Abstract
Aurantiochytrium sp. strain T66 was grown in batch bioreactor cultures in a defined glutamate- and glycerol-containing growth medium. Exponentially growing cells had a lipid content of 13% (w/w) of dry weight. A fattening of cells fed excess glycerol occurred in the post-exponential growth phase, after the medium was depleted of N or P. Lipid accumulation was also initiated by O2 limitation (below 1% of saturation). N starvation per se, or in combination with O2 limitation, gave the highest lipid content, i.e., 54% to 63% (w/w) of dry weight. The corresponding maximum culture density was 90 to 100 g/l dry biomass. The content of docosahexaenoic acid (22:6n-3) in N starved, well-oxygenated cells reached 29% (w/w) of total fatty acids but increased to 36% to 52% in O2-limited cells, depending on the time span of the limitation. O2-limited cells did not accumulate the monounsaturated fatty acids that were normally present. We inferred that the biological explanation is that O2 limitation hindered the O2-dependent desaturase(s) and favored the O2-independent polyunsaturated fatty acid synthase. The highest overall volumetric productivity of docosahexaenoic acid observed was 93 mg/l/h. Additionally, we present a protocol for quantitative lipid extraction, involving heat and protease treatment of freeze-dried thraustochytrids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thraustochytrids are heterotrophic protists that are widespread in marine and estuarine environments (Raghukumar 2002). Adl et al. (2005) rank thraustochytrids under Labyrinthulomycetes, which again is ranked under Stramenophiles (Chromista). Thraustochytrium and Schizochytrium are two genera of thraustochytrids commonly encountered in the scientific literature. However, the genus-level classification of thraustochytrids is problematic due to a poor correlation between the classic taxonomy based on morphological features and the taxonomy based on 18S ribosomal DNA phylogeny (Honda et al. 1999; Huang et al. 2003; Yokoyama and Honda 2007). Yokoyama and Honda (2007) have, therefore, proposed a reorganization of the genus Schizochytrium and erected two new genera; Aurantiochytrium and Oblongichytrium. Many strains of thraustochytrids accumulate large amounts of triacylglycerol (e.g., 50% to 80% of dry biomass) with a high proportion of long chain polyunsaturated fatty acids (PUFA), particularly docosahexaenoic acid (DHA; 22:6n-3) and docosapentaenoic acid (DPA; 22:5n-6). DHA commonly accounts for 20% to 40% of total fatty acids. Depending on the strain, thraustochytrids may also accumulate lesser amounts of arachidonic acid (AA; 20:4n-6), eicosapentaenoic acid (EPA; 20:5n-3), and other PUFA (Burja et al. 2006; Huang et al. 2003; Jakobsen et al. 2007).
DHA is the most abundant fatty acid in the human brain and is together with AA the major structural components of the central nervous system. Birch et al. (2007) reported that diet supplements of DHA and AA in early infancy have positive long term effects on cognitive development and visual acuity. An imbalance in adult intake of n-6 and n-3 PUFA is linked to many diseases including cancer, cardiovascular diseases, and mental illnesses (Bergé and Barnathan 2005 and references therein). PUFA from microorganisms are now widely accepted in the international market (Ward and Singh 2005), e.g., Martek Biosciences Corporation, MD, USA, markets DHA from Schizochytrium sp. and the marine dinoflagellate Crypthecodinium cohnii and AA from the fungus Mortierella alpina (Arterburn et al. 2007).
Oleaginous filamentous fungi and yeasts typically start to accumulate storage lipids when they have excess C but are deprived of N or presumably also other key nutrients (Ratledge 2004). In contrast, Ratledge and Wynn (2002) have proposed that thraustochytrids may accumulate storage lipids during the growth period. This view has been supported by Ganuza and Izquierdo (2007) who studied growth of Schizochytrium sp. G13/2S in minimal medium with glutamate and glucose. Similarly, Bajpai et al. (1991) did not detect any lipid accumulation in the post-exponential growth phase of Thraustochytrium aureum ATCC 34304. On the other hand, several studies have shown that a high C-to-N ratio is advantageous for high-level lipid accumulation in thraustochytrids grown in complex media containing yeast extract (Bowles et al. 1999; Burja et al. 2006; Unagul et al. 2006) or corn steep liquor (Yaguchi et al. 1997). However, in these studies, the kinetics of cell growth, N depletion, and high-level lipid accumulation were not studied in sufficient detail to ascertain the pattern of lipid accumulation.
Schizochytrium sp. ATCC 20888 possesses a PUFA synthase complex which has an O2-independent, polyketide synthase-like mode of action. Among eukaryotes, such a PUFA synthase appears to be unique for thraustochytrids, but it is similar to the so-called anaerobic pathways for DHA or EPA synthesis of marine bacteria. It has, therefore, been proposed that Schizochytrium has obtained the PUFA synthase genes from bacteria by lateral gene transfer (Hauvermale et al. 2006; Metz et al. 2001). The three genes encoding the PUFA synthase of Schizochytrium have been coexpressed together with a gene for phosphopantetheinyl transferase, required to activate acyl carrier protein domains, and shown to confer synthesis of both DPA and DHA in Escherichia coli. From the Schizochytrium sp., a gene for a fatty acid synthase of type I has also been isolated, which produces C14:0 and C16:0 fatty acids when expressed in E. coli. Thus, the combined action of the PUFA and the fatty acid synthases can account for the simple fatty acid profile of the parental Schizochytrium sp. (Hauvermale et al. 2006). A characteristic feature of this profile is the lack of the precursor fatty acids, which are normally found in organisms that produce PUFA by the classical aerobic pathway utilizing fatty acid synthase in combination with elongases and O2-dependent desaturases (Napier and Michaelson 2001). But, as mentioned above, many thraustochytrids have a rather complex PUFA profile, and Qiu (2003) has proposed an aerobic pathway for DHA synthesis in a Thraustochytrium sp. A key enzyme in this pathway is a Δ4 desaturase that confers synthesis of DHA from C22:5 (n-3) fatty acid and DPA from C22:4 (n-6) fatty acid (Qiu et al. 2001). Wu et al. (2005) have also described a Δ6 elongase from Thraustochytrium sp. that shows activity against both C18 and C20 fatty acids but prefers the shorter ones. Thus, different pathways for synthesis of long chain PUFA may exist in thraustochytrids.
In the present investigation, we studied strain T66 which has been isolated and characterized by us previously (Jakobsen et al. 2007), and which we have now assigned to the genus Aurantiochytrium (see “Discussion” below; Yokoyama and Honda 2007). By use of a previously developed defined medium for T66 (Jakobsen et al. 2007), we have studied the kinetics of growth, nutrient depletion, and lipid accumulation in batch-bioreactor grown cells which were fed a surplus of glycerol. We report that lipid accumulation was initiated in T66 cells deprived of N, P, or O2 or combinations of these key growth requirements. Furthermore, the fatty acid composition of T66 could be modulated by the growth conditions, and the relative content of DPA and DHA increased in both O2 limited and P starved T66 cells. Additionally, we present a new protocol for lipid extraction, involving heat treatment and protease digestion, overcoming a previously described problem of lipid extraction from thraustochytrids (Burja et al. 2007; Lewis et al. 2000).
Material and methods
Microorganism, growth media, and growth conditions
Strain T66 (ATCC PRA-276) was isolated from a mixture of marine sediment and seawater sampled from the coast of Madeira, Portugal (Jakobsen et al. 2007). The strain was preserved in 15% (v/v) glycerol at −80 °C. The standard medium used for cultivation (28 °C) of T66 contained (g/l): 145 glycerol, 25 monosodium glutamate hydrate, 14.4 NaCl, 0.5 CaCl2·2H2O, 0.8 MgCl2, 0.4 KCl, 4.0 KH2PO4, 3.0 Na2SO4, 5.8 maleic acid, 6.1 Tris base, 5·10−6 cyanocobalamin, 5·10−5 thiamine·HCl, 2.5 ml trace mineral solution (Jakobsen et al. 2007), 0.3 sodium ampicillin, and 0.3 streptomycin sulfate. The antibiotics were used as a general precaution against bacterial contamination. The concentration of glycerol was always kept above 20 g/l by addition of batches of glycerol during the fermentation period. Modifications of the medium for P starvation are stated in the text. Antifoam (Clerol FBA 622) was added as required.
Actively growing cells used for inoculum (5%; v/v) were cultivated in 500 ml baffled shake flasks incubated on an orbital shaker at 200 rpm. The growth media and the incubation temperature (28 °C) were the same as used in the following fermentation experiments. In these experiments of 6–8 days duration, we used stirred reactors (Applicon) of 1.8-l working volume that were equipped with two six-blade turbine impellers. The pH was maintained at 7.0 using a pH controller with automatic addition of 3.0 M NaOH and 3.0 M H2SO4. The aeration was 0.30 volume air/volume medium/min and the selected level of dissolved O2 in the medium was achieved by automatic or manual regulation of the stirring rate (see text). CO2 in the exhaust gas was measured online by mass spectroscopy (Balzers Omnistar GSD 300 02) and the CO2 evolution rate (CER; millimole per liter per hour) was calculated. Cell doubling times were calculated by linear regression of semi logarithmic plots of CER versus time in the exponential growth phase of three independent cultures. Furthermore, temperature, pH, airflow, stirring rate and dissolved oxygen were recorded throughout the fermentations.
Each of the four experiments with O2 limitation was performed two times and the experiments with N starvation or P and N starvation were performed three times each (see text).
All product and substrate concentrations have been corrected for dilution due to feeding of substrates and H2SO4/NaOH and for volume decrease due to sampling. The correction factors used for end-point samples were between 1.1 and 1.3, and the actually measured concentrations in the bioreactors were therefore correspondingly lower.
Cells were harvested by centrifugation (3,200×g for 10 min; 4 °C) and washed once with an isotonic solution of NaCl. The cells were dried at 105 °C for 20–24 h for determination of dry weight. Cells used for lipid and fatty acid analyses were freeze-dried. The supernatants were filtered through 0.2 μm syringe filters and used for determination of glycerol, sodium glutamate and phosphate concentrations in the fermentation broth (see below).
Lipid extraction
In our standard procedure for lipid extraction, freeze-dried cells (28–32 mg) were suspended in 0.7 ml 0.1 M Tris HCl (pH 7.5 at 50 °C) and heated at 98 °C for 10 min in a test tube with a tight screw cap. The tube was then cooled to 50 °C, added 0.1 ml freshly made protease solution containing 10 mg protease from Streptomyces griseus (Sigma) per ml buffer, and incubated for 1 h. After cooling to ambient temperature, the cell mass was extracted by using a scaled-down version of the modified Bligh and Dyer (1959) extraction procedure of Hardy and Keay (1972), as outlined in the following. Two milliliter methanol and 1.0 ml chloroform was added to the tube, and the mixture homogenized vigorously for 1 min using an Ultra-Turrax T25 homogenizer (IKA Labortechnik). One milliliter chloroform was added and the content homogenized for 20 s before 1.0 ml distilled water was added, and the mix homogenized for another 20 s. After centrifugation (3,200×g, 5 min, 4 °C), a carefully measured volume of the bottom layer in the tube was sampled, dried by N2 flushing (40 °C), and weighed. In some instances, the heat and protease treatments were omitted (see text), and the freeze-dried cells (28–32 mg) were then added 0.8 ml water and otherwise extracted with chloroform-methanol as described above.
Chemical analyses
Fatty acids
Fatty acids of extracted lipids were hydrolyzed in 0.5 M NaOH and methylated in methanol with 14% (w/v) BF3 as catalyst as described by Metcalfe et al. (1966). Direct transesterification of fatty acids of freeze-dried cells and extraction of fatty acid methyl esters were carried out as described previously by Lewis et al. (2000), except that HCl of the transesterification mixture was replaced with H2SO4 to get an essentially water free system as recommended by Christie (2003); freeze-dried cells (30–50 mg) were added 3.0 ml transesterification solution (30 ml methanol, 3.0 ml chloroform and 1.0 ml conc. H2SO4). Quantitative analysis of the fatty acid methyl esters was performed using an Agilent 6890 series gas chromatograph as described previously (Jakobsen et al. 2007).
Glycerol
The concentration of glycerol in the cultures was determined by high-pressure liquid chromatography (HPLC) analysis. A Shimadzu chromatograph was used, equipped with an autoinjector (SIL-9A, Shimadzu), an Aminex HPX-87-H column (BioRad Laboratories) at 45 °C, and a refractive index detector (RID-6A, Shimadzu). Five millimolar H2SO4 was used as mobile phase at 0.6 ml/min.
Glutamate
The supernatant of centrifuged growth medium was treated with 10% sulfosalicylic acid to remove protein and then diluted with 0.1 M sodium phosphate (pH 7.0) prior to reversed-phase HPLC analysis. The samples were precolumn fluorescence derivatized with o-phthaldialdehyde (Sigma; Flynn 1988; Lindroth and Mopper 1979) and separated in a NovaPak C18 3WATO86344 column (Waters). Gradient runs were performed with buffer [0.08 M sodium acetate, pH 5.9, with 2.5% (v/v) tetrahydrofuran] and methanol. The HPLC was equipped with an autoinjector (Gilson 233XL) and a fluorescence detector (RF-530, Shimadzu). Standard samples (AA-S-18, Sigma) were used for calibration.
Phosphate
The concentration of phosphate in the cultures was determined using a photometric malachite green procedure as described by Baykov et al. (1988).
Results
Lipid extraction
We have previously reported that strain T66 is able to accumulate large amounts of lipid in which DHA and DPA are the only PUFA (Jakobsen et al. 2007). To study the effects of nutrient limitations on lipid accumulation, an accurate procedure for lipid extraction from cells with a wide range of lipid content was required. We initially used a modified Bligh and Dyer extraction protocol (Bligh and Dyer 1959; Hardy and Keay 1972), similar to the so-called miniaturized Bligh-Dyer method of Burja et al. (2007), which, they found, gave the highest yield of lipid from freeze-dried thraustochytrids. However, the yield of fatty acid methyl esters (FAME) obtained by this protocol was lower than by the direct transesterification of freeze-dried T66 cells (see Fig. 1). Furthermore, as also illustrated in Fig. 1, an increased yield of lipid was obtained by suspending freeze-dried cells in 0.1 M Tris-HCl (pH 7.5) and then subjecting the cells to heat treatment (10 min, 98 °C) and protease digestion (1 h, 50 °C) prior to the extraction in the following called the treatment, resulting in the treated cells. The yield of FAME obtained by transesterification of lipid extracted from the treated cells was the same as the yield of FAME obtained by direct transesterification of freeze-dried cells, indicating that the extraction of treated cells was complete.
Comparison of the yield of FAME (open bars) and total lipid (hatched bars) from freeze-dried T66 cells obtained by using different methods. FAME was determined by three different methods: A, transesterification of lipid extracted with the B&D method from freeze-dried cells; B, transesterification of lipid extracted with the B&D method from freeze-dried cells subjected to heat treatment and protease digestion (so-called treated cells; see text); and C, direct transesterification of freeze-dried cells. Total lipid was determined by two different methods: D, B&D extraction of freeze-dried cells; and E, B&D extraction of treated cells. The values given are the means ± standard deviations of five measurements. Abbreviations: FAME, fatty acid methyl esters; B&D, the extraction method of Bligh and Dyer (1959) as modified by Hardy and Keay (1972)
The data presented in Fig. 1 was obtained with N starved cells from a 4 days old culture (see below). Further comparisons between the lipid yield from untreated and treated cells showed that the treatment was especially important for cells harvested in the exponential growth phase and the early lipid accumulation phase. For such cells, the treatment increased the yield of extracted lipid up to 2.5-fold. The difference between untreated and treated cells diminished throughout the fermentation period; e.g., for cells from 5- to 8-day-old cultures with high lipid content, the lipid yield was essentially the same. In thraustochytrids, lipids are stored in lipid bodies surrounded by a protein containing membrane (Morita et al. 2006 and references therein). This membrane may be weakened in aged cells with high lipid content.
With respect to lipid extraction, we obtained the same results for a new isolate called S61, which is phylogenetically related to T66. The effect of the treatment was somewhat less pronounced for the previously described strain T29 which is closely related T. aureum ATCC 34304 (Jakobsen et al. 2007; data not shown).
N starvation induces lipid accumulation in T66 cells
In order to study the effect of N starvation on lipid accumulation, T66 was grown in defined medium with glutamate and glycerol as the only sources of N, C, and energy. Monosodium glutamate was only supplied at start, whereas glycerol was supplied at start and at all times kept above 20 g/l by subsequent batch feedings. When presented in the figures, the glycerol consumption is plotted only until the first time point of glycerol addition. The cells were cultivated in bioreactors where dissolved O2 was set at 20% of saturation and automatically controlled by regulation of the stirring rate, while the supply of air to the bioreactor was kept constant. Representative data of one fermentation are presented in Fig. 2.
Effects of N starvation on growth characteristics (A) and lipid and PUFA accumulation (B) of strain T66. The cells were grown in batch bioreactor culture in a defined medium containing a limiting amount of glutamate and excess glycerol (Experiment 1; see text). Dissolved O2 (not shown) was controlled at 20% of saturation. A: Concentrations of products formed and substrates consumed as function of cultivation time. Symbols: (filled square) dry cell mass (g/l); (empty square) nonlipid cell mass (g/l); (filled triangle) lipid (g/l); (empty triangle) DHA (g/l); (multiplication sign) glutamate (g/l); (filled circle) glycerol consumption (g/l,);——CER (mmol/l/h). B: cell content of lipid and PUFA as a function of cultivation time. Symbols: (filled triangle) lipid [% (w/w) of cell dry weight], (empty triangle) DHA, and (inverted empty triangle) DPA [% (w/w) of total fatty acids]
The cells and the growth medium were sampled and analyzed at intervals throughout the incubation period (Fig. 2A) and CO2 in the exhaust gas, the dissolved O2 and the stirring rate were measured continuously (data not shown). While the cells catabolized glycerol and glutamate, CER (mmol CO2 evolved/l/h) increased exponentially (Fig. 2A) and indicated a cell doubling time of 5 h. The same doubling time has previously been determined by turbidity measurements of T66 cells cultivated in the same medium in shake flasks (Jakobsen et al. 2007). In all growth experiments of this kind, there was a temporary drop in CER at the time of glutamate exhaustion, resulting in a distinct side peak on the plotted CER graph. Presumably, this side peak indicated N depletion and signaled a change in the metabolism of the organism. Analysis of the free ammonia concentration in the culture media verified N depletion at this time point (data not shown). CER reached its maximum value about 5 h after glutamate was depleted and at the same time the stirring rate peaked at approximately 1,300 rpm. While glutamate was consumed, large amounts of acid had to be added (0.86 mmol H2SO4 per mmol glutamate consumed) to maintain pH at 7.0. Afterwards, the culture required small amounts of NaOH to stabilize the pH.
After glutamate was consumed, there was a modest increase in nonlipid biomass for approximately 20 h. Then, this parameter, which was the calculated difference between the weight of dry biomass and the weight of extracted lipid, was practically constant in the period between 67 h and the end of the fermentation (182 h). The yield of nonlipid cell mass on added N was 17 g/g N. Provided that all added N was catabolized, this corresponded to an N-content of the nonlipid biomass of 5.9% (w/w).
In the period after glutamate exhaustion, the lipid content of the cells increased from about 13% to 55% (w/w) of dry biomass (Fig. 2B). The decreases in CER (Fig. 2A) and the automatically controlled stirring rate (data not shown), which followed after the exhaustion of glutamate, clearly demonstrated reduced metabolic activity and O2 consumption during the lipid-accumulation phase of the T66 cells. The relative contents of DHA and DPA increased slightly during the lipid-accumulation phase and reached about 29% and 8% (w/w) of total fatty acids, respectively (Fig. 2B). No other PUFA occurred in amounts above 1%. Experiment 1 of Table 1 summarizes data for the fatty acid composition and other growth characteristics of the three independent fermentations of this kind. In the experiment presented in Fig. 2, the maximum volumetric DHA content of the culture was 9 g/l. This was achieved after 182 h with a corresponding cell density of 90 g/l dry biomass.
Besides the noted changes in the PUFA content, the main changes in the relative fatty acid composition of T66 during the fermentation were a decrease in C16:0 from 43% to 34% (w/w) of total fatty acids and increases in C16:1 from 5.0% to 9.5% (w/w) and in C18:1 from 2.4% to 8.3% (w/w; data not shown).
O2 limitation induces lipid accumulation and increases the relative content of PUFA
We performed four growth experiments where T66 was exposed to O2 limitation at different stages of growth. In these experiments, the O2 level was at first set to 20% of saturation, resulting in increasing stirring rate as the biomass of the cultures increased. When the stirring rate reached a predetermined value, the rate was either fixed or reduced as described below. It should be noted that the term O2 limitation is used when the dissolved O2 was below 1% of saturation and the air supply was kept constant. The growth medium described above with the same regime of glutamate and glycerol supply was used.
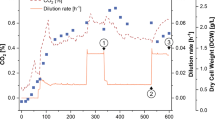
In the experiment with the shortest period of exponential growth (Experiment 2 of Table 1), the stirring rate was fixed when it reached 600 rpm, which occurred after 13 h of growth. As depicted in Fig. 3A, this resulted in a drop in dissolved O2 to about 5% of saturation within a few minutes and to <1% within 12 h. From then on, the increase in nonlipid biomass and CER and the decrease in glutamate content of the medium were practically linear with time. Because the cell density of the culture was low, glutamate was not exhausted during the fermentation period of 143 h. Despite this, the lipid content of the O2 limited cells increased from 13% to 33% (w/w) of dry biomass. This was a lesser increase than that seen for the N-starved cells (cf. Fig. 2), but the relative PUFA content became higher, i.e., DHA and DPA reached 52% and 13% (w/w) of total fatty acids, respectively (Fig. 3B). The cells also contained a small amount of EPA (>1%), but they did not contain measurable amounts of C16:1 and C18:1 fatty acids.
Effects of O2 limitation on growth characteristics (A) and lipid and PUFA accumulation (B) of strain T66. The cells were grown in bioreactor under the same conditions as described in the legend of Fig. 2, except that the O2 transfer to the medium was restricted after 13 h of growth by fixing the stirring rate at 600 rpm (Experiment 2; see text). Symbols: (gray curve) dissolved O2 (% of saturation) of A; other symbols of A and B are the same as in Fig. 2
We also performed two additional experiments where the O2 limitation was imposed while the medium still contained glutamate, but in these cases, the cell density was sufficiently high to consume the remaining glutamate within 15 to 30 h after the stirring rate was fixed. Thus, the cells experienced first O2 limitation and then N starvation. In Experiment 3 (Table 1), the stirring rate was fixed when it reached 800 rpm (occurred after ∼19 h) and then further reduced to 600 rpm after 73 h of cultivation. In Experiment 4, the stirring rate was allowed to reach 1,000 rpm (occurred after ∼26 h) and then it was reduced to 800 rpm and further to 600 rpm, after 54 and 73 h, respectively. Experiments 3 and 4 gave essentially the same results (Table 1), which showed that the system was robust and not influenced by moderate changes in the regime of O2 limitation.
In Experiment 5 depicted in Fig. 4, the cells were allowed to consume all glutamate and to reach the peak value of CER before the stirring rate was reduced from 1,300 to 750 rpm after 49 h of growth. From then on, the O2 content of the growth medium stayed below the detection level. It is noteworthy that the time course of cell growth and lipid accumulation was essentially the same as that observed in Experiment 1 with N limitation and high O2 supply (cf. Fig. 2A), i.e., essentially all increase in biomass was due to lipid accumulation during the last 95 h. Furthermore, the maximum yield of biomass and the maximum lipid content were similar. A comparison of Experiments 3 to 5 showed that delayed onset of O2 limitation gave increased biomass yields, as would be expected. Furthermore, the relative DHA and DPA contents of these cells were at an intermediate level between that of fully aerated cells (Experiment 1) and cells exposed to an earlier onset of O2 limitation (Experiment 2). In the end, all O2-limited cells were essentially devoid of monounsaturated fatty acids. Experiment 5 gave the highest volumetric concentration of DHA; i.e., 13.7 g/l obtained after 182 h, with a corresponding cell density of 88 g/l dry biomass (Fig. 4).
Effects of combined N starvation and O2 limitation on growth characteristics (A) and lipid and PUFA accumulation (B) of strain T66. The cells were grown in bioreactor under the same conditions as described in the legend of Fig. 3, except that the O2 transfer to the medium was reduced at the time point of N exhaustion (49 h) by reducing the stirring rate from 1,300 to 700 rpm (Experiment 5; see text). Symbols: (gray curve) dissolved O2 (% of saturation) of A; other symbols of A and B are the same as in Fig. 2
Effects of sequential P and N starvation
The growth medium was modified to achieve sequential P and N starvation of the T66 cells. Whereas the medium used above contained 4.0 g/l KH2PO4 from start, the present medium contained only 85 mg/l. The initial concentration of monosodium glutamate was 25 g/l as before, but a total of 6.5 g/l monosodium glutamate was added in three similar batches at the time points 22, 46, and 79 h. Glycerol was fed in excess, and the O2 level was maintained at 20% of saturation. Data from one representative fermentation are presented in Fig. 5.
Effects of sequential P and N starvation on growth characteristics (A) and lipid and PUFA accumulation (B) of strain T66. The cells were grown in bioreactor under the same conditions as described in the legend of Fig. 2, except that the medium concentration of KH2PO4 was reduced from 4.0 g/l to 85 mg/l and small amounts of mono sodium glutamate were added during growth (Experiment 6; see text). Dissolved O2 (not shown) was controlled at 20% of saturation. Symbols: (filled diamond) KH2PO4 (mg/l) of A; other symbols of A and B are the same as in Fig. 2
Under these growth conditions, phosphate was completely exhausted already at the first time point of sampling 22 h after inoculation (Fig. 5A). Glutamate was still present in the medium when measured after 50 h but completely exhausted at the time points of 76 and 100 h. Our interpretation of the growth data is that the cells must have had an internal storage of P because the calculated values for nonlipid biomass increased 12-fold after extracellular phosphate was exhausted. At the end of the fermentation period, the calculated P content was 0.04% of dry biomass.
The effect of P starvation on the cell growth was evident from the finding that despite the fact that the cells were given more glutamate than in Experiment 1 (N starvation), the growth yield and maximum CER were strongly reduced. Furthermore, the above-mentioned side peak on the CER graph and the onset in accumulation of lipid occurred before glutamate was exhausted, and the stirring rate peaked at approximately 700 versus 1,300 rpm in Experiment 1. The lipid content reached only 40% (w/w) of dry biomass, but the relative content of PUFA was high, i.e., DHA reached 40% (w/w) and DPA reached 16% (w/w) of total fatty acids (Fig. 5B and Experiment 6 of Table 1). The relative amount of total monounsaturated fatty acids was similar to that observed in Experiment 1.
Discussion
In the present investigation, we show that the lipid content and the fatty acid profile of Aurantiochytrium sp. T66 can be manipulated by changing the growth conditions. The T66 cells maintained a lipid content of 13% of dry weight and a DHA content of 25% of total fatty acids during exponential growth. Depending on the regime of nutrient starvation (N or P) or O2 limitation which we imposed on the cells, the lipid content of the glycerol-fed cells could be increased up to 63% of dry weight or, alternatively, the relative DHA content could be increased up to 52% of total fatty acids. At its best, cells with maximum lipid content displayed a relative DHA content of 39% of total fatty acids.
Similarly to the N starvation induced lipid accumulation in oleaginous yeasts and fungi (Ratledge 2004), a fattening of the T66 cells took place after the medium had run out of the N source (glutamate), and the regular cellular growth had ended. This finding is in accordance with the common observation that a high C-to-N ratio of complex growth media is advantageous for high-level lipid accumulation in thraustochytrids (Bowles et al. 1999; Unagul et al. 2006; Yaguchi et al. 1997). In contrast, Ganuza and Izquierdo (2007) were unable to show this pattern of lipid accumulation for Schizochytrium G13/S2 cells grown in batch cultures with a defined medium containing glutamate and glucose, but they concluded that N limitation is a key parameter for lipid accumulation in cells grown in continuous culture with ammonium and glucose. The main difference between their and our experimental set up of batch cultures, is that we fed the T66 cells an excess of glycerol for nearly 8 days, while their G13/S2 cells ran out of glucose after about 2 days.
Bailey et al. (2003) describe cultivation with low level of dissolved O2 in the fermentation broth (e.g., below 3% of saturation) as a method for increasing the DHA production of thraustochytrids in industrial processes. We observed a pronounced effect of reducing the dissolved O2 level to <1% of saturation after an initial growth period with high level of aeration. Besides increasing the relative content of both DHA and DPA in T66, such O2 limitation caused a depletion of the monounsaturated fatty acids of the cells. Our interpretation of these data is that the long-chain PUFA of T66 was made by the PUFA synthase, since this enzyme system is known to be O2-independent (Metz et al. 2001). Furthermore, the shorter fatty acids that were made by the fatty acid synthase of T66 remained unsaturated because its O2-dependent desaturase(s) did not function under severe O2 limitation. This interpretation also implies that growth with O2 limitation represents a simple experimental method to ascertain the route of DHA synthesis in thraustochytrids.
In oleaginous eukaryotic microorganisms, N starvation leads to activation of AMP deaminase that reduces the cellular AMP level by deamination to IMP. Low level of AMP in the mitochondria initiates a cascade of reactions that causes citrate to accumulate. After being translocated to the cytosol, citrate then serves as a precursor of acetyl-CoA during a prolonged period of fatty acid synthesis. How the AMP deaminase is activated is not known, but it is has been proposed that the incentive for the activation may be to scavenge ammonia from AMP (Ratledge 2004; Ratledge and Wynn 2002). Our finding that also P starvation or O2 limitation induces lipid accumulation in T66, points to the notion that the activation of AMP deaminase may rather be a general stress or starvation response in oleaginous microorganisms.
It was not the primary aim of this study to optimize biomass yield and DHA productivity. However, with N starvation, excess C, and constant high level of dissolved O2 (20% of saturation), up to 100 g/l dry biomass was achieved (Experiment 1). This is higher than any other published values for thraustochytrids in laboratory-scale experiments, but de Swaaf et al. (2003) reached 109 g/l dry biomass with high-density cultures of the DHA producing dinoflagellate C. cohnii ATCC 30772. The highest overall volumetric productivity of DHA observed by us was 93 mg/l/h, obtained for N starved and O2 limited cultures of T66 (Experiment 5), which approaches the highest values reported previously for thraustochytrids, i.e., 138 mg/l/h for Schizochytrium limacinum SR21 (Yaguchi et al. 1997), 115 mg/l/h for Schizochytrium mangrovei Sk-02 (Unagul et al. 2006), and 117 mg/l/h for strain 12B (Perveen et al. 2006).
It has been reported previously by Lewis et al. (2000) and Burja et al. (2007) that the standard lipid extraction procedure of Bligh and Dyer (1959) does not give a complete lipid extraction from thraustochytrids. They have also reported that small modifications of the method may alter the yield of lipid, but it is not obvious why it is so. We showed that treatment with heat and protease digestion facilitated quantitative lipid extraction from T66 and other strains of thraustochytrids. The procedure we employed may be especially useful for determination of the lipid classes of thraustochytrids, which requires a total extraction of lipid. Many researchers routinely produce FAME from thraustochytrids by direct transesterification of freeze-dried cells and may thereby avoid the extraction problems. The commonly used transesterification system is HCl–methanol–chloroform (Burja et al. 2006; Lewis et al. 2000; Yaguchi et al. 1997). Since the researchers did not state that their transesterification system was water-free and since also the transesterification process may be ineffective (Burja et al. 2007), we mention that water will compete with methanol in an esterification reaction and should be avoided. Since it is laborious to prepare water-free HCl–methanol–chloroform, we used H2SO4 in our transesterification mixture, which became practically water-free when made from standard laboratory chemicals, as suggested by Christie (2003).
Strain T66 has been characterized previously (Jakobsen et al. 2007), but it was not assigned to any genus due to the previous uncertainty in the genus-level classification of Schizochytrium spp. and other thraustochytrids. Yokoyama and Honda (2007) have recently proposed a rearrangement of the genus Schizochytrium and erected two new genera; Aurantiochytrium and Oblongichytrium. Based on morphology, 18S ribosomal DNA phylogeny and PUFA profile (approximately 80% DHA) of T66 we have now assigned the organism to the new genus Aurantiochytrium. Even though the variable insert found in 18S rRNA gene of all thraustochytrids (Honda et al. 1999) is not used as a taxonomic criterion by Yokoyama and Honda (2007), it is noteworthy that this signature sequence (CTTTTCGCTGCTCGCAG) of T66 (accession number DQ836628) is identical with the sequence of several strains assigned to the genus Aurantiochytrium, e.g., SEK 209 (AB290574), N1-27 (AB073308), and FJN-10 (AY773276).
References
Adl SM, Simpson AGB, Farmer MA, Andersen RA, Anderson OR, Barta JR, Browser SS, Brugerolle G, Fensome RA, Fredericq S, James TY, Karpov S, Kugrens P, Krug J, Lane CE, Lewis LA, Lodge J, Lynn DH, Mann DG, McCourt RM, Mendoza L, Moestrup Ø, Mozley-Standridge SE, Nerad TA, Shearer CA, Smirnov AV, Spiegel FW, Taylor MFJR (2005) The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J Eukaryot Microbiol 52:399–451
Arterburn LM, Oken HA, Hoffman JP, Bailey-Hall E, Chung G, Rom D, Hamersley J, McCarthy D (2007) Bioequivalence of docosahexaenoic acid from different algal oils in capsules and in a DHA-fortified food. Lipids 42:1011–1024
Bailey RB, DiMasi D, Hansen JM, Mirrasoul PJ, Ruecker CM, Veeder GT III, Kaneko T, Barclay WR (2003) Enhanced production of lipids containing polyenoic fatty acid by very high density cultures of eukaryotic microbes in fermentors. United States Patent 6, 607, 900
Bajpai PK, Bajpai P, Ward OP (1991) Optimization of production of docosahexaenoic acid (DHA) by Thraustochytrium aureum ATCC 34304. JAOCS (J Assoc Oil Chem Soc) 68:509–514
Baykov AA, Evtushenko OA, Avaeva SM (1988) A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem 171:266–270
Bergé JP, Barnathan G (2005) Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv Biochem Eng Biotechnol 96:49–125
Birch EE, Garfield S, Castañeda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D (2007) Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev 83:279–284
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bowles RD, Hunt AE, Bremer GB, Duchars MG, Eaton RA (1999) Long-chain n-3 polyunsaturated fatty acid production by members of the marine protistan group the thraustochytrids: screening of isolates and optimisation of docosahexaenoic acid production. J Biotechnol 70:193–202
Burja AM, Radianingtyas H, Windust A, Barrow CJ (2006) Isolation and characterization of polyunsaturated fatty acid producing Thraustochytrium species: screening of strains and optimization of omega-3 production. Appl Microbiol Biotechnol 72:1161–1169
Burja AM, Armenta RE, Radianingtyas H, Barrow CJ (2007) Evaluation of fatty acid extraction methods for Thraustochytrium sp. ONC-T18. J Agric Food Chem 55:4795–4801
Christie WW (2003) Lipid analysis: isolation, separation, identification and structural analysis of lipids. Oily, Bridgewater
Flynn KJ (1988) Some practical aspects of measurements of dissolved free amino acids in natural waters and within microalgae by the use of HPLC. Chem Ecol 3:269–293
Ganuza E, Izquierdo MS (2007) Lipid accumulation in Schizochytrium G13/2S produced in continuous culture. Appl Microbiol Biotechnol 76:985–990
Hardy R, Keay JN (1972) Seasonal variations in the chemical composition of Cornish mackerel, Scomber scombrus (L), with detailed reference to the lipids. J Food Technol 7:125–137
Hauvermale A, Kuner J, Rosenzweig B, Guerra D, Diltz S, Metz JG (2006) Fatty acid production in Schizochytrium sp.: involvement of a polyunsaturated fatty acid synthase and a type I fatty acid synthase. Lipids 41:739–747
Honda D, Yokochi T, Nakahara T, Raghukumar S, Nakagiri A, Schaumann K, Higashihara T (1999) Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of 18S ribosomal RNA gene. J Eukaryot Microbiol 46:637–647
Huang J, Aki T, Yokochi T, Nakahara T, Honda D, Kawamoto S, Shigeta S, Ono K, Suzuki O (2003) Grouping newly isolated docosahexaenoic acid-producing thraustochytrids based on their polyunsaturated fatty acid profiles and comparative analysis of 18S rRNA genes. Mar Biotechnol 5:450–457
Jakobsen AN, Aasen IM, Strøm AR (2007) Endogenously synthesized (-)-proto-quercitol and glycine betaine are principal compatible solutes of Schizochytrium sp. strain S8 (ATCC 20889) and three new isolates of phylogenetically related thraustochytrids. Appl Environ Microbiol 73:5848–5856
Lewis T, Nichols PD, McMeekin TA (2000) Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J Microbiol Methods 43:107–116
Lindroth P, Mopper K (1979) High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal Chem 51:1667–1674
Metcalfe LD, Schmitz AA, Pelka JR (1966) Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal Chem 38:514–515
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293
Morita E, Kumon Y, Nakahara T, Kagiwada S, Noguchi T (2006) Docosahexaenoic acid production and lipid-body formation in Schizochytrium limacinum SR21. Mar. Biotechnol. 8:319–327
Napier JA, Michaelson LV (2001) Genomic and functional characterization of polyunsaturated fatty acid biosynthesis in Caenorhabditis elegans. Lipids 36:761–766
Perveen Z, Ando H, Ueno A, Ito Y, Yamamoto Y, Yamada Y, Takagi T, Kaneko T, Kogame K, Okuyama H (2006) Isolation and characterization of a novel thraustochytrid-like microorganism that efficiently produces docosahexaenoic acid. Biotechnol Lett 28:197–202
Qiu X (2003) Biosynthesis of docosahexaenoic acid (DHA, 22:6–4, 7,10,13,16,19): two distinct pathways. Prostaglandins Leukot Essent Fatty Acids 68:181–186
Qiu X, Hong H, MacKenzie SL (2001) Identification of a Δ4 fatty acid desaturase from Thraustochytrium sp. involved in the biosynthesis of docosahexanoic acid by heterologous expression in Saccharomyces cerevisiae and Brassica juncea. J Biol Chem 276:31561–31566
Raghukumar S (2002) Ecology of the marine protists, the Labyrinthulomycetes (Thraustochytrids and Labyrinthulids). Eur J Protistol 38:127–145
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 86:807–815
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51
Swaaf de ME, Sijtsma L, Pronk JT (2003) High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol Bioeng 81:666–672
Unagul P, Assantachai C, Phadungruengluij S, Pongsuteeragul T, Suphantharika M, Verduyn C (2006) Biomass and docosahexaenoic acid formation by Schizochytrium mangrovei Sk-02 at low salt concentrations. Bot Mar 49:182–190
Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of production. Process Biochem 40:3627–3652
Wu G, Truksa M, Datla N, Vrinten P, Bauer J, Zank T, Cirpus P, Heinz E, Qiu X (2005) Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat Biotechnol 23:1013–1017
Yaguchi T, Tanaka S, Yokochi T, Nakahara T, Higashihara T (1997) Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. JAOCS (J Assoc Oil Chem Soc) 74:1431–1434
Yokoyama R, Honda D (2007) Taxonomic rearrangement of the genus Schizochytrium sensu lato based on morphology, chemotaxonomic characteristics, and 18S rRNA gene phylogeny (Thraustochytriaceae, Labyrinthulomycetes): emendation for Schizochytrium and erection of Aurantiochytrium and Oblongichytrium gen. nov. Mycoscience 48:199–211
Acknowledgements
We are grateful to Kristin B. Antonsen for help with glutamate analysis and Trond E. Ellingsen for valuable comments to the manuscript. This work was supported by grants from the Research Council of Norway.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jakobsen, A.N., Aasen, I.M., Josefsen, K.D. et al. Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain T66: effects of N and P starvation and O2 limitation. Appl Microbiol Biotechnol 80, 297–306 (2008). https://doi.org/10.1007/s00253-008-1537-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1537-8