Abstract
A bacterial strain E21 was isolated from a sample of water collected in the salt lake located close to Ain Salah, Algeria. The analysis of 16S rRNA gene sequence had indicated that the strain had 93 % sequence similarity with the genus Natrialba sp. strain E21 (GenBank, FR750525.1) and was considered extremely halophilic. Production of biosurfactant by the strain E21 with free and entrapped cells was investigated using soluble starch in the saline conditions. Biosurfactant synthesis was followed by measuring the surface tension and emulsifying index 9 days under optimal conditions (40 °C, pH 7). Some diffusional limitations in alginate and agar beads affected the kinetics of biosurfactant production when compared to that obtained with free cells culture. The minimum values of surface tension were 27 and 30 mN m−1 achieved after 9 days with free and immobilized cells, respectively, while the corresponding maximum E24 values were 65.3 and 62.3 %, respectively. The re-use of bacterial cells along with the limited cell losses provided by the immobilized system might lead to significant reduction of the biosurfactant production cost.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The surfactants are amphipathic molecules which reduce the interfacial tensions between the liquids, solids and gases. They confer an excellent detergency, emulsifying, foaming and other versatile chemical processes. The biosurfactants are surface active molecules produced by various organisms; they have benefits in the structural diversity, better foaming properties and higher selectivity. They are active at extreme temperatures, pH and salinity as well, and can be produced from industrial wastes and by-products. This last feature makes the cheap production of biosurfactant, allows the use of waste substrates and reduction of their polluting effect at the same time (Banat et al. 2000; Tambekar and Gadakh 2013). In addition, the biosurfactants have various tremendous potential for applications in the cosmetics, pharmaceutical, and food industries, as surfactants, emulsifiers, dispersants and they show advantages over chemical surfactants (Desai and Banat 1997). Still, the biosurfactant production could be limited by several factors such as downstream processing which is in many biotechnological processes responsible for up to 60 % of the total production cost. For the economic considerations, most biosurfactants would have to involve either whole-cell spent culture broths or other crude preparations. Hence, their activity may be affected by other materials present in these preparations and their recovery depends chiefly on its ionic charge, water solubility, and location (intracellular, extracellular or cell bound) (Desai and Banat 1997). For this purpose, the immobilization of living cells in porous support gives best advantages in continuous production of biosurfactant. It is an efficient way to reduce the cost of product recovery, as the growth and the product formation phases can be separated and the substrate inhibition can be avoided owing to diffusional limitations (Abouseoud et al. 2008; Anisha and Prema 2008; Tapingkae et al. 2010; Lee et al. 2011).
Therefore, the entrapment methods have many advantages for the study of extracellular biosurfactant produced by immobilized cells to cross the bead wall into the medium without being extracted. Various immobilization matrices, such as agar, alginate, pectin, polyacrylamide, and carrageenan, are used. Thus, the use of alginate is preferred for its cheapness, simplicity and biocompatibility (Lee et al. 2011). Different microbial species have been encapsulated in various matrices for different applications. On many, one might mention the encapsulation of the fungal strains for biocontrol and other applications (Lee et al. 2011; Cruz et al. 1998); the thermophilic and methanogenic bacteria encapsulation (Kanasawud et al. 1989) has also been investigated for potential commercial uses. The variety of microorganisms which have been successfully encapsulated and the wide range of applications which have been explored attest to the utility and versatility of this technology and its potential for use in the environment (Quek et al. 2006; Barreto et al. 2010). Pursing this further, the complex organic and inorganic composition of many industrial waste brines can make them difficult to be treated. High salinities or wide salinity gradients may limit microbial degradation of organic compounds and make conventional biodegradation techniques ineffective. In these cases, halotolerant or halophilic microorganisms have to be used as they have the ability to grow in salinity. Moderately halophilic bacteria are extremophilic microorganisms adapted to live in saline environments. These halophiles optimally grow in media containing salt concentrations between 3 and 15 % (NaCl) (Oren et al. 1997), although the main characteristic of this group of organisms is their ability to grow in a very wide range of salt concentrations. They constitute a very interesting group of microorganisms with a great potential for use in biotechnology (Litchfield 2011). Thus, the encapsulation of the extremes halophilic microorganisms in a gel matrix can be used as a carrier for the environmental application than bioremediation of recalcitrant compound in the wastewater. Besides, the biological treatment of high saline effluents, such as decolorization of acid orange in synthetic wastewater, has been studied (Ogugbue et al. 2012). Hence, Haloferax volcanii D1227, a halophilic archaeon isolated from oil-brine-contaminated soil, was shown to degrade mono-aromatic compounds such as benzoate, cinnamate and 3-phenylpropionate (Emerson et al. 1994; Timpson et al. 2013). The present work aims at studying the growth of immobilized cells of an extremely halophilic bacteria Natrialba sp. strain E21 in the calcium alginate gel bead to identify the factors affecting the production of extracellular biosurfactant by the immobilized system and to assess its operational stability in batch fermentations. So, some strains of Halobacteria obtained from an Algerian culture collection were screened for biosurfactant production in a standard medium using the qualitative drop-collapse test and emulsification activity assay, and five of these Halobacteria strains (strains A21, B21, C21, D21 and E21) reduced the growth medium surface tension below 40 mN m−1 (Kebbouche-Gana et al. 2009). In the present work, we investigated the biosurfactant production by the halophilic bacteria Natrialba sp. strain E21 which exhibited high emulsion-stabilizing capacity using the crude date syrup as growth medium in the extremes conditions of salinity by free and entrapped cells; thereby, this would be the first report about that.
Materials and methods
Source of organism and phenotypic characterization
The strain E21 was isolated from a sample of water collected at 1-m intervals in the salt lake located close to Ain Salah, Algeria (Kebbouche-Gana et al. 2009); the bacterial isolate was routinely cultured in a standard medium (SH), containing (g l−1): 125 g of NaCl, 160 g of MgCl2·6HO2, 5.0 g of K2SO4, 0.1 g of CaCl2·2H2O, 1.0 g of yeast extract (Difco), 1.0 g of Casamino Acids (Difco), 2.0 g of soluble starch (BDH) and 15.0 g agar. The pH of the medium was adjusted to 7.0 ± 0.2. However, tests for phenotypic properties were carried out as indicated in the proposed minimal standards for the description of new taxa in the order Halobacteriales (Oren et al. 1997). Cell motility and shape were examined by phase contrast microscopy without fixation and Gram staining with acetic acid fixation. Tests for catalase and oxidase activities and hydrolysis of starch, gelatin, casein and Tween 80 were performed as described previously by Gutiérrez and Gonzàlez (Gutiérrez and González 1972). Growth response to NaCl was examined in liquid standard medium using serial NaCl concentrations ranging from 50 to 350 g l−1 and influencing growth at pH 5–10. The growth response to temperature was examined by testing growth in liquid medium up to 50 °C. Antibiotic susceptibility and H2S production were tested as previously described (Colwell et al. 1979). The requirement for Mg2+ for growth was tested qualitatively by growing the strains in standard liquid medium with and without MgSO4·7H2O. The use of sugars and the acid production from these compounds were determined in standard medium. This medium was buffered at pH 7.0 and modified as follows: the starch was omitted, the yeast extract and casamino acids concentrations were reduced to 0.25 g l−1 each or yeast extract and casamino acids were omitted, as described below. In the latter case, the media were amended with 0.1 g of NH4Cl per liter and 0.01 g of KH2PO4 per liter (Oren et al. 1995). Each potential carbon source was added to a final concentration of 5 g l−1 from a concentrated sterile solution. Appropriate positive and negative controls were included in all performed tests. Growth was monitored by determining the optical density of each culture at 600 nm, and the pH culture was compared to the pH of a control culture. Consequently, decrease in the pH to a value less than 6.0 was considered as evidence of the acid production. Starch hydrolysis was tested by flooding colonies grown on agar plates containing the standard growth medium with an iodine solution.
DNA extraction, DNA amplification, sequencing 16S ribosomal DNA and phylogenic analysis
Genomic DNA extraction, PCR-mediated amplification of the 16S rDNA and purification of PCR products were carried out as previously described (Rainey et al. 1996). Purified DNA was amplified using specific 16S rRNA archaeal primers (21f 5′-TTCCGGTTGATCCYGCCGGA-3′) and (958r 5′-YCCGGCGTTGAMTCCAATT-3′) (Cytryn et al. 2000). Each 50 μl reaction mixture contained 5 μl of 10 × PCR buffer, 5 μl of deoxynucleoside-triphosphate mix (2.5 nM each), 2.5 μl of bovine serum albumin, 0.5 μl of 21f primer (50 μM), 0.5 μl of 958r primer (50 μM), 0.5 μl of Taq polymerase (TaKaRa, Otsushiga, Japan), 1 μl of template DNA and RNase/DNase-free water to a final volume of 50 μl. PCR was performed in 50-μl glass capillaries using a Perkin-Elmer 480 thermal cycler. The following PCR program was used: 94 °C for 30 s, followed by 30 cycles of 94 °C for 15 s, 55 °C for 20 s and 72 °C for 45 s, followed by 72 °C for 30 s. The strain E21 sequence phylogenetic analysis was performed using the BLAST search program against Genbank database (http://www.ncbi.nlm.nih.gov) and the Ribosomal Data Base Project (http://rdp.cme.smu.edu). The phylogenetic tree was constructed by the neighbor-joining method using MEGA version 5.05 software (Tamura et al. 2011). Bootstrap resembling analysis for 1,000 replicates was performed to estimate the confidence of tree topologies. Methanospirillum hungatei (NR042789) was used as outgroup.
Production of biosurfactant by the free and immobilized cells
Media and cultivation conditions
The nutrient agar was used for inoculum preparation and biosurfactant synthesis. Two loops of agar culture were used to inoculate 50 ml of the liquid standard medium (SH). Seed culture was carried out for 72–76 h on a rotary shaker at room temperature. An inoculum aliquot was used to inoculate culture medium at 4 % (v/v) level. The cultivations were performed in 250-ml flasks containing 50 ml medium at room temperature and stirred in an orbital shaker at 200 rpm and 40 °C.
Entrapment in Ca-alginate
The culture broth was centrifuged at 10,000×g for 30 min, and the pellets were used for the immobilization. Cells were entrapped using sodium alginate at different concentrations ranging from 2 to 10 %. The process was carried out according to the modified method described by Eikmeier et al. 1984. To prepare the alginate beads, four percent gel solution (w/v) was prepared by dissolving 4 g Na-alginate in 90 ml sterile saline water (15 % NaCl), then autoclaved at 105 °C for 10 min. 15 g wet weight cells (strain E21) in 50 ml saline was added to the sterile gel solution, after that 10 ml of the gel-bacterial cell mixture was drawn with the aid of a sterile syringe and allowed to drop through a hypodermic needle into a cross-linking solution (100 ml of 15 % CaCl2 solution at pH 6.9 and 5 °C in 250 ml Erlenmeyer flask) to obtain spherical beads. The bead diameter was 3.0 ± 0.1 mm determined according to the modified method of R.V.G (Barreto et al. 2010). The beads were left in calcium chloride solution for 1 h and then washed several times with sterile saline water 15 % NaCl. Beads resulted from 10 ml alginate were added to 50 ml sterile standard medium (SH) in a 250 ml Erlenmeyer flask. The flasks were incubated on a rotary shaker (200 rpm) at 40 °C. When the beads were not used, they were preserved in 15 % sodium chloride solution in the refrigerator. All the operations were carried out aseptically under laminar flow unit. The effect of Na-alginate and NaCl concentration on fermentation, during 8 at 9 days, was conducted at various concentrations of Na-alginate ranging from 2.0, 4.0, 6.0, 8.0 and 10 % and at various concentrations of NaCl ranging from 10 at 30 %. The initial pH of the fermentation medium was 7.0, with 3.0 mm bead diameter at 40 °C.
Agar entrapment of cells
The procedure was carried out according to the modified method described by Nilsson et al. (1983). A 6 % agar solution (w/v in sterile saline water 10 % NaCl) was homogenized by boiling, sterilized at 121 °C for 15 min and cooled to 50 °C. The bacterial cell suspension (15 g wet weight cells in 50 ml saline) was added to 200 ml agar solution, mixed by stirring on a magnetic stirrer. The beads were prepared as described for alginate entrapment but with paraffin oil as the immobilizing phase instead of CaCl2. The agar beads of 2 mm diameter thus obtained were washed with saline and distilled water. In all cases, cultures were also carried out in triplicates.
Biosurfactant production by the immobilized cells in batch culture
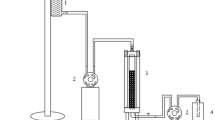
The strain E21 was used in all experiments. Firstly, the batch culture technique experiments of the immobilized cells were preformed in 250 ml Erlenmeyer flasks, each containing 50 ml of sterile medium (SH). The flasks were inoculated with calculated amounts of beads. The flasks were incubated in rotary shaker (200 rpm) at 40 °C for 4–5 days. The culture medium was filtered off and the clear filtrate was then taken for the analytical assay. In the second time, fermentations were (Fig. 1) conducted in a Minifors Bioreactor (INFORS HT) equipped with a polarographic 02 electrode. The working volume for batch fermentations was 1.8 L. Fermentations in bioreactor were conducted in the optimal conditions using crude date syrup medium supplemented with sodium chloride (150 g l−1) and MgCl2·6H2O (100 g l−1). The medium pH was maintained at 7.0 by the addition of 1 M NaOH or 1 M HC1 using an external pH controller, the temperature was adjusted at 40 °C and the agitation rate was set at 200 rpm. For inoculation, the glass bottle contains 1.8 L of the saline crude date syrup medium and 10 g of immobilized cells beads. The date syrup was prepared from date fruits (Phoenix dactylifera L.) variety(Degla-Beida) (Table 1), which is considered a low good quality obtained from the local market (Boumerdes). Dates were chopped into small particles and heated in distilled water (solid/liquid ratio, 1:3) at 80 °C for 2 h and the mixture was maintained at room temperature for 24 h. After that, the mixture was homogenized in warring Blender for 3 min. The extract was filtered in a filter press. Another part of dates was pitted and the flesh was minced just before chemical analysis.
Repeated batch experiments
To get the stabilities in the sugars degradation by immobilized cells, repeated batch experiments were carried out. The Erlenmeyer flasks containing 60 ml of crude date syrup supplemented with sodium chloride (150 g l−1), MgCl2·6HO2 (100 g l−1) and 300 mg of immobilized cells beads were kept in an incubator shaker (200 rpm) maintained at 40 °C. After every 3 days of incubation, crude date syrup was taken out and the oligosaccharide concentration was determined. The beads were separated by filtration, washed with sterile saline water (NaCl 15 %), and transferred into another batch of the crude date syrup (60 ml) supplemented with sodium chloride (150 g l−1) and MgCl2·6HO2 (100 g l−1) for 3-day incubation. The reaction was carried out under identical conditions.
Analytical methods
Biosurfactant production kinetics
The biosurfactant production kinetics by free and immobilized cells were followed in batch cultures during the growth under the optimal conditions, and the surface tension and the emulsification index E24 of supernatant samples obtained, after removing cells as well, were measured.
Biomass and pH measurements
The content of the cells entrapped in the alginate beads was measured by dissolving the gel beads in 10 ml sodium pyrophosphate (1 % w/v) followed by serial dilution and plating on nutrient agar plates (standard and SH medium) using a Lapiz bacteriological colony counter. The content of the cells entrapped in agar beads was measured by dissolving the gel beads at 90 °C for 3 min and reading the absorbance of the gel solution at 600 nm using a UV–Vis spectrophotometer (Perkin-Elmer, USA). The pH of the supernatant was measured with a digital pH meter.
Emulsification activity assay and Surface tension measurement
Drop test and oil spread test were carried out according to Youssef et al. (2004). The emulsification index (E24) was identified through the addition of the hydrocarbon to the same volume of cell-free culture broth, mixed with a vortex for 2 min and left to stand for 24 h. The emulsification activity was determined as the percentage of height of the emulsified layer (mm) divided by the total height of the liquid column (mm). To study the emulsion stability, the emulsified solutions were allowed to stand at room temperature and emulsification index was analyzed at different time intervals. During the growth in the media (saline crude date syrup and SH medium), the surface tension of the cell-free supernatant (50 ml) collected at different time intervals after centrifugation (4,500×g) for 10 min was determined using a KRUSS F6 tensiometer following the Wilhelmy plate measurement technique at room temperature. The values reported are the average of the three measurements.
Surfactant isolation and critical micellar concentration
A crude biosurfactant preparation was obtained according to the method of Cooper and Goldenberg (1987). Hence, the lyophilized material was extracted three times with chloroform/methanol (2:1, v/v) solvent system. The extract was dried with the aid of a rotary evaporator under vacuum. The used method for the purification of biosurfactant was modified from the work of Kim et al. (2000). Instead of the column chromatography steps used by Kim et al. (2000), further purification was achieved by the preparative thin-layer chromatography (TLC) of the extract. The extract was dissolved in distilled water (250 μl) and spotted onto preparative silica gel TLC plates (Whatman, Clifton, NJ, USA) with a solvent system of chloroform/methanol (2:1, v/v). The components were observed under UV light (wavelength of 280 nm). Each fraction was scraped off the plate, dissolved in 250 μl of water, and tested for surface activities using the qualitative drop-collapse test. Surface active fractions were lyophilized.
The extract material was analyzed by thin-layer chromatography according to the modified method described by Kebbouche-Gana et al. (2009). To study the critical micellar concentration, the purified biosurfactant from the strain E21 was dissolved in aqueous solutions at concentrations ranging from 0 to 6 g l−1. The critical micellar concentration (CMC) was determined by plotting the surface tension as a function of the biosurfactant concentration (Kim et al. 2000). For each concentration, surface tension measurement was carried out until a constant value was reached.
Results and discussion
Isolate characterization
The enrichment process was used to select Halobacteria. Colonies from the enrichment that developed on solid media were about 1 mm in diameter, circular, entire and pigmented red–orange after 1 week of incubation at 40 °C. The strain E21 had a salt concentration growth requirement that was always between 1.5 and 4.0 M, the optimum of salts is about 2.54 M and was considered extremely halophilic. The strain was presumptively identified as a family Halobacteriaceae member based on the phenotypic characteristics as shown in Table 2. The strain E21 was gram negative, motile, catalase and oxidase positive. On the basis of the tested phenotypic features, the strain E21 showed phenotypic features resembling members of the genera Natrialba. The obtained partial gene sequence was 847 nucleotides in length (GenBank, FR750525.1) and the phylogenic analysis showed that strain E21 had a sequence identity higher than 93 % with those of the genus Natrialba (Fig. 2). A significant similarity (based on partial 16rRNA sequence) for possible species relatedness (98 %) was found with the two validly described species: Natrialba aegyptia 40 (99 %) (Hezayen et al. 2001) and Natrialba taiwanensis strain B1 (98 %) (Xu et al. 2001), thus indicating that the strain E21 approaches the genus Natrialba to a great extent. The distance matrix indicated that strain E21 might belong to one of these species. The complete 16S rDNA sequence analysis and DNA: DNA hybridizations were suggested for an extensive study for the identification of this strain.
Phylogenetic dendrogram of some Halobacteria based on 16S rRNA gene sequence data, indicating the position of the strain E21. The tree was constructed using the neighbor-joining method. The sequence data used for the following strains were obtained from the sequences collected from Genbank database. Methanospirillum hungatei (NR042789) was used as outgroup. The values at the tree nodes indicate percentages of recurring branches (1,000 bootstraps for resampling)
Production of biosurfactant by free cells
The biosurfactant production kinetic by free cells was followed in batch cultures on crude date syrup medium supplemented with sodium chloride (150 g l−1), during 9 days under the optimal condition. Aqueous solutions of biosurfactant showed good foaming stability. Total foam extinction was detected after 2 h. In addition to the surface and interfacial tension, the emulsion between oil and water was commonly used as a surface activity indicator (Abouseoud et al. 2008). The strain E21 free cells produced extracellular biosurfactant capable to generate a stable emulsion over several hours, according to Abouseoud et al. (2008); a cited criterion for the emulsion-stabilizing capacity was the ability to maintain at least 50 % of the original emulsion 24 h after formation. Hence, the cell-free supernatant from this strain exhibited reduced surface tension (ST) as shown in Fig. 3.
In the growth kinetic of curve, from the second day of the growth, we can clearly see that the production of biosurfactant takes place during the lag phase and being spread out during the stationary growth phase. The cell division occurs with increasing frequency until the maximum growth rate (μmax) for the specific conditions of the batch fermentation is reached. At this point exponential growth begins and cell numbers/biomass increase at a constant rate. The biosurfactants are, therefore, produced during the logarithmic growth phase and the stationary growth phase.
The reduced surface tension of the medium occurred during the lag phase and the stationary growth phase (shown in Fig. 3). Thus, we saw a decrease of ST value until reached 27 mN m−1, it is the lowest value reached at the fermentation end. Also, we note that the pH remains practically unchanged during the fermentation time of strain E21. This result shows that there is a production of biomolecules with surface activity as biosurfactant that causes the reduction of the ST of the medium. The fermentation course and biosurfactant synthesis by the local isolate Strain E21 at different incubation periods (1–9 days) were tested. Following the fermentation time course of the strain E21 adopting the shacked culture technique (Fig. 3), the production of the biosurfactant is initiated from the early exponential phase and maximal production (65.3 %) is reached at 9 days.
Production of biosurfactant by immobilized cells
NaCl and Na-alginate concentration effects
In this part of the study, we report the influence of the concentrations of Na-alginate and NaCl on the properties of the beads (general size, external appearance and texture) encapsulating the halophilic bacteria strain E21. So, high salinity is toxic to most cells; however, the extreme halophiles are well adapted to their hypersaline environment, as evident by their predominance in these habitats, requiring at least 1.5 M NaCl for growth and 3.5–4.5 M NaCl for optimal growth (Tapingkae et al. 2010; Kebbouche-Gana et al. 2009). To prevent the dehydration and maintain the osmotic balance with their surroundings, the extreme halophiles have a high intracellular concentration of salts, as high as the NaCl concentration in their immediate environment. Thus, in this study, salt solution is used to identify whether the alginate beads would increase the production of biosurfactant in this environment. The study of the impact of the NaCl on the formation of beads in the various concentrations of Na-alginate showed that, when we increase the concentration of the NaCl (10–30 %), the beads are increasingly less formed (Fig. 4). To find a solution to this problem, we increase the concentration of Na-alginate which may render the beads more resistant. Indeed, with 2–4 % alginate, the beads are not formed, but from 6 to 10 % of alginate, they shape perfect spherical shape (Fig. 5).
According to Haug (1959a, b) and Smidsrød and Haug (1967), any change of ionic strength in an alginate solution has a profound effect on polymer behavior, especially on polymer chain extension and enhanced also the solution viscosity. At high ionic strengths, the solubility is also affected. Alginate may be precipitated and fractionated to give a precipitate enriched in mannuronate residues by high concentrations of inorganic salts like potassium chloride. Haug (1959b) claims also that the drive of the dissolution process of alginate in water is most probably the gradient in the chemical potential of water between the bulk solvent and the solvent in the alginate particle due to a very high concentration in the particle. This drive becomes severely reduced when the alginate is attempted to be dissolved in an aqueous solvent already containing ions. If alginates are to be applied at high salt concentrations, the polymer should first be fully hydrated in pure water followed by the addition of salt under shear.
In addition, the Halophilic bacteria strain E21 is immobilized in Ca-alginate beads prepared from different concentrations of Na-alginate (2.0, 4.0, 6.0, 8.0 and 10.0 %) and their fermentations are efficiently investigated in the standard liquid medium (SH). Figure 6 shows the growth pattern for the five concentrations of sodium alginate. The increase in the Na-alginate concentration below and above 8 % prolongs the lag phase and the bacteria do not exhibit improved growth.
This longer lag phase could be due to the bacteria requiring adapting to their environment. The exponential growth can be seen in all the flasks except for the flask containing 2.0 % of Na-alginate concentration. The 8.0 % Na-alginate concentration produces more cells compared to other samples. The exponential phase begins after 2 days and the cell grows gradually until 6 days where the stationary phase begins. Therefore, the presence of only 8.0 % Na-alginate concentration in the calcium alginate beads creates the optimum condition for the strain E21. The effect of Na-alginate concentration on the emulsion stability is shown in Fig. 7. The highest emulsion stability is obtained for the 8.0 % of Na-alginate concentration with a value 62.4 %.
The increase in the Na-alginate concentration above 8.0 % decreases the biosurfactant production due to the lower stability of the beads. Thus, when only 2.0, 4.0, and 6.0 % of Na-alginate concentration is used, the beads are disrupted in the medium at the end of the fermentation.
According to Guiseley (1989), typical alginate concentrations used in encapsulation vary between 1 and 8 % (w/v) of calcium alginate in water. The carrier/cell suspension is extruded dropwise into a 0.05–2.0 % CaC12 solution. The ionic bonding between the interacting strands of polymers and Ca +2 ions occurs immediately on contact with the solution, and the resulting polymer network has been depicted as an ‘egg-crate’ matrix, in which the cells are entrapped. In the present report, we use 15 % CaCl2 concentration for gelation because of the excess of NaCl (15 %) used in all experiments. In fact, the complete gelling of alginate beads without cells has been reported to be less than 30 min gelling time increased with cell addition prior to gelation, but decreased with increasing CaC12 concentration up to 1 M, with increasing temperature over the range of 0–50 °C (Guiseley 1989). After the gelation, the encapsulated cells can be used as they are, or placed in a nutrient solution to encourage additional cell growth inside the gel-matrix prior to use (Tapingkae et al. 2010). Hence, the extracellular matrices are known to influence the behavior of encapsulated cells. Therefore, it is not surprising that the strain E21 cells encapsulated in alginate gels of varying composition exhibit differences in their growth dynamics, which correlate with the overall metabolic and secretory activities of the cultures. Those changes are a function of chemical composition, molecular weight, and concentration of alginate; however, the exact cause responsible for these effects is still unclear. The bacterial cells at their logarithmic phase of growth are immobilized on different carriers (i.e. agar, calcium alginate). In another set of experiments, the same amounts of the free cells are grown for comparison with the immobilized cells. As shown in Fig. 8, the culture was grown at 40 °C in the crude date syrup medium supplemented with sodium chloride (150 g l−1). The production of biosurfactant obtained with the immobilized cells is of lower magnitudes than that of the free cells, whereby the effectiveness factor of the immobilization is less than one. The lower surface activity is marked (30 mN m−1) for the immobilized cells of the strain E21. However, we note that the ST in the 1st day of fermentation is quite high, but by the end of the 7th day, we score a decline in value until it reaches 36.9 mN m−1. The production of the biosurfactant is initiated from the early exponential phase and maximal emulsification index E24 (62.3 %) is reached at 8 days. However, as for the pH of the culture medium, it remains stable and stationary all along the microbial growth, as shown in Figs. 3 and 8. Accordingly, the production of biosurfactant obtained with the immobilized cells is lower than that of the free cells. More specifically, the immobilization of cells could cause a decrease in biochemical activity, which induces a reduction in the production of biosurfactant, in which the activity or rather the synthesis of primary or secondary metabolites is dependent on external and internal mass transfer and adequate oxygen supply. This restriction of diffusion can exert a negative effect on the activity of the immobilized cells (Anisha and Prema 2008). The recorded average rates during immobilized cell culture, if compared to free cell culture, are caused by diffusional limitations of nutrients from the bulk of the culture through the gel bead on the one hand, and product (biosurfactant) diffusion from the immobilized cells to the surrounding medium on the other hand. It is worth to note that after reaching its minimum value, the surface tension remains, thus, constant throughout culture (Fig. 8).
On the other hand, Abouseoud et al. (2008) report that minimum values of surface tension are 30 and 35 dynes cm−1 achieved after 40 and 72 h with free and immobilized cells of Pseudomonas fluorescens, respectively, while the corresponding maximum E24 values are 67 and 62 %, respectively. Further, Abouseoud et al. (2008) have alluded that the diffusional limitations in alginate beads affect the kinetics of biosurfactant production when compared to that obtained with free cells culture. Nevertheless, the emulsion stability is improved and fewer by-products interfered with the biosurfactant activity. The microbial cells grow less when they are free in the liquid suspension than inside the Ca-alginate and agar beads. This can be pretty clarified by factors such as oxygen limitation and nutrient availability (Lee et al. 2011). Both immobilized and free cells display a typical growth curve with the stationary phase being reached after 1 at 2 days of incubation. However, Stewart and Robertson (1989) studied Escherichia coli cells encapsulated in alginate and observed that when cells were physically stressed, the porosity of the bead decreased, cell shape was distorted, cell size was reduced and dewatering of encapsulated cells was possible due to the compression generated by cell growth.
Production of biosurfactant with immobilized cells in both matrices by entrapment techniques
Several methods for cell immobilization exist. The choice of the method depends on the specific task; it is determined by the factors that can be assessed only from practical experiences. However, certain general aspects should be taken into account. The cells survive in mild immobilization methods, grow easily in matrix; the diffusion coefficients of substrates are high, relatively cheap; the matrixes are soluble and biodegradable. Comparative data on biosurfactant production of fermentations by free cells and different carrier immobilized cells in the various matrices (agar and in calcium alginate beads) are shown in Fig. 9. There are some remarkable differences between free and the immobilized cells with respect to fermentation yields. Yet, there were noticeable differences in the emulsification activity of fermentations. The emulsification activity and stability of Ca-alginate and agar immobilized cells were 62.3 and 56.2 %, respectively, whereas it was 65.3 % for conventional free cell fermentation. So, it was observed that, during the fermentation process, some cells were released from the agar gel beads into the medium, because of the high porosity of the agar gel.
We venture think that there are no detailed reports on immobilization of extremes halophilc cells. Generally, bacterial cell entrapment in agar gel is an insufficiently studied field. Kanasawud et al. (1989) performed immobilization of the thermophilic bacterium Thermus aquaticus strain YT-1 in agar gel, but their studies did not show clearly enough the effect of this procedure on the proteinase synthesis and bacterial growth. According to Darah and Ibrahim (1998), the cells entrapped in scouring mesh cubes of agar–agar also showed lower growth and lower activity, compared to cells entrapped in calcium alginate beads. Other support materials were employed by other workers for the immobilization of P. chrysosporium for LiP production. They found that polyurethane foams and nylon fibers gave significant results. Also, Nilsson et al. (1983) suggested that probably the most widely used technique for the immobilization of cells with preserved viability to date had been their entrapment in Ca2+-alginate or K-carrageenan. One of the major advantages of this technique has been the simplicity with which spherical particles could be obtained simply by dripping a polymer-cell suspension into a medium containing positively charged ions. Yet, there are other polymers with desirable properties which are also useful for the immobilization of viable cells. These polymers have the advantage over alginate in that they do not require relatively high concentrations of counterions for gel formation. In fact, the difficulties involved in the preparation of spherical particles with these polymers have significantly hampered their wider use.
Chromatographic behavior and CMC
Protein and sugar gave a positive test on TLC plates, indicating that there is a production of the extracellular compounds by the strains E21. Lipids are presented with a regular mobility in the solvent SS2 (RF, 0.34) and t gave a positive test on TLC plates. According to these results, the crude biosurfactants produced by the halophilic bacteria E21 are glycoproteins, glycolipids or lipopeptides. It is currently known that the type made of biosurfactant is dictated by the producing microorganism. These results are in concordance with those obtained by Kebbouche-Gana et al. 2009. In this study, the authors used some strains of Halobacteria from an Algerian culture collection and were screened for biosurfactant production using the qualitative drop-collapse test and emulsification activity assay, Halovivax (strain A21) and Haloarcula (strain D21) reduced the growth medium surface tension below 40 mN m−1. Moreover, the components of the biosurfactant were identified; it contained sugar, protein and lipid. One major class of the biosurfactant was glycolipids, which included rhamnolipids (Abouseoud et al. 2008; Ohlendorf et al. 2008; Rooney et al. 2009), trehalose lipids, sophorose lipids and lipopeptide (Janek et al. 2010). So, polymeric biosurfactant producers were isolated from Eubacteria, Eukaryotes and Archaea (Bodour et al. 2003). The CMC for the isolated biosurfactant was 270 mg l−1 and the corresponding surface tension was 31 mN/m. Biosurfactant concentrations above the CMC produced only 1 weak decrease of the surface tension, indicating that biosurfactant molecules began to aggregate (Abouseoud et al. 2008).
The halobacteria comprise a well-defined, monophyletic group of aerobic or facultatively anaerobic micro-organisms that require high salt concentrations for growth and are recognized as part of the Archaea (Woese et al. 1990). Currently, members of the aerobic, extremely halophilic archaea are classified into 15 genera: Halobacterium, Halococcus, Haloarcula, Haloferax, Halorubrum, Halobaculum, Natrialba, Natronomonas, Natronobacterium, Natronococcus, Halogeometricum, Natrinema, Haloterrigena, Natronorubrum and Halorhabdus (Xu et al. 2001). Most halobacteria are neutrophilic, but some of these genera including the species which are haloalkaliphilic require not only high NaCl concentrations but also high pH and low Mg2+ concentrations for growth. Extremely halophilic archaea are biotechnologically important organisms, particularly because they grow under high salt concentrations and because simple carbon sources such as sugars, starch, acetate or butyrate are sometimes used for growth. We isolate several extremely halophilic archaea from hypersaline water (Ain Salah, Algeria). These isolates are screened with respect to the formation of extracellular emulsifying polymers. In this study, we attempt to describe the taxonomic characterization of strain E21 and the analysis of the extracellular biosurfactant it produces. We find that this strain is a member of the genus Natrialba, but it is sufficiently different from the only described neutrophilic and alcalophilic species, N. aegyptiaca, N. asiatica, N. taiwanensis and Natrialba to justify classification in a new species. Furthermore, we obtained evidence that Natrialba sp. strain E21 should be classified in a new species.
Production of biosurfactant by immobilized cells with repeated batch experiments
On 3rd day, production medium was stopped and the beads were continuously washed with sterile saline solution (NaCl, 15 %). Later, the rejuvenated cells were again subjected for the production of biosurfactant on the crude date syrup medium. As a result, the continuous cultivation for biosurfactant production was able to carry out for 33 days using the alginate beads entrapped Natrialba sp. strain E21 cells and the optimized production medium by intermittent treatment with saline solution and reactivation of the cells. The immobilized beads should be porous enough to allow the transportation of substrates and products in and out of the beads. The immobilized cells were reactivated as shown by the increased production of biosurfactant which gradually decreased with the increase in operational time. Probably, this was because of the decrease in cell viability caused by either aeration or agitation or both. The immobilized cells in alginate beads before fermentation and after fermentation are shown in Fig. 10. Whereas, in the case of oxytetracycline production with polyurethane entrapped Streptomyces cells, the cells were intermittently treated with saline to improve the metabolite productivity (Ogaki et al. 1986). Still, the continuous operation of the reactor with the immobilized Streptomyces cells showed gradual decrease in actinomycin production (Dalili and Chau 1988). The overall results indicated that the continuous fermentation with immobilized cells was found to be the most economical than the batch process. The immobilized beads showed a half-life of 33 days during continuous fermentation. The immobilization provided high cell concentration and eliminates the costly processes of cell recovery and cell recycles. Thus, this process offered a favorable microenvironmental and improved genetic stability and protected against shear damage.
Conclusions
Natrialba sp. strain E21 was used to produce the biosurfactant by free and entrapped cells using the crude date syrup as growth medium. The study showed that when varying the concentration of NaCl (less than 10 %) and the concentration of Na-alginate (higher than 6 %), the formed beads showed perfectly spherical shapes. The immobilized whole cells of Natrialba sp. strain E21 on the alginate and agar supports were promising for biosurfactant production in the high salinity and the biosurfactant can be useful in remediation of pesticides contaminated soils. Saline and hyper-saline environments are frequently contaminated with organic compounds as a result of industrial activities. Contamination of these habitats constitutes a serious environmental problem mainly due to the high toxicity exhibited by aromatic hydrocarbons. Henceforth, this study demonstrated that inexpensive, increased higher cell densities, repeated use of biocatalyst and minimal cost for cell separation, accessible waste materials to significant degradation of organic contaminants in seawater and saline effluents.
References
Abouseoud M, Yataghene A, Amrane A, Maachi R (2008) Biosurfactant production by free and alginate entrapped cells of Pseudomonas fluorescens. J Ind Microbiol Biotechnol 35:1303–1308
Anisha GS, Prema P (2008) Cell immobilization technique for the enhanced production of alpha-galactosidase by Streptomyces griseoloalbus. Bioresour Technol 99:3325–3330
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Barreto RV, Hissa DC, Paes FA, Grangeiro TB, Nascimento RF, Rebelo LM, Craveiro AA, Melo VM (2010) New approach for petroleum hydrocarbon degradation using bacterial spores entrapped in chitosan beads. Bioresour Technol 101:2121–2125
Bodour AA, Drees KP, Maier RM (2003) Distribution of biosurfactant-producing bacteria in undisturbed and contaminated arid Southwestern soils. Appl Environ Microbiol 69:3280–3287
Colwell RR, Litchfield CD, Vreeland RH, Kiefer LA, Gibbons NE (1979) Taxonomic studies of red halophilic bacteria. Int J Syst Bacteriol 29:379–399
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microbiol 53:224–229
Cruz R, Cruz VD, Belini MZ, Belote JG, Vieira CR (1998) Production of fructo-oligosaccharides by the mycelia of Aspergillus japonicus immobilized in calcium alginate. Bioresour Technol 65:139–143
Cytryn E, Minz D, Oremland RS, Cohen Y (2000) Distribution and diversity of archaea corresponding to the limnological cycle of a hypersaline stratified lake (Solar Lake, Sinai, Egypt). Appl Environ Microbiol 66:3269–3276
Dalili M, Chau PC (1988) Production of Actinomycin B with immobilized Sterptomyces parvullux under nitrogen and carbon starvation conditions. Biotechnol Lett 10:331–336
Darah I, Ibrahim CO (1998) Laboratory-scale production of lignin-degrading enzymes by free and entrapped cells of Phanerochaete chrysosporium in a tubular air-lift bioreactor. Folia Microbiol 43:161–168
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Eikmeier H, Westmeier F, Rehm HJ (1984) Morphological development of Aspergillus niger immobilized in Ca-alginate and K-carrageenan. Appl Microbiol Biotechnol 19:10–12
Emerson D, Chauhan S, Oriel P, Breznak J (1994) Haloferax sp. D1227, a halophilic Archaeon capable of growth on aromatic compounds. Arch Microbiol 161:445–452
Guiseley K (1989) Chemical and physical properties of algal polysaccharides used for cell immobilization. Enzyme Microb Technol 11:706–716
Gutiérrez C, González C (1972) Method for simultaneous detection of proteinase and esterase activities in extremely halophilic bacteria. Appl Microbiol 24:516–517
Haug A (1959a) Fractionation of alginic acid. Acta Chem Scand 13:601–603
Haug A (1959b) Ion exchange properties of alginate fractions. Acta Chem Scand 13:1250–1251
Hezayen FF, Rehm BH, Tindall BJ, Steinbüchel A (2001) Transfer of Natrialba asiatica B1T to Natrialba taiwanensis sp. nov. and description of Natrialba aegyptiaca sp. nov., a novel extremely halophilic, aerobic, non-pigmented member of the Archaea from Egypt that produces extracellular poly (glutamic acid). Int J Syst Evol Microbiol 51:1133–1142
Janek T, Łukaszewicz M, Rezanka T, Krasowska A (2010) Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresour Technol 101:6118–6123
Kanasawud P, Hjorleifsdottir S, Holst O, Mattiasson B (1989) Studies on immobilization of the thermophilic bacterium Thermus aquaticus YT-1 by entrapment in various matrices. Appl Microbiol Biotechnol 31:10–12
Kebbouche-Gana S, Gana ML, Khemili S, Fazouane-Naimi F, Bouanane NA, Penninckx M, Hacene H (2009) Isolation and characterization of halophilic Archaea able to produce biosurfactant. J Ind Microbiol Biotechnol 36:727–738
Kim SH, Lim EJ, Lee SO, Lee JD, Lee TH (2000) Purification and characterization of biosurfactant from Nocardia sp. L-417. Biotechnol Appl Biochem 31:249–253
Lee K, Choi I, Kim Y, Yang D, Bae H (2011) Enhanced production of bioethanol and ultrastructural characteristics of reused Saccharomyces cerevisiae immobilized calcium alginate beads. Bioresour Technol 102:8191–8198
Litchfield CD (2011) Potential for industrial products from the halophilic Archaea. J Ind Microbiol Biotechnol 38:1635–1647. doi:10.1007/S.10295-011-1021-9
Nilsson K, Birnbaum S, Flygare S, Linse L, Schröder U, Jeppsson U, Larsson PO (1983) A general method for the immobilization of cells with preserved viability. Eur J Appl Microbiol Biotechnol 17:319–326
Ogaki M, Sonomoto K, Nakajirna H, Tanaka A (1986) Continuous production of oxyteracycline by immobilized growing Streptomyces rimosus cells. Appl. Microb. Biotechnol 24:6–11
Ogugbue CJ, Akubuenyi F, Ibiene AA (2012) Bacterial decolourization of acid orange 10 in synthetic wastewater under saline conditions: effect of process parameters. Singap J Sci Res 2:1–13
Ohlendorf B, Lorenzen W, Kehraus S, Krick A, Bode HB, König GM (2008) Myxotyrosides A and B, unusual rhamnosides from Myxococcus sp. J Nat Products. 72:82–86
Oren A, Gurevich P, Gemmell RT, Teske A (1995) Halobaculum gomorrense gen. nov., sp. nov., a novel extremely halophilic archaeon from the Dead Sea. Int J Syst Bacteriol 45:747–754
Oren A, Ventosa A, Grant WD (1997) Proposed minimal standards for description of new taxa in the order Halobacteriales. Int J Syst Bacteriol 47:233–238
Quek E, Ting YP, Tan HM (2006) Rhodococcus sp. F92 immobilized on polyurethane foam shows ability to degrade various petroleum products. Bioresour Technol 97:32–38
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage; proposal of Nocardiopsaceae fam. nov. Int. J. Syst Bacteriol 46:1088–1092
Rooney AP, Price NP, Ray KJ, Kuo TM (2009) Isolation and characterization of rhamnolipid-producing bacterial strains from a biodiesel facility. FEMS Microbiol Lett 295:82–87
Smidsrød O, Haug A (1967) Precipitation of acidic polysaccharides by salts in ethanol–water mixtures. J Polym Sci Part C Polym Symp 16:1587–1598
Stewart P, Robertson C (1989) Microbial growth in a fixed volume: studies with entrapped Escherichia coli. Appl Microbiol Biotechnol 30:10–12
Tambekar DH, Gadakh PV (2013) Biochemical and molecular detection of biosurfactant producing bacteria from soil. Int. J. LifeSci Biotechnol Pharm. Res 2:204–211
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol
Tapingkae W, Parkin KL, Tanasupawat S, Kruenate J, Benjakul S, Visessanguan W (2010) Whole cell immobilization of Natrinema gari BCC 24369 for histamine degradation. Food Chem 120:842–849
Timpson LM, Liliensiek AK, Alsafadi D, Cassidy J, Sharkey MA, Liddell S, Allers T, Paradisi FA (2013) Comparison of two novel alcohol dehydrogenase enzymes (ADH1 and ADH2) from the extreme halophile Haloferax volcanii. Appl Microbiol Biotechnol 97:195–203
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87:4576–4579
Xu Y, Wang Z, Xue Y, Zhou P, Ma Y, Ventosa A, Grant WD (2001) Natrialba hulunbeirensis sp. nov. and Natrialba chahannaoensis sp. nov., novel haloalkaliphilic archaea from soda lakes in Inner Mongolia Autonomous Region, China. Int J Syst Evol Microbiol 51:1693–1698
Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, Mcinerney MJ (2004) Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Methods 56:339–347
Acknowledgments
This work was supported by the assistance of the Algerian Ministry of Education grant PNR project (14/u35/1360) http://www.nasr-dz.org/dprep/pnr2/projets-pnr/PNR_efghi.htm.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Antranikian.
Rights and permissions
About this article
Cite this article
Kebbouche-Gana, S., Gana, M.L., Ferrioune, I. et al. Production of biosurfactant on crude date syrup under saline conditions by entrapped cells of Natrialba sp. strain E21, an extremely halophilic bacterium isolated from a solar saltern (Ain Salah, Algeria). Extremophiles 17, 981–993 (2013). https://doi.org/10.1007/s00792-013-0580-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0580-2