Abstract
Biosurfactants have been considered as promising candidates for oil spill cleanup as they are generally more biodegradable, less toxic, and better in enhancing biodegradation than chemical surfactants. This study targeted the marine microbial biosurfactants to examine their enhanced production methods and application for the removal of crude oil from soil. The biosurfactants generated by Bacillus subtilis, which was isolated from the Atlantic Ocean, were investigated in this study. The economic production medium using different carbon (n-hexadecane, diesel oil, glycerol, glucose, starch, and sucrose) and nitrogen sources (NaNO3, (NH4)2SO4, and yeast extract) was studied. The best performance of biosurfactant production was achieved when using glycerol as carbon source and sodium nitrate and yeast extract as nitrogen sources in the substrate. The production rate was enhanced five times compared with that of the original screening recipe. The fermentative production of the generated biosurfactants could reduce the surface tension of water to 27 mN/m and with strong surface activity (∼36.4 mN/m) even after dilution for 10 times. The critical micellar concentration (CMC) of the product was 507 mg/L. A thin layer chromatography (TLC) analysis indicated that the purified product was a mixture of lipopeptide and glycolipid. The microbially produced biosurfactants were further examined as a soil-washing agent to enhance crude oil removal in a soil column system. The removal rates of 58 and 65 % were achieved using the biosurfactant solution with concentrations of 4 and 8 g/L, respectively. The results demonstrated the potential of marine microbial biosurfactants in cleaning crude oil-contaminated soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biosurfactants are a group of surface active molecules synthesized by microorganisms (Cai et al. 2015). They have amphipathic molecules that tend to accumulate at the interfaces between fluid phases with different polarities (e.g., oil-water or air-water); thus, they are capable of reducing surface tension (ST) and interfacial tension (Ghribi et al. 2012) between individual molecules. In addition, they are able to form emulsion where hydrocarbons can solubilize in water or where water can solubilize in hydrocarbons (Desai and Banat 1997; Joshi et al. 2008). In recent years, much attention has been directed toward biosurfactants due to their advantages such as lower toxicity, higher biodegradability, better environmental compatibility, stronger foaming ability, and selectivity than chemical surfactants (Pacwa-Plociniczak et al. 2011). They exhibit stable performance even at extreme temperature, pH and salinity, and the ability to be synthesized from renewable feedstocks (Ilori et al. 2005). Furthermore, biosurfactants have high surface activities together with low critical micelle concentrations (CMCs), in some cases even lower than most of the traditional chemical surfactants (Mulligan 2005). The aforementioned advantages allow their use and possible replacement of chemically synthesized surfactants in environmental and petro-chemical industries and as antimicrobial agents in health care and food-processing industries (Banat et al. 2000; Gudina et al. 2015a). In recent years, the application of biosurfactant has been regarded as a cost-effective and eco-friendly approach in environmental remediation such as soil washing (Mulligan et al. 2001; Silva and Sarubbo 2015; Urum et al. 2003). Although soil washing has been widely applied to remediate soil contaminated with crude oil, only a few studies focused on the removal of crude oil from contaminated soil through a soil-washing process with biosurfactants (Uhmann and Aspray 2012; Urum and Pekdemir 2004).
Biosurfactants display a wide variety of chemical structures including small-molecular-weight biosurfactants such as glycolipids, phospholipids, and lipopeptides and high-molecular-weight biosurfactants such as amphipathic, polysaccharides, proteins, lipopolysaccharides, and lipoproteins (Pacwa-Plociniczak et al. 2011). Surfactin is a lipopeptide biosurfactant mostly produced by Bacillus subtilis strains. Kuyukina et al. (2005) examined the enhanced crude oil desorption and dispersion through soil system with the injection of biosurfactant solution. Gudina et al. (2015b) and Pereira et al. (2013) reported the enhanced solubilization of crude oil from soil with the injection of biosurfactants and the reduction of ST to 27 mN/m. Surfactin also shows a high emulsifying activity and high antimicrobial, antiviral, and antitumor activities (Gudina et al. 2013). However, the biosurfactants produced by B. subtilis strains were not well commercialized mainly due to the high production cost (Marin et al. 2015). The expected breakthrough in terms of their applications remains to be achieved. Research indicated that proper selection of culture conditions for biosurfactant production, especially the carbon and nitrogen sources, can promote the production rate, thus reducing the production cost (e Silva N.M.P.R. et al. 2014; Fonseca et al. 2007). Aiming at production cost reduction and effectiveness improvement, selection of proper nutrient sources was suggested to be further explored (Daverey and Pakshirajan 2009; Reis et al. 2004; Saikia et al. 2014).
In this study, biosurfactant production by a B. subtilis strain previously isolated from Atlantic Ocean (Cai et al. 2014) was studied through a proper manipulation of carbon and nitrogen sources. Biosurfactant production with different media composition was investigated using evaluating parameters including ST, emulsification activity, and solution dilution as an indirect measurement of productivity. Biosurfactant product generated by the selected growth media was characterized for the composition with thin layer chromatography (TLC). The ionic charge of generated biosurfactant product and the stability was further studied. Finally, the effectiveness and applicability of the biosurfactant product in enhanced oil removal was evaluated.
2 Materials and Methods
2.1 Biosurfactant-Producing Microorganisms
The bacterium used in this study was screened in the Northern Region Persistent Organic Pollution Control (NRPOP) Laboratory from oily contaminated seawater samples, named as B. subtilis N3-4P (Cai et al. 2014). Bacillus strains are a group of well-recognized biosurfactant producers, which can lower the water ST to 27 mN/m. Among screened Bacillus strains in NRPOP lab, B. subtilis N3-4P was identified as a promising biosurfactant producer, whose products possessed strong surface activity and high emulsification capacity. B. subtilis 21332 was a well-known commercialized biosurfactant producer for the product of surfactin. It was obtained from the American Type Culture Collection (ATCC) as a performance comparison with lab-generated bacteria.
2.2 Media and Cultivation Conditions
The composition of the inoculum broth used was as follows: BD Difco™ Nutrient Broth 23400 (Fisher Scientific Company, Ottawa, Canada) 8.0 g and NaCl 5.0 g in 1 L of distilled water. A loopful of bacteria colony was transferred to 125-mL Erlenmeyer flask containing 50-mL inoculum broth. The culture was initially grown on a rotary incubator shaker (Thermo MaxQ 4000) at 200 rpm for 24 h under room temperature. This seeded culture media was used as inoculum at the 1 % (v/v) level. For biosurfactant production, a mineral salt medium modified from Cai et al. (2014) was listed as follows (g L−1): hexadecane (1 %), glucose (0.5) and sucrose (0.5), (NH4)2SO4 (10), NaCl (15), FeSO4‧7H2O (2.8 × 10−4), KH2PO4 (3.4), K2HPO4‧3H2O (4.4), MgSO4‧7H2O (1.02), yeast extract (0.5), and trace element solution 0.5 mL L−1 of distilled water. The trace element solution contained ZnSO4 (0.29), CaCl2 (0.24), CuSO4 (0.25), and MnSO4 (0.17) g L−1 of distilled water and was sterilized separately.
Carbon and nitrogen sources were added separately. In order to study the effect of carbon source on biosurfactant production, the original carbon source (hexadecane, glucose, and sucrose) in this growth media was replaced by sodium acetate (SA), sodium citrate (SC), glycerol (GLY), glucose (GLU), sucrose (SUC), starch (STA), n-hexadecane (HEX), and diesel (de Faria et al. 2011) separately at a concentration of 10 g L−1 or 1 % (v/v). Similarly, while exploring the nitrogen effect on biosurfactant production, the nitrogen sources in original recipe ((NH4)2SO4) and yeast extract were replaced by ammonium sulfate (AS), yeast extract (YE), and sodium nitrate (SN) at a concentration of 10 g L−1 separately.
The effect of different carbon and nitrogen sources on the production of biosurfactants was evaluated using ST, emulsification index (EI), and series solution dilution as an index of productivity, respectively. Medium without bacteria was used as the abiotic control. The selected carbon and nitrogen sources were further used for biosurfactant production by lab-screened bacteria B. subtilis N3-4P.
2.3 Biosurfactant Production and Purification
Selected biosurfactant production medium are listed as follows (g L−1) based on the results from Sect. 2.2: glycerol (10), nitrogen source (NH4)2SO4 (10), NaCl (15), FeSO4‧7H2O (2.8 × 10−4), KH2PO4 (3.4), K2HPO4‧3H2O (4.4), MgSO4‧7H2O (1.02), and yeast extract (0.5) as additive and trace element solution 0.5 mL L−1 of distilled water. The trace element solution contained ZnSO4 (0.29), CaCl2 (0.24), CuSO4 (0.25), and MnSO4 (0.17) g per 1 L of distilled water and was sterilized separately. Fermentation was performed in 1-L flasks containing 600-mL production medium. The medium was incubated in a shaking incubator at 200 rpm for 5 days. The culture broth was centrifuged at 12,000 rpm for 10 min to remove all the cells. Afterward, biosurfactant solution was further purified through solvent extraction with an equal volume of chloroform–methanol (1:2 v/v) solvent and the organic layer of liquid was collected. Crude biosurfactant product was concentrated by rotary evaporation and then subjected to freeze dry. Crude biosurfactant product was analyzed by TLC on silica gel plates to get its composition. The chemical content and ionic character of generated product was also determined.
2.4 Biosurfactant-Enhanced Soil Washing
Lab-scale, biosurfactant-enhanced soil-washing experiments were carried out in a bench-scale column as Fig. 1 illustrated. The soil was air dried, homogenized, and kept in an oven overnight at 105 °C. Physical and chemical characterization of the soil was performed in accordance with methods of soil analysis (Page 1982). The results presented in Table 1 suggested that the soil is a fine silty loam. The preparation of crude oil-contaminated soil was modified from Urum et al. (2003) and Banks and Schultz (2005). Five grams of crude oil was spiked into 1 kg of soil at room temperature, and the crude oil-contaminated soil was shaken vigorously for 30 min and allowed to age naturally for 1 week before use. Contaminated soil was then determined for its original oil concentration. Five-hundred grams of crude oil-contaminated soil sample was layered into a cylindrical column with a diameter of 3.8 cm and height of 30 cm. To prevent soil from being washed from the column, the bottom was covered with a layer of fiberglass. Additionally, a layer of glass beads with a 6-mm diameter were laid at the bottom and top of the soil column. Soil-washing experiments were conducted with lab-generated biosurfactant solutions at two different concentrations of 4 and 8 g L−1. A control experiment was run in parallel, where the soil was treated with distilled water. The biosurfactant solution or water was continuously pumped through the column for 8.5 h. The washing effluent from the column was collected and analyzed for flushed crude oil concentration. Soil samples were collected before and after the experiment for the removal rate.
2.5 Sample Analysis
ST
A 15-mL cell-free culture was subjected to the determination of ST in petri dish. The ST was measured by the ring method using a Du Nouy Tensiometer (CSC Scientific Company). To ensure the reliability of tested results, the average of two independent measurements were taken.
CMC
CMC is defined as the surfactant concentration necessary to initiate micelle formation. The CMC of generated biosurfactant product was determined by plotting the surface tensions as a function of biosurfactant concentration, and it was found from the intercept of two straight lines extrapolated from the concentration-dependent and concentration-independent sections as Fig. 2 illustrated (de Oliveira et al. 2013; Sheppard and Mulligan 1987).
Dilution of Biosurfactant Solution
Cell-free culture media with surface tension lower than 40 mN/m was further subjected to dilution test. The biosurfactant concentration was estimated by measuring the ST for varying dilutions (twofold, fivefold, and tenfold) of the sample (Das et al. 2009; Joshi et al. 2008). The dilution at which the ST began to increase indicated that the effective biosurfactant concentration exceeded the CMC (Ghurye et al. 1994). The dilution factor obtained was related to the crude surfactant concentration (Nitschke and Pastore 2006).
Emulsification Activity (EI24)
The emulsification activity of the culture broth was determined by addition of 2-mL culture aliquot to 2 mL hexadecane and vortexed for 2 min to create an optimum emulsion. Tests were performed in duplicate for quality assurance purpose, and the results were expressed using the average of two measurements.
By repeating the reading after 24 h, an indication of the stability of the emulsions will be obtained.
TLC
A 0.01 g of purified biosurfactant sample was dissolved in 1 mL methanol and was subjected to TLC analysis. Ten-microliter sample was applied to a 20 × 20 silica gel TLC plate (Sigma-Aldrich). Carbohydrate and lipid were developed in a chloroform:methanol:acetate acid (95:15:2) solvent system, and protein was developed in an n-butanol:acetic acid:water (4:3:0.5) solvent system. The spots were revealed with color reagents. For detection of amino acids, the dry plates were sprayed with a solution of 0.5 g ninhydrin in 100 mL acetone and kept at 105 °C for 5 min. Lipid content was visualized by iodine chamber. Carbohydrates were visualized by spraying phenol-sulfuchromic acid and heated at 105 °C for 5 min.
Composition Analysis
Of biosurfactant sample, 0.01 g was dissolved in 1 mL distilled water and then tested for its chemical composition. The protein content was determined by the method of Bradford (1976). Total carbohydrate content was estimated using the phenol–sulfuric acid method by Dubois et al. (1956). Lipid content was determined based on the method described by Pande et al. (1963).
Stability Characterization
The stability of generated biosurfactants was determined at different temperature, pH, and salinity following Abouseoud et al. (2008). Generally, 1 CMC of biosurfactant solution was prepared and maintained at a constant temperature of 0, 25, 50, 75, and 100 °C for 120 min and cooled at room temperature. Similarly, pH stability was determined by adjusting the pH value of biosurfactant solution to 2, 4, 6, 8, 10, and 12 using HCl or NaOH. The effect of salinity on the stability of the biosurfactant was investigated by adding NaCl at concentrations of 1, 2, 3, and 4 % (w/v). Stability was determined by the change of ST values in duplicate.
Ionic Charge Determination
The ionic charge of generated biosurfactants was characterized using agar double diffusion tests (Meylheuc et al. 2001). This test was based on the passive diffusion of two compounds bearing the same or opposite type’s charges in an agar plate. A low-hardness agar plate (1 %) was prepared with two regularly spaced rows of wells. The bottom hole was filled with lab-generated biosurfactant solution, and the upper well was filled with selected pure compound with known ionic charge. The appearance of a precipitation lines with known compounds indicated the ionic character of lab-generated biosurfactants. The selected anionic compounds, sodium dodecyl sulfate (SDS; Sigma-Aldrich) was prepared at a concentration of 20 mmol L−1. The cationic compounds barium chloride (Sigma-Aldrich) and cetyltrimethylammonium bromide (CTAB; Sigma-Aldrich) were prepared at 50 and 20 mmol L−1, respectively, following Meylheuc et al. (2001).
Chemical Analysis of Crude Oil in Soil
Soil samples were collected before and after the soil-washing process to test the crude oil concentration. Generally, soil samples were taken from three spots of the reactor and mixed well before test. The concentration of crude oil in collected soil was determined using the method adapted from Urum and Pekdemir (2004) and Han et al. (2009). Ten milliliter of hexane was mixed with 5 g of collected soil sample. The mixture was shaken laterally for 5 min, and all the n-hexane/crude oil extract was removed by centrifugation for 10 min at 3000 rpm. The extraction process was repeated for five times until the final extract had the same absorbance as that of the pure n-hexane. All five-time extract was collected into a volumetric flask and made up to 50 mL with n-hexane. Concentration of crude oil was determined by measuring the absorbance of extract at the wavelength of 229 nm at room temperature with Sigma spectrophotometer. Test was performed in duplicate. The concentration of crude oil in soil system was determined using equation as follows:
where O is the concentration of crude oil in soil (mg g−1 dry soil), A is the absorbance of the diluted crude oil/n-hexane solution at 229 nm, and m is the weight of soil collected (g).
The crude oil removal efficiency was determined using the following equation:
where O i is the initial crude oil concentration in the crude oil-contaminated soil (mg g−1 dry soil) before washing and O r is the residual crude oil concentration in the soil (mg g−1 dry soil) after washing.
Chemical Analysis of Crude Oil in Washing Solution
A 10 mL of soil-washing solution was collected every 30 min and analyzed for flushed crude oil concentration. Ten milliliter of n-hexane was added into the washing solution and was shaken laterally for 30 min. Samples were then centrifuged at 3000 rpm for 10 min. The centrifuged supernatant was analyzed for crude oil content using a spectrophotometer at 229 nm. The concentration of crude oil was then determined following the method mentioned previously.
2.6 Statistical Analysis
All the tests were performed in duplicate to ensure the reliability of results, and the results were expressed as the average of two measurements. Biosurfactant production and their performance were analyzed using OriginalPro 9.0 with paired t tests for the statistical evaluation of differences between treated groups and control. A p value that is less than 0.05 indicated the significant difference between the tested groups.
3 Experimental Results
3.1 Effects of Carbon and Nitrogen Sources on Biosurfactant Production
Different water-miscible and water-immiscible carbon substrates were investigated for their capacity to support bacterial growth and biosurfactant production by lab-screened marine origin bacteria B. subtilis N3-4P and one commercial bacterium B. subtilis 21332 in this work. Hexadecane and diesel were employed as water-insoluble carbon sources, and sodium acetate, sodium citrate, glucose, sucrose, starch, and glycerol were selected as water-soluble carbon sources. An important indication for the production of biosurfactant is the reduction of surface tension of growth medium (Youssef et al. 2004). The surface tension reduction ability of generated biosurfactants was illustrated in Fig. 3. A surface tension reduction range between 35 and 40 mN/m could indicate a promising biosurfactant production (Jaraz et al. 2012). From the figure, it can be found that both B. subtilis 21332 and B. subtilis N3-4P were hardly able to use carbon substrates such as sodium acetate and sodium carbonate for biosurfactant production, given the limited reduction of surface tension. In the meantime, limited biosurfactant production was detected by B. subtilis 21332 in the medium using diesel and hexadecane as carbon sources. Instead, other tested water-soluble carbon sources, such as glucose, sucrose, starch, and glycerol, were more preferred for its biosurfactant production. The surface tension of starch-based growth media can be reduced to as low as 28 mN/m. Biosurfactants produced by B. subtilis 21332 mostly displayed a good EI value; the one generated by glucose can reach to a value of 55.2 %. On the contrary, biosurfactant production by lab-screened B. subtilis N3-4P with glucose, sucrose, and starch as carbon sources was insignificant, whereas glycerol, hexadecane, and diesel are preferable. Among them, glycerol-based cell-free culture broth had the lowest surface tension with the value of 27.8 mN/m. This media also exhibited a highest EI value of 38.3 % among biosurfactant products generated by B. subtilis N3-4P.
The effect of nitrogen sources on biosurfactant production rate was also investigated in study, and the results were listed in Fig. 3. The studied nitrogen sources were classified into organic (yeast extract) and inorganic (sodium nitrate and ammonium sulfate) sources, and the role of nitrogen source in influencing biosurfactant production is quite evident. The organic nitrogen source yeast extract was a promising nitrogen source for both bacteria. A decrease in surface tension of the culture broth was observed for B. subtilis 21332 using AS- and SN-based growth media. However, neither of them assisted biosurfactant production by B. subtilis N3-4P. In addition, comparing the biosurfactant production by B. subtilis N3-4P using different carbon and nitrogen sources, it can be found that a biosurfactant production was observed in GLY-, HEX-, and DIE-based media using AS and yeast extract as co-nitrogen sources, and yeast-based media using hexadecane as carbon source, yet AS as sole nitrogen source with hexadecane as carbon source resulted in a poor biosurfactant production. Therefore, it can be concluded that a mixture of both organic and inorganic nitrogen sources can greatly promote the biosurfactant production and this combination was used for production test.
The selection of proper carbon sources are highly related with biosurfactant production rate (Panilaitis et al. 2007). Therefore, cell-free culture media with surface tension lower than 40 mN/m was further diluted for 2, 5, and 10 times and examined for surface tension reduction as an indirect measurement of the relative biosurfactant concentration in growth media. The recorded results were illustrated in Fig. 4. From the figure, it can be found that the effect of carbon source on biosurfactant production by B. subtilis N3-4P was as follows: glycerol > hexadecane > diesel. After 10 times of dilution, the concentration of biosurfactant in the glycerol-based cell-free solution was able to reduce its surface tension to lower than 40 mN/m. Sucrose, starch, and glycerol can be served as promising carbon sources for B. subtilis 21332. The surface tension of those carbon source based cell-free growth media were able to reduce to lower than 35 mN/m after 10 times of dilution. The most effective carbon source for B. subtilis 21332 was sucrose, whose surface tension remained unchanged event after 10 times of dilution. Yeast and AS were identified as a favorable nitrogen source for both Bacillus strains.
The mechanisms of carbon source utilization for biosurfactant production are closely related with selected bacteria. This study indicated that for strain B. subtilis N3-4P, biosurfactant production rate was much higher when using glycerol or hydrocarbon as carbon sources. Abouseoud et al. (2007) reported that the addition of carbohydrate was capable of reducing pH of growth media via promoting the production of secondary acid metabolites such as uronic acid. Biosurfactant production was therefore hindered. In this study, the pH of glucose-based media reduced to 3.53. This may explain the inhabitation of biosurfactant production by B. subtilis N3-4P using carbohydrate carbon source such as starch and sucrose in this study. The correlation of oily carbon source metabolism with biosurfactant production is well documented. On the other hand, bacteria B. subtilis 21332 was found to have an opposite preference on the selected carbon source. A poor biosurfactant production was discovered using hydrocarbon as their carbon sources, yet this rate was much higher on water-soluble carbon substrate. The ability of using water-soluble carbon sources for the production of biosurfactants was reported by previous studies (Fox and Bala 2000; Patel and Desai 1997). Research conducted by Abdel-Mawgoud et al. (2008) and Das et al. (2009) reported an inhibitory effect on the use of hydrocarbons (including n-hexadecane and diesel) for bacterial growth and biosurfactant production. In their study, glucose- and sucrose-based growth media had a better biosurfactant production rate. Similarly, Joshi et al. (2008) reported the uses of hydrocarbons as the sole carbon source resulted in no biosurfactant production. Besides that, addition of mineral contents is also important for biosurfactant production; the addition of nutrients such as yeast extract will simulate the production of biosurfactant even with the existence of hydrocarbons (Cai et al. 2014). In conclusion, various cheaper carbon sources can be used as an alternative to support the growth of lab-screened Bacillus strains for biosurfactant production. They can even be identified as an industry waste. For instance, glycerol is a by-product of biodiesel industry, starch and glucose can be widely found in agro-industrial waste, and sucrose commonly existed in sugar-processing industry (Das et al. 2009). A proper selection of corresponding industrial waste can further reduce the production cost.
Research indicated that the conditions of nitrogen metabolism played an important role in surfactin production (Davis et al. 1999). Many different sources of nitrogen had been investigated for biosurfactant production, and the most frequently used substrates were nitrate salts and ammonia. Among the inorganic salts tested, nitrate ions supported maximum biosurfactant production in B. subtilis (Makkar and Cameotra 1998). A recent research conducted by Abdel-Mawgoud et al. (2008) indicated that sodium nitrate was the best nitrogen source for surfactin production, while other tested nitrogen sources decreased surfactin production with different degrees. Although utilization of NaNO3 as nitrogen source greatly simulated biosurfactant production by B. subtilis 21332, its performance was inferior to that of AS for lab screened bacteria B. subtilis N3-4P. In this study, the production of biosurfactant by B. subtilis N3-4P only occurred with the addition of yeast extract. Otherwise, neither inorganic nitrogen source was able to support biosurfactant production by B. subtilis N3-4P. This is probably due to the lack of some essential nutrients (Gudiña et al. 2011). Qazi et al. (2013) believed that yeast extract that contained amine groups either triggered the biosynthesis of peptide-containing biosurfactant, such as lipopeptide, or stimulated the growth of the enzymes regulating the biosynthesis of other biosurfactant type. This explained the importance of yeast extract in growth media. In contrast to the carbon source used in biotechnological processes, complex or less well-defined sources of nitrogen (e.g., yeast extract or protein hydrolysates) are relatively less researched, yet proved to have promising productivity as Fig. 4 illustrated. The utilization of protein hydrolysates as an alternative for nitrogen source was attractive for biosurfactant production. In conclusion, a balance between organic and inorganic nitrogen sources should be considered for biosurfactant synthesis by microorganisms (Ghribi and Ellouze-Chaabouni 2011). Ammonium sulfate and yeast extract were therefore used as nitrogen sources for biosurfactant production and characterization by B. subtilis N3-4P. Glycerol was used as the carbon source.
3.2 Characterization of Lab-Generated Biosurfactant Product
Biosurfactant product generated by lab-screened bacteria B. subtilis N3-4P was further characterized for its chemical composition and stability using glycerol as the selected carbon source and (NH4)2SO4 and yeast extract as nitrogen sources. The biosurfactant product was able to reduce the surface tension of distilled water from 72 to 27 mN/m. The CMC value of the product was determined by separately measuring the surface tension of different concentrations of the product, and the value was 0.507 g L−1.
A TLC analysis indicated that the biosurfactant product was a mixture of carbohydrate, lipid, and protein. This mixture contained 21 % (w/w) of protein, a 35 % (w/w) of lipid, and 18 % of carbohydrate (w/w). This result indicated that the product was very likely to be a mixture of lipopeptide and glycolipid biosurfactants. No precipitation lines were observed between lab-generated biosurfactants and selected chemical compounds (barium chloride, CTAB, SDS). Therefore, lab-generated biosurfactant product had non-ionic character. Mulligan (2005) and Cameotra and Makkar (1998) also proved that most of the biosurfactants were either with neutral or anionic character.
Biosurfactant stability at difference environment conditions, such as various temperature, pH, and salinity, are highly related with its applicability in the fields. Therefore, the stability of biosurfactant product generated by B. subtilis N3-4P was tested over a wide range of temperature, pH value, and salinity (Fig. 5). Enhanced surface tension reduction ability during heating process was observed in this study. An enhanced surface activity of biosurfactant product was observed as the increase of temperature. Lab-generated biosurfactant solution achieved the lowest surface tension when temperature reaches 100 °C, yet it still had a remarkable surface tension reduction capacity even at 0 °C. Therefore, it can be concluded that this product maintains its surface properties unaffected in the range of temperatures between 0 and 100 °C. The activity of lab-generated biosurfactant was low at a pH value of 2. Surface tension of generated biosurfactants decreased with the increase of pH value, indicating that the products had a better stability at a higher pH level. The unchanged surface tension of biosurfactant solution under various salinities demonstrating generated biosurfactant has a stable performance between salinity of 1–4 %.
3.3 Biosurfactant-Enhanced Soil Washing for Crude Oil Removal
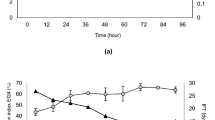
Figure 6 illustrated the effect of biosurfactant concentration on enhanced crude oil removal in soil-washing systems. From this figure, it can be found that both biosurfactant solutions significantly enhanced the removal rate of crude oil as compared to control column using distilled water as washing agent. Paired t test result (p < 0.05) indicated that biosurfactant is highly effective in cleanup crude oil in soil system. Biosurfactant concentration with 4 g/L had a lower crude oil removal rate and a longer washing time than the one with 8 g/L. It can also be found that biosurfactant-enhanced aqueous systems were much faster to reach saturation than control. As the biosurfactant concentration was increased, an improved percentage of crude oil removal was observed. With the application of 4 and 8 g/L crude biosurfactant solutions, 58 % and 65.2 % of crude oil can be removed from soil system, respectively, while the control system (water only) can only clean up 36.9 % crude oil in the contaminated soil.
Given that crude oil was complex in nature and compose over 50–80 % aliphatic hydrocarbons, the cleanup of crude oil-contaminated soils required more efforts compared with other petroleum-contaminated soils (NRC 1985). The result obtained in this study was expected, and in accordance with previous studies, Lai et al. (2009) examined biosurfactant-enhanced total petroleum hydrocarbon (TPH) removal from low TPH-contaminated (LTC) soil (3 mg/g) and high TPH-contaminated (HTC) soil (9 mg/g). Their result indicated that biosurfactant had a better performance when treating HTC soil. Addition of rhamnolipid and surfactin solution can increase the removal rate from 20.4 to over 60 % from HTC soil. This study suggested that lab-generated biosurfactants can be used as an effective washing agent to clean up crude oil in soil system. Urum et al. (2006) provided some insight on the removal of crude oil from soil system. Their research indicated that the preference for crude oil removal highly depended on selected surfactant. A significant amount of oil compounds can be removed from the system; however, high-molecular-weight aromatic hydrocarbons such as dibenzothiophenes, an organic compound widely used in heavier fractions of petroleum, can hardly be removed by studied surfactants. Similar conclusion was confirmed by Zhang (2015) as well. This study helped to explain the relationship between residue oil in soil column and limited oil concentration in eluent in this study.
In this study, compared with using distilled water as washing agent, crude oil removal rate was significantly increased using biosurfactant-based washing solution. The mechanism of biosurfactant-enhanced crude oil removal is closely related with its concentration. When the concentration of biosurfactant solution is below its CMC value, the mechanism of biosurfactant-enhanced crude oil removal mainly relied on the reduced surface and interfacial tension at the water-air and crude oil-water interface due to its amphiphilic structure (Abdul et al. 1990). The lowered interfacial tension thus led to an increase contact angle and reduced capillary force holding the crude oil and soil particles and consequently enhanced the mobility of crude oil (Kavitha et al. 2014; Pacwa-Plociniczak et al. 2011). This is also known as the mobilization mechanism (Pacwa-Plociniczak et al. 2011). When the concentration of biosurfactant is above its CMC value, the formation of biosurfactant micelle can greatly increase the solubilization process and help to solubilize the residue oil compounds left in the soil system and enhanced the removal of organic contaminants (Urum and Pekdemir 2004). Moreover, a recent research conducted by Zhang et al. (2014) indicated that an increased structural disjoining pressure in the wedge film caused by biosurfactant micelles is another reason for the promotion to detach oil droplets from soil surface. The extent of this pressure is correlated with the micelle size, particle size, and surface charge of particles (Zhang et al. 2014). Therefore, it can be concluded that the enhanced solubilization and the structural disjoining pressure was the major reason for crude oil removal in this study. The increased concentration of biosurfactant would accelerate the formation of micelles in system, and those micelles could replace the biosurfactant monomers adsorbed to the soil, and increase the effective biosurfactant concentration in the system. In this study, compared with using 4 g/L of biosurfactant solution as washing agent, a higher crude oil removal rate was reported using the biosurfactant solution at 8 g/L.
Last but not least, the removal of hydrocarbon from soil system was reported to be closely related with soil texture and mineralogy (Lee et al. 2002). The reported removal rate in this study was lower than the ones treated with sandy soils. Researches also proved that biosurfactants had a better performance in sandy soils (Lee et al. 2001). The effectiveness of surfactant-based remediation can be limited by adsorption of surfactants to clay, silt, and organic soil contents (Lee et al. 2001). Furthermore, given that most of the soil surface was negatively charged, the adsorption process was even worse for the cationic surfactants. They tended to a higher affinity on the soil particles, thus affecting its removal efficiency. Lab-generated biosurfactants by B. subtilis N3-4P were proved to have a non-ionic character, thus believed to have a better performance. This result was proved by other studies. Kavitha et al. (2014) and Zhang (2015) found that the solubility of crude oil was proved to be proportional to the concentration of biosurfactants with a non-ionic nature, such as rhamnolipid. Urum and Pekdemir (2004) found that with the injection of rhamnolipid biosurfactant at 25 CMCs, the removal rate of crude rate can reach up to 80 %. In this study, 58 and 65.2 % of crude oil were removed from soil system using biosurfactant solution at 4 and 8 g/L, respectively. Considering that the lab-generated biosurfactant is a non-ionic lipopeptide complex, a higher removal rate is expected when a higher concentration of biosurfactant solution was adopted as washing agent.
4 Conclusion
The enhanced biosurfactant production by marine-originated bacteria B. subtilis under different carbon and nitrogen sources were studied by comparing the ST, EI, and ST after several times of dilution. The results proved the capability of marine bacteria B. subtilis N3-4P in producing biosurfactant, which was a mixture of lipopeptide and glycolipid. The production rate and emulsion capacity were compatible with those generated by commercial strain B. subtilis 21332. The highest production rate was achieved when using glycerol as carbon source and yeast extract and sodium nitrate as nitrogen sources. The biosurfactant solution could reduce the surface tension of distilled water to as low as 27 mN/m, with a CMC value of 500 mg/L. Even after dilution for 10 times, a surface tension of 36.4 mN/m was still observed. The biosurfactant product was found to have non-ionic character and had a stable performance with a duration up to 24 h at various temperature (0–100 °C), pH (2–8), and salinity (1–4 %) values.
This study further evaluated the effectiveness and applicability of the generated biosurfactants in crude oil soil washing. The results showed that the removal rates reached at 58 and 65.2 % by introducing the generated biosurfactants with the concentrations of 4 and 8 g/L, respectively. A paired t test with p value less than 0.05 indicated that biosurfactant is highly effective in cleanup crude oil in soil system. In comparison, only 36.9 % of crude oil were washed out by water only. Given the adsorption of anionic surfactant onto negatively charged soil particles, the injection of non-ionic Bacillus biosurfactant was considered as more suitable for soil-washing agent since they were less likely to be adsorbed to the soil and thus were mobile and effective. Overall, the results demonstrated the potential of Bacillus biosurfactants for applications in petroleum-contaminated site remediation. Ongoing studies are being carried out analyzing soil samples and effluent for residue components to better understand the removal mechanism by using biosurfactant as a soil-washing agent.
References
Abdel-Mawgoud, A. M., Aboulwafa, M. M., & Hassouna, N. A. H. (2008). Optimization of surfactin production by Bacillus subtilis isolate BS5. Applied Biochemistry and Biotechnology, 150(3), 305–325.
Abdul, A. S., Gibson, T. L., & Rai, D. N. (1990). Selection of surfactants for the removal of petroleum-products from shallow sandy aquifers. Ground Water, 28(6), 920–926.
Abouseoud, M., Maachi, R., & Amrane, A. (2007). Biosurfactant production from olive oil by Pseudomonas fluorescens. Comm. Curr. Res. Educ. Top. Trends Appl. Microbiol, 1, 340–347.
Abouseoud, M., Yataghene, A., Amrane, A., & Maachi, R. (2008). Biosurfactant production by free and alginate entrapped cells of Pseudomonas fluorescens. Journal of Industrial Microbiology & Biotechnology, 35(11), 1303–1308.
Banat, I. M., Makkar, R. S., & Cameotra, S. S. (2000). Potential commercial applications of microbial surfactants. Applied Microbiology and Biotechnology, 53(5), 495–508.
Banks, M., & Schultz, K. (2005). Comparison of plants for germination toxicity tests in petroleum-contaminated soils. Water, Air, and Soil Pollution, 167(1–4), 211–219.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Cai, Q., Zhang, B., Chen, B., Song, X., Zhu, Z., & Cao, T. (2015). Screening of biosurfactant-producing bacteria from offshore oil and gas platforms in North Atlantic Canada. Environmental monitoring and assessment, 187(5), 4490.
Cai, Q., Zhang, B., Chen, B., Zhu, Z., Lin, W., & Cao, T. (2014). Screening of biosurfactant producers from petroleum hydrocarbon contaminated sources in cold marine environments. Marine Pollution Bulletin, 86(1–2), 402–410.
Cameotra, S., & Makkar, R. (1998). Synthesis of biosurfactants in extreme conditions. Applied Microbiology and Biotechnology, 50(5), 520–529.
Das, P., Mukherjee, S., & Sen, R. (2009). Substrate dependent production of extracellular biosurfactant by a marine bacterium. Bioresource Technology, 100(2), 1015–1019.
Daverey, A., & Pakshirajan, K. (2009). Production of sophorolipids by the yeast Candida bombicola using simple and low cost fermentative media. Food Research International, 42(4), 499–504.
Davis, D. A., Lynch, H. C., & Varley, J. (1999). The production of surfactin in batch culture by Bacillus subtilis ATCC 21332 is strongly influenced by the conditions of nitrogen metabolism. Enzyme and Microbial Technology, 25(3–5), 322–329.
de Faria, A. F., Teodoro-Martinez, D. S., de Oliveira Barbosa, G. N., Vaz, B. G., Silva, I. S., Garcia, J. S., Tótola, M. R., Eberlin, M. N., Grossman, M., & Alves, O. L. (2011). Production and structural characterization of surfactin (C 14/Leu 7) produced by Bacillus subtilis isolate LSFM-05 grown on raw glycerol from the biodiesel industry. Process Biochemistry, 46(10), 1951–1957.
de Oliveira, D. W. F., Franca, I. W. L., Felix, A. K. N., Martins, J. J. L., Giro, M. E. A., Melo, V. M. M., & Goncalves, L. R. B. (2013). Kinetic study of biosurfactant production by Bacillus subtilis LAMI005 grown in clarified cashew apple juice. Colloids and Surfaces B-Biointerfaces, 101, 34–43.
Desai, J. D., & Banat, I. M. (1997). Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews, 61(1), 47–64.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
e Silva, N.M.P.R., Rufino, R.D., Luna, J.M., Santos, V.A., Sarubbo, L.A. 2014. Screening of Pseudomonas species for biosurfactant production using low-cost substrates. Biocatalysis and Agricultural Biotechnology, 3(2), 132–139
Fonseca, R., Silva, A., De França, F., Cardoso, V., & Sérvulo, E. (2007). Optimizing carbon/nitrogen ratio for biosurfactant production by a Bacillus subtilis strain. Applied biochemistry and biotechnology, 137(1–12), 471–486.
Fox, S. L., & Bala, G. A. (2000). Production of surfactant from Bacillus subtilis ATCC 21332 using potato substrates. Bioresource Technology, 75(3), 235–240.
Ghribi, D., Abdelkefi-Mesrati, L., Mnif, I., Kammoun, R., Ayadi, I., Saadaoui, I., Maktouf, S., Chaabouni-Ellouze, S. 2012. Investigation of antimicrobial activity and statistical optimization of Bacillus subtilis SPB1 biosurfactant production in solid-state fermentation. Journal of Biomedicine and Biotechnology.
Ghribi, D., Ellouze-Chaabouni, S. 2011. Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnology research international, 2011.
Ghurye, G. L., Vipulanandan, C., & Willson, R. C. (1994). A practical approach to biosurfactant production using nonaseptic fermentation of mixed cultures. Biotechnology and Bioengineering, 44(5), 661–666.
Gudiña, E.J., Teixeira, J.A., Rodrigues, L.R. 2011. Biosurfactant-producing lactobacilli: screening, production profiles, and effect of medium composition. Applied and Environmental Soil Science, 2011.
Gudina, E.J., Fernandes, E.C., Rodrigues, A.I., Teixeira, J.A., Rodrigues, L.R. 2015a. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Frontiers in Microbiology, 6
Gudina, E. J., Pereira, J. F. B., Costa, R., Coutinho, J. A. P., Teixeira, J. A., & Rodrigues, L. R. (2013). Biosurfactant-producing and oil-degrading Bacillus subtilis strains enhance oil recovery in laboratory sand-pack columns. Journal of Hazardous Materials, 261, 106–113.
Gudina, E. J., Rodrigues, A. I., Alves, E., Rosario Domingues, M., Teixeira, J. A., & Rodrigues, L. R. (2015a). Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresource Technology, 177, 87–93.
Han, M., Ji, G., & Ni, J. (2009). Washing of field weathered crude oil contaminated soil with an environmentally compatible surfactant, alkyl polyglucoside. Chemosphere, 76(5), 579–586.
Ilori, M. O., Amobi, C. J., & Odocha, A. C. (2005). Factors affecting biosurfactant production by oil degrading Aeromonas spp. isolated from a tropical environment. Chemosphere, 61(7), 985–992.
Jaraz, A., Alencarz, A., de Campos-Takaki, G., Gusmao, N. 2012. Property and stability. Microbes in Applied Research: Current Advances and Challenges, 358.
Joshi, S., Bharucha, C., Jha, S., Yadav, S., Nerurkar, A., & Desai, A. J. (2008). Biosurfactant production using molasses and whey under thermophilic conditions. Bioresource Technology, 99(1), 195–199.
Kavitha, V., Mandal, A. B., & Gnanamani, A. (2014). Microbial biosurfactant mediated removal and/or solubilization of crude oil contamination from soil and aqueous phase: an approach with Bacillus licheniformis MTCC 5514. International Biodeterioration & Biodegradation, 94, 24–30.
Kuyukina, M. S., Ivshina, I. B., Makarov, S. O., Litvinenko, L. V., Cunningham, C. J., & Philp, J. C. (2005). Effect of biosurfactants on crude oil desorption and mobilization in a soil system. Environment International, 31(2), 155–161.
Lai, C. C., Huang, Y. C., Wei, Y. H., & Chang, J. S. (2009). Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. Journal of Hazardous Materials, 167(1–3), 609–614.
Lee, D. H., Cody, R. D., & Hoyle, B. L. (2001). Laboratory evaluation of the use of surfactants for ground water remediation and the potential for recycling them. Ground Water Monitoring and Remediation, 21(1), 49–57.
Lee, D. H., Cody, R. D., Kim, D. J., & Choi, S. (2002). Effect of soil texture on surfactant-based remediation of hydrophobic organic-contaminated soil. Environment International, 27(8), 681–688.
Makkar, R. S., & Cameotra, S. S. (1998). Production of biosurfactant at mesophilic and thermophilic conditions by a strain of Bacillus subtilis. Journal of Industrial Microbiology & Biotechnology, 20(1), 48–52.
Marin, C. P., Kaschuk, J. J., Frollini, E., & Nitschke, M. (2015). Potential use of the liquor from sisal pulp hydrolysis as substrate for surfactin production. Industrial Crops and Products, 66, 239–245.
Meylheuc, T., van Oss, C. J., & Bellon-Fontaine, M. N. (2001). Adsorption of biosurfactant on solid surfaces and consequences regarding the bioadhesion of Listeria monocytogenes LO28. Journal of Applied Microbiology, 91(5), 822–832.
Mulligan, C. N. (2005). Environmental applications for biosurfactants. Environmental Pollution, 133(2), 183–198.
Mulligan, C. N., Yong, R. N., & Gibbs, B. F. (2001). Surfactant-enhanced remediation of contaminated soil: a review. Engineering Geology, 60(1–4), 371–380.
Nitschke, M., & Pastore, G. M. (2006). Production and properties of a surfactant obtained from Bacillus subtilis grown on cassava wastewater. Bioresource Technology, 97(2), 336–341.
NRC, N.R.C. 1985. Oil in the sea: inputs, fates, and effects. National Academies.
Pacwa-Plociniczak, M., Plaza, G. A., Piotrowska-Seget, Z., & Cameotra, S. S. (2011). Environmental applications of biosurfactants: recent advances. International Journal of Molecular Sciences, 12(1), 633–654.
Page, A.L. 1982. Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Soil Science Society of America.
Pande, S. V., Venkitasubramaniam, T. A., & Khan, R. P. (1963). Microdetermination of lipids and serum total fatty acids. Analytical Biochemistry, 6(5), 415–423.
Panilaitis, B., Castro, G., Solaiman, D., & Kaplan, D. (2007). Biosynthesis of emulsan biopolymers from agro-based feedstocks. Journal of applied microbiology, 102(2), 531–537.
Patel, R. M., & Desai, A. J. (1997). Biosurfactant production by Pseudomonas aeruginosa GS3 from molasses. Letters in Applied Microbiology, 25(2), 91–94.
Pereira, J. F. B., Gudina, E. J., Costa, R., Vitorino, R., Teixeira, J. A., Coutinho, J. A. P., & Rodrigues, L. R. (2013). Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel, 111, 259–268.
Qazi, M.A., Malik, Z.A., Qureshi, G.D., Hameed, A., Ahmed, S. 2013. Yeast extract as the most preferable substrate for optimized biosurfactant production by rhlB gene positive Pseudomonas putida SOL-10 isolate. Journal of Bioremediation & Biodegradation, 2013.
Reis, F., Servulo, E. F. C., & De Franca, F. P. (2004). Lipopeptide surfactant production by Bacillus subtilis grown on low-cost raw materials. Applied Biochemistry and Biotechnology, 113, 899–912.
Reis, R. S., Pereira, A. G., Neves, B. C., & Freire, D. M. (2011). Gene regulation of rhamnolipid production in Pseudomonas aeruginosa—a review. Bioresource technology, 102(11), 6377–6384.
Saikia, R. R., Deka, H., Goswami, D., Lahkar, J., Borah, S. N., Patowary, K., Baruah, P., & Deka, S. (2014). Achieving the best yield in glycolipid biosurfactant preparation by selecting the proper carbon/nitrogen ratio. Journal of Surfactants and Detergents, 17(3), 563–571.
Sheppard, J. D., & Mulligan, C. N. (1987). The production of surfactin by Bacillus subtilis grown on peat hydrolysate. Applied Microbiology and Biotechnology, 27(2), 110–116.
Silva, E. J., & Sarubbo, L. A. (2015). Application of bacterial and yeast biosurfactants for enhanced removal and biodegradation of motor oil from contaminated sand.
Uhmann, A., & Aspray, T. J. (2012). Potential benefit of surfactants in a hydrocarbon contaminated soil washing process: fluorescence spectroscopy based assessment. Journal of hazardous materials, 219, 141–147.
Urum, K., Grigson, S., Pekdemir, T., & McMenamy, S. (2006). A comparison of the efficiency of different surfactants for removal of crude oil from contaminated soils. Chemosphere, 62(9), 1403–1410.
Urum, K., & Pekdemir, T. (2004). Evaluation of biosurfactants for crude oil contaminated soil washing. Chemosphere, 57(9), 1139–1150.
Urum, K., Pekdemir, T., & Gopur, M. (2003). Optimum conditions for washing of crude oil-contaminated soil with biosurfactant solutions. Process Safety and Environmental Protection, 81(B3), 203–209.
Youssef, N. H., Duncan, K. E., Nagle, D. P., Savage, K. N., Knapp, R. M., & McInerney, M. J. (2004). Comparison of methods to detect biosurfactant production by diverse microorganisms. Journal of Microbiological Methods, 56(3), 339–347.
Zhang, H., Nikolov, A., & Wasan, D. (2014). Enhanced oil recovery (EOR) using nanoparticle dispersions: underlying mechanism and imbibition experiments. Energy & Fuels, 28(5), 3002–3009.
Zhang, W. (2015). Batch washing of saturated hydrocarbons and polycyclic aromatic hydrocarbons from crude oil contaminated soils using bio-surfactant. Journal of Central South University, 22(3), 895–903.
Acknowledgments
The authors would like to express their gratitude to Natural Science and Engineering Research Council of Canada (NSERC), Canada Foundation for Innovation (CFI), and Research and Development Corporation (RDC) of Newfoundland and Labrador for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Z., Zhang, B., Chen, B. et al. Biosurfactant Production by Marine-Originated Bacteria Bacillus Subtilis and Its Application for Crude Oil Removal. Water Air Soil Pollut 227, 328 (2016). https://doi.org/10.1007/s11270-016-3012-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3012-y