Abstract
An isolation program targeting Thraustochytrids (marine fungoid protists) from 19 different Atlantic Canadian locations was performed. Sixty-eight isolates were screened for biomass, total fatty acid (TFA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) content. Analysis of fatty acid methyl ester results discerned four distinctive clusters based on fatty acid profiles, with biomass ranging from 0.1 to 2.3 g L−1, and lipid, EPA, and DHA contents ranging from 27.1 to 321.14, 2.97 to 21.25, and 5.18 to 83.63 mg g−1 biomass, respectively. ONC-T18, was subsequently chosen for further manipulations. Identified using 18S rRNA gene sequencing techniques as a Thraustochytrium sp., most closely related to Thraustochytrium striatum T91-6, ONC-T18 produced up to 28.0 g L−1 biomass, 81.7% TFA, 31.4% (w/w biomass) DHA, and 4.6 g L−1 DHA under optimal fermentation conditions. Furthermore, this strain was found to produce the carotenoids and xanthophylls astaxanthin, zeaxanthin, canthaxanthin, echinenone, and β-carotene. Given this strain’s impressive productivity when compared to commercial strains, such as Schizochytrium sp. SR21 (which has only 50% TFA), coupled with its ability to grow at economical nitrogen and very low salt concentrations (2 g L−1), ONC-T18 is seen as an ideal candidate for both scale-up and commercialization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Omega-3 long chain fatty acids have, in recent years, become increasingly popular in the nutritional supplement and nutraceutical arena, with the release of an increasing number of new nutritional supplements and functional foods. Over the past decade, consumers have begun to recognize the importance of these highly unsaturated fatty acids within their diets. Of the omega-3 oils, eicosapentaenoic acid (EPA; C20:5n-3) and docosahexaenoic acid (DHA, C22:6n-3), constitute the largest number of beneficial health claims.
Currently, the major source of these essential fatty acids is the consumption of oily fish such as sardines, salmon, tuna, and herring, as well as that of their processed oils. These oils, accumulated by fish, are ultimately derived from microorganisms concentrated up the food chain. One area receiving considerable interest at present is the cultivation of polyunsaturated fatty acid (PUFA)-producing microheterotrophs. There are two groups of marine microbes that have been found to produce these oils, namely, the marine diatoms, Cryptheconidiaceae, and the marine protists, Thraustochytriidae. The later is considered to hold particular potential as the basis of a viable industry to supply vegetative PUFA-rich biomass and oils rich in essential omega-3 fatty acids (Burja and Radianingtyas 2005), and will be the focus of this study.
Thraustochytrids are marine protists that have been classified into the class Labryinthula of the kingdom Chromista, and include genera such as Thraustochytrium, Aplanochytrium, Japonochytrium, Ulkenia, and Schizochytrium. Thraustochytrids are found throughout the world in estuarine and marine habitats. Their nutritional mode is primarily saprotrophic (that is, they obtain nutrients by absorbing dissolved organic matter), and as such, they are generally found associated with organic detritus, decomposing algal and plant material, and in sediments. Thraustochytrids play an important part in enriching nutritionally poor mangrove areas by acting as decomposers of mangrove litter (Bremer 2000; Kamlangdee and Fan 2003). Furthermore, they can utilize a wide range of carbon and nitrogen sources (Goldstein 1963) and are able to secrete catabolic enzymes, such as cellulases and amylases (Bremer 2000; Raghukumar 2002). The presence of carotenoid pigments amongst the Thraustochytrid group of protists has also been reported (Carmona et al. 2003). In addition, these microbes, when grown on nitrogen-deprived medium, sequester varying amounts of fatty acid (Nakahara et al. 1996; Yokochi et al. 1998). Furthermore, there exists a great deal of variability in biomass, lipid, and DHA yields within strains, mostly dependent upon fermentation conditions. To date, the most promising DHA-producing strain, Schizochytrium limacinum SR21, has been shown to produce 0.84 g DHA L−1 day−1 (Yaguchi et al. 1997). This research therefore focuses on the isolation of a collection of Thraustochytrid species from a range of habitats and ecological niches in Atlantic Canada that produce greater biomass, total fatty acid (TFA), DHA, and carotenoid levels than those previously reported.
Materials and methods
Isolation and maintenance of Thraustochytrids
Seventy marine samples including Spartina alterniflora, Zostera marina, and sediment were collected in eastern Canadian coastal sites from Nova Scotia, Prince Edward Island, New Brunswick, Newfoundland, and Labrador between July and August 2002 (Fig. 1). Samples were placed in 20-mL vials containing 10 mL of sterile, 0.2-μm-filtered, natural seawater, 300 mg L−1 penicillin, and 500 mg L−1 streptomycin. Suspensions were baited with sterile pollen (Acer sp.) and incubated for 48 h at 18°C, according to Bremer (2000). Pollen grains were then transferred by loop and streaked onto B1 agar plates (1 g L−1 yeast extract, 1 g L−1 peptone, 10 g L−1 agar to 1 L natural seawater) containing antibiotics, and were incubated. Single, irregular, hyaline colonies made up of spherical or limaciform cells and atypical of either yeast or bacterial colonies were picked and subcultured at least three times on B1 plates for purity.
Biomass production for fatty acid screening
To screen isolates for growth and fatty acid production, liquid medium was prepared using 0.2-μm-filtered natural seawater containing 2 g L−1 peptone (BD, Franklin Lakes, NJ, USA) and 2 g L−1 yeast extract (BD), which was sterilized by autoclaving, followed by the addition of 5 g L−1, 0.2-μm-filtered sterilized glucose (Sigma-Aldrich, St. Louis, MO, USA) (Bowles et al. 1999). A 30-mL-volume culture was inoculated by loop from an agar plate and grown for 4 days at 18°C on a shaker at 100 rpm. Of this culture, 5 mL was then used to inoculate a 95-mL culture incubated for a further 4 days (stationary phase). Cells were harvested by centrifugation at 4,500 rpm, rinsed with 5 mL of distilled water, and recentrifuged. The cell pellets were freeze-dried, weighed, and stored at −80°C prior to derivatization for fatty acid analysis.
Preparation of fatty acid methyl esters (FAME)
Fatty acid methyl ester (FAME) extraction was performed via the direct transesterification method, modified from Lewis et al. (2000). Specifically, 20 mg of freeze-dried material and 3 mL of transesterification reaction mix [methanol/hydrochloric acid/chloroform (10:1:1 vol/vol)] were added. Cells were vortexed for 10 s to ensure even dispersal of biomass and were placed at 90°C for 120 min. Once transesterification was complete, the samples were removed and allowed to cool to room temperature. Water (1 mL) was then added and vortexed for 10 s. FAMEs were then extracted via the addition of 3×2-mL aliquots of hexane and chloroform (4:1), vortexed for 10 s, and allowed to sit until clear liquid separations were achieved.
GC analysis of FAMEs
GC analysis of FAMES was carried out using two internal standards (200 μL each). One, hexacosanoic acid (C23:0), is added prior to transesterification and the other, nonadecanoic acid (C19:0), is added directly before analysis. Analyses was performed using an Agilent 6890 GC (Agilent, Palo Alto, CA, USA) equipped with a 30×0.32-m-internal diameter (0.25 μm film thickness) OMEGAWAX 320 fused-silica capillary column (Sigma-Aldrich) and a flame ionization detector (injection volume of 1 μL, carrier gas H2 with a constant flow of 5.0 mL min−1, and set at 250°C, split ratio 50:1 to flame ionization detector at 275°C). Confirmation of FAME identity was performed using a Trace GC-DSQ mass spectrometer (Thermo Electron, Boston, MA, USA) and by comparison of retention times for laboratory standards.
Genetic identification
The genomic DNA was extracted using the MoBio UltraClean Microbial DNA Isolation Kit (MoBio, Carlsbad, CA, USA) according to manufacturer instructions. The oligonucleotide primers used in amplifying the 18S rRNA gene were modified from Honda et al. (1999), namely, T18S1F 5′-CAACCTGGTTGATCCTGCCAGTA-3′ and T18S5R 5′-TCACTACGGAAACCTTGTTACGAC-3′. A 20-μL PCR reaction mixture contained 2 U Biolase DNA polymerase (Bioline, Boston, MA, USA), 1×NH4 reaction buffer, 3 mM MgCl2, 1 M Betaine (Sigma-Aldrich), 200 μM of mixed PCR nucleotides (Promega, Madison, WI, USA), 1 μM of each forward and reverse primer (MWG Biotech, High Point, NC, USA), and 100 ng of genomic DNA template. After an initial denaturation step for 3 min at 94°C, PCR amplification was performed using an Eppendorf Master Cycle Gradient thermal cycler (Eppendorf, Westbury, NY, USA), using a program of 45 s at 94°C, 30 s at 64°C, and 2 min at 72°C, for 30 cycles, followed by a 10-min final extension at 72°C. The PCR product was purified using the MoBio UltraClean PCR Clean-up Kit (MoBio) for direct sequencing (MWG Biotech) using primers FA2, FA3, RA1, R (Mo et al. 2002), T18S1F, and T18S5R. The resulting sequences were aligned and compared to nucleotide sequences of similar microorganisms stored in GenBank (Benson et al. 2005) using DS Gene (Accelrys, San Diego, CA, USA). A phylogenetic tree was subsequently generated using the neighbor-joining method (Saitou and Nei 1987), with the statistical significance assessed using 1,000 bootstrap resamplings (Felsenstein 1985).
Identification of carotenoids
Cells were harvested by centrifugation at 3,800×g and washed with phosphate buffered saline. Then, the cells were resuspended in 10×volume of acetone (Sigma-Aldrich), agitated for 5 min at 200 rpm, centrifuged at 3,800×g for 5 min, and concentrated to dryness by N2 evaporation. This was followed by resuspension in a minimal amount of 10% acetone in hexane, prior to high-performance liquid chromatography (HPLC) analysis. Identifications were carried out on an Agilent 1100 HPLC (Agilent) equipped with a variable wavelength detector set at 470 nm. Samples were injected through a Symmetry C18 guard column (Waters, Milford, MA, USA) to a Bondclone C18 reverse-phase column (Phenomenex, Torrance, CA, USA; 10-μm particles; 3.9×300 mm i.d.). The injection volume was 10 μL, and a flow of 1 mL min−1 of 10% acetone in hexane over a 25-min period was used. Carotenoid identity was further confirmed with mass spectrometry analysis (Micromass ESI-QTOF MS, Waters). Quantitative data for each carotenoid were based on the development of a calibration curve using standards (astaxanthin, zeaxanthin, canthaxanthin, echinenone, and β-carotene) and comparing the peak area with defined concentrations.
Fermentation optimization
The effect of carbon, nitrogen, and sea salt on fatty acid and DHA production were examined using batch cultures in 250-mL Erlenmeyer flasks shaken at 130 rpm for 3 days at 25°C. Further cultivation studies were carried out using a Biostat Bplus Twin 5L Bioreactor (Sartorius BBI Systems, Bethlehem, PA, USA). A 100-mL inoculum was used to inoculate 4.9 L of medium in the bioreactor. Glucose concentration was measured using the Glucose (HK) Assay Kit (Sigma-Aldrich) according to the manufacturers instructions. The media constituents and the conditions employed in the bioreactor are detailed with the relevant results.
Results
A collection and screening process was developed whereby members of the protist family Labyrinthulida, especially the genera Schizochytrium and Thraustochytrium, were isolated using pollen-baiting and selective bacteriological media. This study, covering 20 unique collection sites dispersed throughout Atlantic Canada (Fig. 1), produced 68 pure strains, identified microscopically. The selection of oleaginous strains, with more than 20% of their cell dry weight being fatty acids, was based on the results of GC PUFA profiling, biomass productivity, maximal TFA, and DHA and, to a lesser extent, EPA concentrations (Fig. 2), according to the method of Lewis et al. (2000). Values for biomass ranged from 100 to 2,300 mg L−1, and values for TFA and subsequent productivities of DHA and EPA ranged from 27.1 to 321.14, 5.18 to 83.63, and 2.97 to 21.25 mg g−1, respectively (Fig. 2).
All isolates that grew in liquid medium (54 out of 68) produced major amounts of omega-3 PUFA, particularly DHA, which comprised between 22 and 80% of the total C20 to C22 content of these cells (Fig. 2). This confirms previous findings, whereby Thraustochytrids isolated from cold temperate environments have fatty acid profiles, with DHA being up to 53% of the TFA present (Bowles et al. 1999; Huang et al. 2003). Of particular interest is ONC-T18, which produces up to 90% of its C20 to C22 content as DHA, which is approximately 35% of the total intracellular fatty acids. This DHA content was shown to be equivalent to those of several commercial production strains, such as Schizochytrium sp. American Type Culture Collection (ATCC) 20888 (32%) and S. limacinum MYA-1381/SR21 (34%) (Barclay et al. 1994; Yokochi et al. 2003). Furthermore, all of the isolates synthesized EPA, varying between 2 and 20% w/w of total PUFAs identified (Fig. 2). In addition to the omega-3 oils produced, approximately 80% of all isolates synthesized the omega-6 PUFAs arachidonic acid (AA) or docosapentanoic acids (DPA), at concentrations varying between 1 and 18% and 3 and 7% w/w, respectively (Fig. 2).
Huang et al. (2003) suggested that for Thraustochytrids isolated from the tropical coastal waters of Japan and Fiji, five PUFA profiles could be described, namely, DHA/DPA (n-6), DHA/DPA/EPA, DHA/EPA, DHA/DPA/EPA/AA, and DHA/DPA/EPA/AA/docosatetranoic acid (Huang et al. 2003). In the case of this collection of Thraustochytrids, isolated from the temperate waters of Atlantic Canada, four PUFA profiles could be determined, three of which are identical to those mentioned above, namely, DHA/DPA/EPA at 7.4% of collection, DHA/EPA at 13% of collection, and DHA/DPA/EPA/AA at 74% of collection, with a forth comprising a mixture of DHA/EPA/AA at 5.6%.
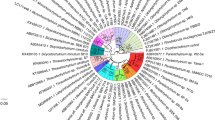
Through direct sequencing of the 18S rDNA gene, ONC-T18 was positively identified as a member of the Thraustochytrid family (GenBank accession number DQ374149). Phylogenetic analysis indicated that ONC-T18 formed a unique group (97.5% identity) with Thraustochytrium striatum T91-6 (Fig. 3) (Leander and Porter 2001). While Thraustochytriidae sp. MBIC 11093, N1-27, and Thraustochytrium sp. CHN-1, collected from the coastal tropical waters of Japan and found to be significant producers of DHA (Carmona et al. 2003; Huang et al. 2003), were shown to be 96, 95.5, and 94.5% similar, respectively. Genetic diversity is quite low between all members of the Thraustochytriidae shown in Fig. 3, ranging from 97.5–91.0% similarity throughout. Yet, these species are globally distributed, with two-thirds isolated from the tropical coastal waters of Japan, China, and Israel, and the rest from temperate waters off America, Europe, and Canada.
The fatty acid profile of ONC-T18 included high contents of C22 PUFA, very low levels of C18 and C20 FA, and the occurrence of odd-chain saturated fatty acids (15:0 and 17:0), similar to that of Schizochytrium sp. KH105 or S. limacinum SR21. Furthermore, analysis of carbon and nitrogen utilization profiles for strains ONC-T18, SR21, and KH105 showed a similar pattern of assimilation (data not shown). The content of n-6 DPA in strain ONC-T18 ranged from 6–10%, which seems to be extremely high when considering the limited occurrence of n-6 DPA in the biosphere. Similar levels of n-6 DPA were reported, however, by Nakahara et al. (1996) in Schizochytrium sp. SR21 (6–10%) and by Ellenbogen et al. (1969) in Thraustochytrium aureum (9.5%) and Thraustochytrium roseum (6.6%).
Analysis of the fatty acid profile of ONC-T18 under three different culture configurations—(1) agar plate, (2) conical flask, and (3) bioreactor and grown on the same medium (Fig. 4)—shows a decrease in the diversity of PUFAs present and an overall increase in TFA from agar plate to bioreactor. Specifically, agar plates exhibited an array of PUFAs, while the flask- and bioreactor-grown cultures were dominated by one or two intermediates (Fig. 4). Therefore, it may be reasonable to conclude that when ONC-T18 is grown for an extended period of time, intermediate PUFAs are able to develop into representative or natural distributions, while those which are grown in flask or bioreactor cultures for a short period of time are predisposed to the production of essential storage PUFAs. That ONC-T18 has DHA as one of these compounds lends ONC-T18 well to further fermentation manipulations and bioprocess optimizations. Compared to T. aureum, which grew better in flask culture than in a stirred tank fermenter (Ilda et al. 1996), ONC-T18 grew better in a bioreactor. This result is in agreement with that of Nakahara et al. (1996), who found that Schizochytrium sp. SR21 showed high resistance to mechanical stirring, and therefore thrived under bioreactor conditions.
Furthermore, carotenoid pigments were found to be produced in plate, flask, and bioreactor fermentations of Thraustochytrium sp. ONC-T18, resulting in a pale orange discoloration. The production of these antioxidants is maximal within bioreactor fermentations concurrently with fatty acid production (data not shown). Moreover, through the use of HPLC mass spectrometry, it was determined that these antioxidant compounds were identified as astaxanthin, zeaxanthin, canthaxanthin, echineone, and β-carotene (Fig. 5), being conjugated to various PUFAs (data not shown). Similar results were reported amongst members of the thraustochytid group of protists. Specifically, Schizochytrium aggregatum was shown to produce echinenone and canthaxanthin (Valadon 1976), while Carmona et al. (2003) demonstrated the production of astaxanthin, echinenone, canthaxanthin, phoenicoxanthin (not zeaxanthin as in ONC-T18), and β-carotene by Thraustochytrium sp. CHN-1, a close relative of ONC-T18 (Fig. 3). In this study, concentrations of these carotenoids were found to be an order of magnitude less than those of CHN-1, with the major compound being β-carotene, rather than astaxanthin. Taking these results into account, it may be reasonable to assume that within Thraustochytrium spp., PUFA and carotenoid production are linked so that the storage fats being produced may be protected from oxidation.
Previously, it has been determined that the relative amounts of the principal fatty acid components (myristic, palmitic, and oleic acids) may be altered somewhat by changing the growth conditions of the culture (Ilda et al. 1996). In this way, one may be able to manipulate the final fatty acid composition and, hence, the physical properties of the desired PUFA in a controlled fashion during fermentation (Sijtsma et al. 1998). In an attempt to limit the factors inhibiting both biomass and omega-3 PUFA production in ONC-T18, carbon, nitrogen, and sea salt components in nutrient media were manipulated (Table 1), along with duration of culture (Fig. 6).
Within this study, as the concentration of nitrogen decreased, TFA content increased, with the highest TFA content (approximately 80%) obtained at 1% concentration of yeast extract and or monosodium glutamate (w/v). Cultures with low nitrogen concentrations, however, also limited cell growth and, hence, TFA production. Optimal production using 8 g L−1 monosodium glutamate and 2 g L−1 yeast extract produced 26.1 g L−1 biomass and 4.5 g L−1 DHA (Table 1). Furthermore, increases in carbon up to 100 g L−1 effectively increased DHA yield; this is in agreement with results obtained for Schizochytrium sp. SR21 (Yokochi et al. 1998), and is contrary to those shown in T. aureum, where glucose concentrations above 10 g L−1 were inhibitory (Ilda et al. 1996). Maximum DHA yields of more than 4.0 g L−1 were obtained in glucose medium, with yields more than five times those of T. aureum (Bajpai et al. 1991) and T. roseum (Li and Ward 1994), and comparable to those of Schizochytrium sp. SR21 and KH105 (Aki et al. 2003). Finally, ONC-T18 exhibited classical euryhaline abilities, being able to withstand salinities ranging from 2.0 to 50.0 g L−1, resulting in biomass productivity of 25–30% variability (Table 1). In the same experiment, DHA gram-per-liter values were found to vary up to 45%, between optimal at 4.6 g L−1 and minimal at 2.5 g L−1 (Table 1).
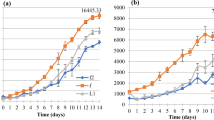
The biomass, TFA, and DHA produced by ONC-T18 over a 168-h period in a 5-L bioreactor are presented in Fig. 6. The growth curve depicted is typical of several achieved under identical conditions. Maximum biomass production was reached after 120 h, close to the point of carbon source (i.e., glucose) depletion. This was also the point at which the TFA content of the biomass reached a maximum at around 70% biomass. Interestingly, after only 24 h of cultivation, DHA content spiked to 30% TFA, thereafter remaining constant at 20–25%. These results are consistent with those of other fatty acid-producing Thraustochytid strains; yet, there is disparity with regards to the rate at which these reactions occur.
Discussion
A collection, screening, and selection process was developed to readily isolate strains of Thraustochytrids with the following combination of economically desirable characteristics: (1) the capability of maximal heterotrophic growth (compared to control strains), (2) the containment of a high percentage of omega-3 highly unsaturated fatty acids, (3) the capability of growing on inexpensive nutrients; (4) thermotolerance, (5) euryhalinity, and (6) the ability to synthesize carotenoids. This differed from previous strategies whereby the strains that were selected had little or no pigmentation present (Barclay et al. 1994). Within this study, strains that produced pigments, and particularly, the carotenoid and xanthophyll family of compounds, were included. Therefore, adhering to these criteria, ONC-T18, isolated from mangrove leaves collected at Advocate Harbour, Nova Scotia, was selected for further optimization, as it possesses a biomass production rate 25% higher than Schizochytrium sp. ATCC 20891. Moreover, the impressive TFA and DHA content of ONC-T18, even when grown on a nonoptimal production medium when compared to S. limacinum MYA-1381/SR21 [such as the general purpose medium used here (Fig. 2)], as well as the presence of carotenoid compounds useful as antioxidants, made this an ideal Thraustochytrid strain for optimization and commercialization.
The optimum culture temperature of 25°C is similar to that of Schizochytrium sp. SR21, T. aureum, and T. roseum (Bajpai et al. 1991; Li and Ward 1994; Yokochi et al. 1998). Furthermore, monosaccharides (glucose, dl-malic acid, d-fructose, d-xylose, fumaric acid, d-cellobiose, pyruvic acid, α-d-lactose, 5-keto-d-gluconic acid, and glycerol) supported good cell growth and DHA yield. A high content of DHA (more than 20%) was obtained with these carbon sources. In contrast, di- and polysaccharides gave poor cell growth. The low utilization of these carbon sources is in complete agreement with results obtained from studies of Schizochytrium sp. SR21 (Nakahara et al. 1996; Yaguchi et al. 1997; Yokochi et al. 1998). Nitrogen utilization by ONC-T18, on the other hand, was consistent with results obtained in other studies, including those performed with T. aureum (Bajpai et al. 1991), in which the strain used organic, elemental, and inorganic sources of nitrogen and ammonium for growth (production of proteins and nucleic acids necessary for cell division). Previously, most studies of Labyrinturomycota identified strains that are unable to store TFA in amounts greater than 20% of biomass. For example, prior to the isolation of Schizochytrium sp. SR 21, which is able to accumulate up to 50% of biomass as fat, T. aureum was the best accumulator at 20% of biomass (Bajpai et al. 1991). ONC-T18, on the other hand, is able to accumulate up to 80% of its biomass as lipid.
For oleaginous microorganisms, such as ONC-T18, to accumulate oil, they must be grown in a culture medium with a limited amount of nitrogen (usually exhausted after 24 to 36 h) and abundant amounts of a carbon source. Once the nitrogen is depleted, the oleaginous microbes continue to assimilate the carbon source but are no longer able to undergo cell division due to a lack of nitrogen (thus preventing protein and nucleic acid synthesis). The result being the conversion of these carbon sources (i.e., sugars such as glucose) into storage oils. In this regard, ONC-T18 is considered to grow more slowly than other Thraustochytrid strains, such as G13 (Bowles et al. 1999), yet it produces DHA at faster rates and demonstrates a unique ability to incorporate elevated amounts of TFAs. Finally, the ability of ONC-T18 to grow at very low salt concentrations with both high biomass and TFA productivity is remarkable. This ability lends ONC-T18 well to scale-up by negating the corrosive nature of salt water on industrial fermentation equipment.
References
Aki T, Hachida K, Yoshinaga M, Katai Y, Yamasaki T, Kawamoto S, Kakizono T, Maoka T, Shigeta S, Suzuki O, Ono K (2003) Thraustochytrids as a potential source of carotenoids. J Am Oil Chem Soc 80:789–794
Bajpai PK, Bajpai P, Ward OP (1991) Optimization of production of docosahexaenoic acid (DHA) by Thraustochytrium aureum ATCC 34304. J Am Oil Chem Soc 68:509–514
Barclay WR, Meager KM, Abril JR (1994) Heterotrophic production of long chain omega-3 fatty acids utilizing algae and algae-like microorganisms. J Appl Phycol 6:123–129
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Wheeler DL (2005) GenBank. Nucleic Acids Res 33:D34–38
Bowles RD, Hunt AE, Bremer GB, Duchars MG, Eaton RA (1999) Long-chain n-3 polyunsaturated fatty acid production by members of the marine protistan group the Thraustochytrids: screening of isolates and optimisation of docosahexaenoic acid production. J Biotechnol 70:193–202
Bremer G (2000) Isolation and culture of Thraustochytrids. In: Hyde K, Pointing S (eds) Marine mycology — a practical approach. Fungal Diversity Press, Hong Kong, pp 49–61
Burja AM, Radianingtyas H (2005) Marine microbial-derived nutraceutical biotechnology: an update. Food Sci Technol 19:14–16
Carmona ML, Naganuma T, Yamaoka Y (2003) Identification by HPLC-MS of carotenoids of the Thraustochytrium CHN-1 strain isolated from the Seto Inland Sea. Biosci Biotechnol Biochem 67:884–888
Ellenbogen BB, Aaronson S, Goldstein S, Belsky MM (1969) Polyunsaturated fatty acids of aquatic fungi: possible phylogenetic significance. Comp Biochem Physiol 29:805–811
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Goldstein S (1963) Development and nutrition of new species of Thraustochytrium. Am J Bot 50:271–279
Honda D, Yokochi T, Nakahara T, Raghukumar S, Nakagiri A, Schaumann K, Higashihara T (1999) Molecular phylogeny of labyrinthulids and Thraustochytrids based on the sequencing of 18S Ribosomal RNA Gene. J Eukaryot Microbiol 46:637–647
Huang J, Aki T, Yokochi T, Nakahara T, Honda D, Kawamoto S, Shigeta S, Ono K, Suzuki O (2003) Grouping newly isolate docosahexaenoic acid-producing Thraustochytrids based on their polyunsaturated fatty acid profiles and comparative analysis of 18S rRNA genes. Mar Biotechnol (NY) 5:450–457
Ilda I, Nakahara T, Yokochi T, Kamisaka Y, Yagi H, Yamaoka M, Suzuki O (1996) Improvement of docosahexaenoic acid production in a culture of Thraustochytrium aureum by medium optimization. J Ferment Bioeng 81:76–78
Kamlangdee N, Fan KW (2003) Polyunsaturated fatty acids production by Schizochytrium sp. isolated from mangrove. J Sci Technol 25:643–650
Leander CA, Porter D (2001) The Labyrinthulomycota is comprised of three distinct lineages. Mycologia 93:459–464
Lewis T, Nichols PD, McMeekin TA (2000) Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J Microbiol Methods 43:107–116
Li ZY, Ward OP (1994) Production of docosahexaenoic acid by Thraustochytrium roseum. J Ind Microbiol 13:238–241
Mo C, Douek J, Rinkevich B (2002) Development of a PCR strategy for Thraustochytrid identification based on 18S rDNA sequence. Mar Biol 140:883–889
Nakahara T, Yokochi T, Higashihara T, Tanaka S, Yaguchi T, Honda D (1996) Production of docosahexaenoic and docosapentaenoic acids by Schizochytrium sp. isolated from Yap Islands. J Am Oil Chem Soc 73:1421–1426
Raghukumar S (2002) Ecology of the marine protists, the Labyrinthulomycetes (Thraustochytrids and Labyrinthulids). Eur J Protistol 38:127–145
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sijtsma L, Springer J, Moesters PAEP, de Swaaf ME, Eggink G (1998) Recent advances in fatty acid synthesis in oleaginous yeasts and microalgae. Recent Res Dev Microbiol 2:219–232
Valadon LRG (1976) Carotenoids as additional taxonomic characters in fungi: a review. Trans Br Mycol Soc 67:1–15
Yaguchi T, Tanaka S, Yokochi T, Nakahara T, Higashihara T (1997) Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J Am Oil Chem Soc 74:1431–1434
Yokochi T, Honda D, Higashihara T, Nakahara T (1998) Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl Microbiol Biotechnol 49:72–76
Yokochi T, Nakahara T, Higashihara T, Tanaka S, Yaguchi T (2003) Microorganisms capable of producing highly unsaturated fatty acids and process for producing highly unsaturated fatty acids by using the microorganisms. US Patent 6,582,941
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burja, A.M., Radianingtyas, H., Windust, A. et al. Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: screening of strains and optimization of omega-3 production. Appl Microbiol Biotechnol 72, 1161–1169 (2006). https://doi.org/10.1007/s00253-006-0419-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0419-1





 Arachidonic acid;
Arachidonic acid;  Eicosapentaenoic acid;
Eicosapentaenoic acid;  Docosapentaenoic acid, n-6; and
Docosapentaenoic acid, n-6; and Docosahexaenoic acid. Results were compared to two reference strains: ATCC 20891 and MYA-1381
Docosahexaenoic acid. Results were compared to two reference strains: ATCC 20891 and MYA-1381


