifying our strains: 1. percentage of total HUFA compared to total fatty acid content, 2. relative amount of n-6 and n-3 DPA, and 3. the presence of C20:3n-6 and C20:4n-6 for grouping and identifying our strains. We have demonstrated here that the result of grouping according to the fatty acid profile is consistent with grouping results according to the traditional morphological characteristics as well as phylogenetic analysis. For researchers are not familiar with observation of cell morphologies and protocols of molecular biology, our method provides another option for fast identification of species within Thraustochytriaceae family. According to the criteria we designed, the Aurantiochytrium, Oblongichytrium, and Schizochytrium strains in other study (Yokoyama et al. 2007) also have HUFAs profiles similar to our strains belonging to the same genera. T. striatum ATCC 24473 (Yokoyama et al. 2007), which forms a monophylogenic group with our HK5 and HK10 strains (Fig. 1) has a similar fatty acid profile to those two strains. So, we believe that the criteria of fatty acid profiling are not only suitable for grouping and identifying strains isolated in our study, but also applicable as a general rule for classifying other strains belonging to Thraustochytriaceae family. Since fatty acid profiles of these strains might be affected by cultural and analytical conditions, these strains should be cultivated and analyzed under the same standardized conditions.
In summary, an easy method based on fatty acid profiling has been proven to be useful for identifying algal strains belonging to Thraustochytriaceae family. From the strains established by us, an Aurantiochytrium strain (BL10) is thought to be a promising candidate for commercial microbial DHA production giving a good level of production as well as a simple fatty acid profile for easy DHA purification.
References
Apt KE, Behrens PW (1999) Commercial developments in microalgal biotechnology. J Phycol 35:215–226
Bajpai PK, Bajpai P, Ward OP (1991) Optimization of production of docosahexaenoic acid (DHA) by Thraustochytrium aureum ATCC 34304. J Am Oil Chem Soc 68:509–514
Barclay WR, Meager KM, Abril JR (1994) Heterotrophic production of long chain omega-3 fatty acids utilizing algae and algae-like microorganisms. J Appl Phycol 6:123–129
Burja AM, Radianingtyas H, Windust A, Barrow CJ (2006) Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: screening of strains and optimization of omega-3 production. Appl Microbiol Biotechnol 72:1161–1169
Cavalier-Smith T, Allsopp MTEP, Chao EE (1994) Thraustochytrids are chromists, not fungi: 18S rRNA signatures of Heterokonta. Phil Trans R Soc Lond B 346:387–397
Chen S-F, Chien C-Y (2002) Six proliferous species of Thraustochytrium from Taiwan. Taiwania 47:106–114
Das UN (2008) Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer's disease—but how and why? Prostaglandins Leukot Essent Fatty Acids 78:11–19
De Swaaf ME, Sijtsma L, Pronk JT (2003) High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol Bioeng 81:666–672
Ganuza E, Anderson AJ, Ratledge C (2008) High-cell-density cultivation of Schizochytrium sp. in an ammonium/pH-auxostat fed-batch system. Biotechnol Lett 30:1559–1564
Grzebyk D, Sako Y, Berland B (1998) Phylogenetic analysis of nine species of Prorocentrum (Dinophyceae) inferred from 18S ribosomal DNA sequences, morphological comparisons, and description of Prorocentrum panamensis, sp. nov. J Phycol 34:1055–1068
Honda D, Yokochi T, Nakahara T, Raghukumar S, Nakagiri A, Schaumann K, Higashihara T (1999) Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of 18S ribosomal RNA gene. J Eukaryot Microbiol 46:637–647
Huang J, Aki T, Yokochi T, Nakahara T, Honda D, Kawamoto S, Shigeta S, Ono K, Suzuki O (2003) Grouping newly isolated docosahexaenoic acid-producing thraustochytrids based on their polyunsaturated fatty acid profiles and comparative analysis of 18S rRNA genes. Mar Biotechnol 5:450–457
Jakobsen AN, Aasen IM, Josefsen KD, Strøm AR (2008) Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain T66: effects of N and P starvation and O2 limitation. Appl Microbiol Biotechnol 80:297–306
Kamlangdee N, Fan KW (2003) Polyunsaturated fatty acids production by Schizochytrium sp. isolated from mangrove. Songklanakarin J Sci Tech 25:643–650
Kris-Etherton PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106:2747–2757
Kumon Y, Yokoyama R, Haque Z, Yokochi T, Honda D, Nakahara T (2006) A new labyrinthulid isolate that produces only docosahexaenoic acid. Mar Biotechnol (NY) 8:170–177
Kuo HC, Su YL, Yang HL, Chen TY (2005) Identification of Chinese medicinal fungus Cordyceps sinensis by PCR-single-stranded conformation polymorphism and phylogenetic relationship. J Agric Food Chem 53:3963–3968
Leipe DD, Wainright PO, Gunderson JH, Porter D, Patterson DJ, Valois F, Himmerich S, Sogin ML (1994) The stramenopiles from a molecular perspective: 16S-like rRNA sequences from Labyrinthuloides minuta and Cafeteria roenbergensis. Phycologia 33:369–377
Moro I, Negrisolo E, Callegaro A, Andreoli C (2003) Aplanochytrium stocchinoi: a new Labyrinthulomycota from the southern ocean (Ross Sea, Antarctica). Protist 154:331–340
Muskiet FA, Fokkema MR, Schaafsma A, Boersma ER, Crawford MA (2004) Is docosahexaenoic acid (DHA) essential? Lessons from DHA status regulation, our ancient diet, epidemiology and randomized controlled trials. J Nutr 134:183–186
Nakahara T, Yokochi T, Higashihara T, Tanaka S, Yaguchi T, Honda D (1996) Production of docosahexaenoic and docosapentaenoic acids by Schizochytrium sp. isolated from Yap Islands. J Am Oil Chem Soc 73:1421–1426
Raghukumar S (2008) Thraustochytrid marine protists: production of PUFAs and other emerging technologies. Mar Biotechnol (NY) 10:631–640
Singh M (2005) Essential fatty acids, DHA and human brain. Indian J Pediatr 72:239–242
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Takao Y, Nagasaki K, Mise K, Okuno T, Honda D (2005) Isolation and characteristion of a novel single-stranded RNA virus infectious to a marine fungoid protist, Schizochytrium sp. (Thraustochytriaceae, Labyrinthulea). Appl Environ Microbiol 71:4516–4522
Takao Y, Tomaru Y, Nagasaki Y, Yokoyama R, Honda D (2007) Fluorescence in situ hybridization using 18S rRNA-targeted probe for specific detection of thraustochytrids (Labyrinthulomycetes). Plankton Benthos Res 2:91–97
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tsui CK, Marshall W, Yokoyama R, Honda D, Lippmeier JC, Craven KD, Peterson PD, Berbee ML (2009) Labyrinthulomycetes phylogeny and its implications for the evolutionary loss of chloroplasts and gain of ectoplasmic gliding. Mol Phylogenet Evol 50:129–140
Volz PA, Hsu Y-C, Liu C-H (1976) The Thraustochytriaceae and other intertidal fungi of Taiwan. Taiwania 21:1–5
Wu S-T, Lin L-P (2003) Application of response surface methodology to optimize docosahexaenoic acid production by Schizochytrium sp. S31. J Food Biochem 27:127–139
Wu S-T, Yu S-T, Lin L-P (2005) Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochem 40:3103–3108
Yokochi T, Honda D, Higashihara T, Nakahara T (1998) Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl Microbiol Biotechnol 49:72–76
Yokoyama R, Honda D (2007) Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience 48:199–211
Yokoyama R, Salleh B, Honda D (2007) Taxonomic rearrangement of the genus Ulkenia sensu lato based on morphology, chemotaxonomical characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Ulkenia and erection of Botryochytrium, Parietichytrium, and Sicyoidochytrium gen. nov. Mycoscience 48:329–341
Acknowledgements
We thank K. Thomas for manuscript editing and Y.-J. Wang for technical advice regarding to phylogenetic analysis. This work was supported by grants (NSC96-2313-B-006-006-, and NSC97-2313-B-006-004-MY3) from National Science Council, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, HL., Lu, CK., Chen, SF. et al. Isolation and Characterization of Taiwanese Heterotrophic Microalgae: Screening of Strains for Docosahexaenoic Acid (DHA) Production. Mar Biotechnol 12, 173–185 (2010). https://doi.org/10.1007/s10126-009-9207-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-009-9207-0