Abstract
Production of biosurfactant by free and alginate-entrapped cells of Pseudomonas fluorescens Migula 1895-DSMZ was investigated using olive oil as the sole carbon and energy source. Biosurfactant synthesis was followed by measuring surface tension and emulsifying index E24 over 5 days at ambient temperature and at neutral pH. Diffusional limitations in alginate beads affected the kinetics of biosurfactant production when compared to that obtained with free cells culture. Nevertheless, the emulsion stability was improved and fewer by-products interfered with the biosurfactant activity. A decrease in pH down to 5 in the case of immobilized cells was observed during the first 3 days, after which it returned to its initial value. The minimum values of surface tension were 30 and 35 dynes cm−1 achieved after 40 and 72 h with free and immobilized cells, respectively, while the corresponding maximum E24 values were 67 and 62%, respectively. After separation by acetone precipitation, the biosurfactant showed a rhamnolipid-type in nature, and had a good foaming and emulsifying activities. The critical micellar concentration was found to be 290 mg l−1. The biosurfactant also showed good stability during exposure to high temperatures (up to 120 °C for 15 min), to high salinity (10% NaCl) and to a wide range of pH (4–9).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
To improve substrate availability, some microorganisms produce amphipathic molecules to reduce the surface tension between phases of different polarities, such as oil and water. Biosurfactants are produced by a wide variety of bacteria, yeast, and filamentous fungi. They include peptides, glycolipids, glycopeptides, fatty acids and phospholipids [3]. These molecules have tremendous potential for applications in the pharmaceutical, cosmetics, and food industries, as emulsifiers, surfactants, and dispersants. Indeed, they show many advantages over chemical surfactants as regards biodegradability, low toxicity, and effectiveness at extreme temperatures, pH, or salinity [4, 17]. Nevertheless, biosurfactant production could be limited by several factors such as limited yield, recovery, and purification difficulties, as well as a limited productivity of batch process or occurrence of substrate inhibition [7]. The development of this area of research is of paramount importance, mainly in view of the present concern with the protection of the environment. Therefore, the most significant advantage of a microbial surfactant over chemical surfactants is its ecological acceptance, because it is biodegradable and nontoxic to natural environments [3, 7, 12].
Biosurfactant production optimization appears to be one of the main fields of research, owing to the low product yields. In this aim, the adequate culture medium, as well as the optimal conditions and mode of culture, are of major importance. Because of the low produced amounts, biosurfactant recovery from the culture medium account significantly in the final cost production. Moreover, the amphiphilic characteristic of these molecules increased the problem of separation, which is generally carried out by precipitation, organic extraction, chromatographic adsorption or ultrafiltration [4]. In this aim, immobilization of living cells in porous support offers enormous advantages in continuous production of biosurfactant. It is an efficient way to reduce the cost of product recovery, as the growth and the product formation phases can be separated and substrate inhibition could be avoided owing to diffusional limitations [23, 25, 26].
Entrapment in insoluble calcium alginate is recognized as a rapid, nontoxic, inexpensive, versatile method, which was successfully used for biosurfactant production [10, 23].
Pseudomonas is one of the most often reported genus for its availability to produce biosurfactant molecules to help in the degradation of several substrates, including glycerol, mannitol, fructose, glucose, n-paraffins, and vegetable oils [15, 20]. The objective of this work was therefore to examine the feasibility and effect of immobilization of Pseudomonas fluorescens on the kinetics of biosurfactant production in comparison to those obtained with free cells. The product will be separated and characterized for its surface activity and stability.
Materials and methods
Organism
Pseudomonas fluorescens Migula 1895 from DSMZ (Braunschweig, Germany) was used in the present study and maintained on nutrient agar.
Media and cultivation conditions
Nutrient agar was used for inoculum preparation. The composition of the nutrient agar used was as follows (g l−1): beef extract 1.0, yeast extract 2.0, peptone 5.0, NaCl 5.0, agar 15.0 g. Two loops of agar culture were used to inoculate 50 ml of nutrient broth (Difco). Seed culture was carried out for 16–18 h on a rotary shaker at room temperature. An aliquot of inoculum was used to inoculate culture medium at 2% (v/v) level.
For biosurfactant synthesis, a mineral salt medium (M) with the following composition (g l−1) was used: Na2HPO4 2.2, KH2 PO4 1.4, MgSO4·7H2O 0.6, FeSO4·7H2O 0.01, NaCl 0.05, CaCl2 0.02, yeast extract 0.02, and 0.1 ml of trace element solution (g l−1) containing the following: ZnSO4·7H2O 2.32, MnSO4·4H2O 1.78, H3BO3 0.56, CuSO4·5H2O 1.0, Na2MoO4·2H2O 0.39, CoCl2·6H2O 0.42, NiCl2·6H2O 0.004, KI 0.66, and EDTA 1.0. The pH of the medium was adjusted to 7.0 ± 0.2.
The medium was optimized in a previous study for carbon and energy source (C), nitrogen source (N) and C/N ratio, respectively, as follows [1]: 2% (v/v) olive oil and 1 g l−1 ammonium nitrate, leading to a C/N ratio of 10.
Cultivations were performed in 250-ml flasks containing 50 ml medium at room temperature and stirred on a rotary shaker (GFL 3500 Burgwedel, Germany) at 150 rpm.
Immobilization technique
The culture broth was centrifuged at 10,000×g for 30 min, and the pellets were used for immobilization. Alginates (Sigma-Aldrich Chemie, Saint-Quentin Fallavier, France) were thoroughly mixed with hot distilled water, and then autoclaved. The mixture was cooled to about 30–40 °C and then added to a solution of about 1.5 g wet cell weight per 100 ml of alginate solution. The resulting mixture was dropped into a solution containing CaCl2 0.05 M, and slowly stirred for 1 h to form beads.
The spherical (approximately 2 mm diameter) formed beads were kept under agitation for about 2 h to harden. The beads were finally washed with sterilized water and used for immobilized cells experiments or conserved at 4 °C in a buffer solution.
The modified culture medium (M) for the immobilized cells contained (g l−1 of distilled water) the following: KH2PO4 (0.2), K2HPO4 (0.1) and CaCl2 (0.01).
Biomass and pH measurements
The dry weight technique was used to quantify microbial growth as bacterial density through the culture’s absorbance at 600 nm using a UV–Vis spectrophotometer (Jenway 6305, Dunmow, Essex, England). The biomass obtained after filtration on a 0.2 μm Millipore (Saint-Quentin-en-Yvelines, France) was dried overnight at 105 °C and weighed. The pH of the supernatant was measured with a digital pH-meter (InoLab, WTW, Weilheim, Allemagne).
Surface tension measurement
The surface tension measurement of cell-free supernatants was determined in a K6 tensiometer (Krüss GmbH, Hamburg, Germany), using the du Nouy ring method. The values reported are the mean of three measurements. All measurements were made on cell-free broth obtained after culture centrifugation at 10,000×g for 25 min.
Emulsification index (E24)
The E24 of culture samples was determined by adding 2 ml of a hydrocarbon (gasoil) to the same amount of culture, mixing with a vortex for 2 min, and leaving to stand for 24 h. The E24 index corresponded to the percentage of the height of the emulsified layer (mm) divided by the total height of the liquid column (mm) [6].
Biosurfactant production kinetics
The kinetics of biosurfactant production by free and immobilized cells were followed in batch cultures during 120 h at the optimal conditions, by measuring surface tension and emulsification index E24 of supernatant samples obtained after removing cells.
Biosurfactant recovery
The culture broth was centrifuged (10,000×g for 15 min) to remove the cells and thereafter sterilized with Millipore membrane filter. The clear sterile supernatant served as the source of crude biosurfactant. The biosurfactant was recovered from the cell-free culture supernatant by cold acetone precipitation as described by Pruthi and Cameotra [19]. The pH of the biosurfactant cell-free solution was adjusted to pH 11 before acetone addition to eliminate secondary metabolites by precipitation. Three volumes of chilled acetone was added and allowed to stand for 10 h at 4 °C. The precipitate was collected by centrifugation and evaporated to dryness to remove residual acetone; it was thereafter redissolved in sterile water.
Biosurfactant characterization
Structural characterization—Rhamnose test
The presence of carbohydrate groups in the biosurfactant molecule was assayed by the rhamnose test using the method of Dubois et al. [8]. A volume of 0.5 ml of cell supernatant was mixed with 0.5 ml of 5% phenol solution and 2.5 ml of sulphuric acid, incubated for 15 min before measuring the absorbance at 490 nm.
Activity characterization
Foaming and emulsifying properties
The foam was produced by hand-shaking 5 g l−1 of crude biosurfactant solution for several minutes. The stability of the foam was monitored during 2 h.
The ability of the biosurfactant to emulsify some liquid hydrocarbons, such as diesel oil, kerosene, n-heptane, and sunflower oil, was determined. The sterile biosurfactant (2 ml) was added into each test tube (in a set of three) containing the substrate (2 ml). The content of the tubes were mixed at high speed for 2 min and left undisturbed for 24 h. The emulsification index (E24) was determined as the height of the emulsion layer divided by the total height and multiplied by 100.
Critical micellar concentration
The critical micellar concentration (CMC) corresponded to the concentration of an amphiphilic component at which the formation of micelles is initiated in the solution.
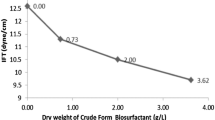
Purified biosurfactant from P. fluorescens was dissolved in aqueous solutions at concentrations ranging from 0 to 6 g l−1. The CMC was determined by plotting the surface tension as a function of the biosurfactant concentration [5, 13]. For each concentration, surface tension measurement was carried out until a constant value was reached.
Stability characterization
It was based on the determination of temperature, pH, and NaCl effects on the activity of the biosurfactant. To determine the thermal stability of the biosurfactant, cell-free broth was maintained at a constant temperature in the range 20–100 °C for 15 min and cooled at room temperature. To determine the effect of pH on the activity, the pH of the biosurfactant was adjusted at a value in the range 2.0–11.0 prior to filter sterilization. The effect of NaCl on the activity of the biosurfactant was investigated by adding NaCl in the range 5–20% (w/v). The biosurfactant was then redissolved after purification with distilled water containing NaCl. In each cases, surface tension and E24 values were performed as described above.
Results and discussion
Kinetics of biosurfactant production
Free cell cultures were carried out in duplicates. During Pseudomonas fluorescens growth in mineral medium containing olive oil as carbon and energy source (C) and NH4NO3 as nitrogen source (N), corresponding to a C/N ratio of 10, the surface tension dropped rapidly after inoculation, reaching its lowest value (30 dyne cm−1) during exponential growth phase, namely after about 40 h of culture (Fig. 1a). The E24 plot, a measure of the biosurfactant concentration, showed that the surfactant was not initially present in a sufficient amount to form micelles. After less than 36 h of growth, the surfactant concentration started to increase, reaching its maximum value after about 56 h (67%) (Fig. 1a). The increase of the surface tension and the decrease of the emulsification index after 56 h of culture (Fig. 1a) characterize cessation of biosurfactant biosynthesis, most likely due to the production of secondary metabolites, which could interfere with emulsion formation and due to the adsorption of surfactant molecules at the oil–water interface [5]. These results indicated that the biosurfactant biosynthesis from olive oil occurred predominantly during the exponential growth phase, suggesting that the biosurfactant was produced as a primary metabolite, accompanying cellular biomass formation (growth-associated kinetics) [18]. This property suggests that biosurfactant could be more efficiently produced under chemostat conditions or by immobilized cells [14].
In the case of immobilized cells, cultures were also carried out in duplicates; the maximum amount of biosurfactant was achieved after 72 h (Fig. 1b). Although the minimum surface tension value recorded (35 dynes cm−1) was slightly greater than that obtained with free cells, the formed emulsion was more stable (E24 = 62%), most likely due to a low content of secondary metabolites, which could interfere as in the case of free cells [5]. The lower rates recorded during immobilized cell culture, if compared to free cell culture, was caused by diffusional limitations of nutrients from the bulk of the culture through the gel bead on one hand, and product (biosurfactant) diffusion from the immobilized cells to the surrounding medium on the other hand. It should be noted that after reaching its minimum value, surface tension remained then constant throughout culture (Fig. 1b), while after reaching a maximum value, the consecutive decrease observed for E24 was clearly less pronounced (Fig. 1b) than that observed during free cell culture (Fig. 1a). This showed that immobilization led to more physiological stability of cells. Moreover, a pH decrease from 7 to 5 was observed during the first 72 h, caused by oxygen deficiency or by the weakness of the buffer capacity of the broth in the case of immobilized cells, as also previously reported by some authors [10, 11, 25].
Biosurfactant separation and characterization
The cold acetone precipitation method used for biosurfactant recovery appeared easy and reliable, and no loss of biosurfactant activity was noted. The yield was approximately 2 g l−1, which was similar to those reported in the available literature [9].
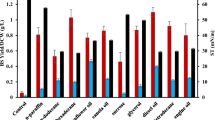
The rhamnose test was positive, indicating a glycolipid type of the produced biosurfactant, as previously reported for this microorganism [18, 26]. Aqueous solutions of biosurfactant showed good foaming stability. Total disappearance of the foam was detected after 2 h. In addition to surface and interfacial tension, stabilization of an emulsion of oil and water is commonly used as a surface activity indicator [6]. All the hydrocarbons tested are suitable substrates for emulsification by the biosurfactant, since their emulsification index values (E24) were in the range of 45–55%. Diesel oil (E24 = 55 ± 2%) and kerosene (54 ± 2%) were the best substrates, while sunflower oil (45 ± 2 %) was less suitable substrate for emulsification (the E24 value for heptane was 50 ± 2%).
As seen in Fig. 2, the critical micellar concentration corresponded to a sudden change in the surface tension. The CMC for the isolated biosurfactant was 290 mg l−1 and the corresponding surface tension was 32 dynes cm−1. Biosurfactant concentrations above the CMC produced only weak decrease of the surface tension, indicating that biosurfactant molecules began to aggregate [12, 17]. Since the yield was approximately 2 g l−1, the biosurfactant concentration in the culture medium was about five to ten times above its CMC. Minimum interfacial tensions were achieved for the CMC concentration and were 2.5 and 12 dynes cm−1 with olive oil and hexadecane, respectively.
The stability of the biosurfactant was tested over a wide range of temperature. The biosurfactant produced by P. fluorescens was shown to be thermostable (Fig. 3a). Heating of the supernatant to 100 °C (or its autoclaving at 120 °C) caused no significant effect on the biosurfactant performances. The surface tension reduction and emulsification activity were quite stable, irrespective of the operating temperature (ST = 35 dynes cm−1; E24 = 40–48%), in comparison with synthetic surfactants such as SDS, which exhibits a significant loss of emulsification activity above 70 °C [13].
Figure 3b shows that pH increase had a positive effect on surface tension, which decreased from 34 to 30 dynes cm−1, and also on emulsion stability (15% increase of E24). This could be caused by a better stability of fatty acids-surfactant micelles in presence of NaOH and the precipitation of secondary metabolites at higher pH values. The effect of pH on surface activity has been reported for biosurfactants produced by various microorganisms [2]. Extreme pH values could possibly transform weak surface-active species into more active emulsifiers by increasing ionization, in agreement with the available literature [16, 21, 22, 24]. The activity of the rhamnolipid produced by Rhodococcus [2] and surfactin produced by Bacillus subtillis [13] was also pH-stable in a wide range from 4 to 11.
Figure 3c shows that the addition of NaCl in the range of concentrations tested (5–20%) had only a weak effect on surface tension and emulsification index of P. fluorescens biosurfactant. Abu-Ruwaidha et al. [2] reported a similar effect on efficiency and effectiveness upon the addition of 10% sodium chloride, for a biosurfactant produced by Rhodococcus strain ST-5.
Conclusion
The capacity of biosurfactant production by Pseudomonas fluorescens for both free and alginate-entrapped cells was proved in batch cultures. The effect of diffusional limitations of substrates and product observed with immobilized cells affected the production kinetics, since the required time to attain maximum yield was delayed. Nevertheless, the emulsion stability was improved and fewer by-products interfered with the biosurfactant activity. The pH decrease showed that cells physiology was also slightly affected when they were immobilized. The immobilization of cells was helpful for biosurfactant recovery, if compared to previous work with free cells [1]. Further studies at laboratory scale might be of great interest for the production of biosurfactant by immobilized cells to optimize additional parameters such as support characteristics and immobilization conditions [10, 23], before conducting experiments in continuous reactors.
The critical micellar concentration (290 mg l−1) obtained was in agreement with other values reported in the literature [5, 10], and the tensioactive properties of these molecules indicate good prospects for industrial applications, when compared to the values of the CMC of chemical anionic surfactants. The emulsifying activity of the biosurfactant revealed that it could be used as an emulsion-forming agent for hydrocarbons and oils, giving stable emulsions. The ability to form stable emulsions with vegetable oils suggests potential applications as cleaning and emulsifying agent in food industry. The product exhibited a high level of thermal stability, a positive effect for increasing pH, and demonstrates a high level of tolerance to ionic strength, which shows clear perspectives for its use in extreme environmental conditions in bioremediation, pharmaceutical formulations and other industrial fields.
References
Abouseoud M, Maachi R, Amrane A, Boudergua S, Nabi A (2008) Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 223:143–151
Abu-Ruwaida AS, Banat IM, Haditirto S, Salem A, Kadri M (1991) Isolation of biosurfactant-producing bacteria product characterization, and evaluation. Acta Biotechnol 11:315–324
Banat IM (2000) Les biosurfactants, plus que jamais sollicités. Biofutur 198:44–47
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Bonilla M, Olivaro C, Corona M, Vazquez A, Soubes M (2005) Production and characterization of a new bioemulsifier from Pseudomonas putida ML2. J Appl Microbiol 98:456–463
Cooper DG, Goldenberg BG (1987) Surface-active agents from two Bacillus species. Appl Environ Microbiol 53(2):224–229
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61(3):47–64
Dubois M, Gills KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugar and related substances. Anal Chem 28:350–356
Haba E, Espuny MJ, Busquets M, Manresa A (2000) Screening and production of rhamnolipids by Pseudomonas aeroginusa 47T2 NCIB 40044 from waste frying oils. J Appl Microbiol 88:379–387
Hye-Sung J, Dong-Jung L, Sun-Hee H, Soon-Duck H, Jai-Yul K (2004) Rhamnolipid production by Pseudomonas aeruginosa immobilised in polyvinyl alcohol beads. Biotechnol Lett 26:35–39
Inoue S, Itoh S (1982) Sophorolipids from Torulopsis bombicola as microbial surfactants in alkane fermentation. Biotechnol Lett 4:3–8
Karsa DR, Bailey RM, Shelmerdine B, McCann SA (1999) Overview: a decade of change in the surfactant industry. In: Karsa DR (ed) Industrial applications of surfactants, vol 4. Royal Society of Chemistry, London, pp 1–22
Kim H, Yoon B, Lee C, Suh H, Oh H, Katsuragi T, Tani Y (1997) Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. J Ferment Bioeng 84(1):41–46
Klein J, Wagner F (1987) Different strategies to optimize the production phase of immobilised cells. Ann N Y Acad Sci 501:306–316
Koch AK, Kappeli O, Fiechter A, Reiser J (1991) Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J Bacteriol 173:4212–4219
Mahanty B, Pakshirajan K, Dasu VV (2006) Production and properties of a biosurfactant applied to polycyclic aromatic hydrocarbon solubilization. Appl Biochem Biotechnol 134:129–141
Meylheuc T, Heary JM, Bellon-Fontaine MN (2001) Les biosurfactants, des biomolécules à forte potentialité d’application. Sci Aliments 21:591–649
Persson A, Österberg E, Dostalek M (1988) Biosurfactant production by Pseudomonas fluorescens 378: growth and product characteristics. Appl Microbiol Biotechnol 29(1):1–4
Pruthi V, Cameotra SS (1995) Rapid method for monitoring maximum biosurfactant production obtained by acetone precipitation. Biotechnol Lett 9(4):271–276
Santos AS, Sampaio AW, Vasquez GS, Santa Anna LM, Pereira N, Freire MG (2002) Evaluation of different carbon and nitrogen sources in production of rhamnolipids by a strain of Pseudomonas aeruginosa. Appl Biochem Biotechnol 98(100):1025–1035
Sarrubo LA, Luna JM, Campos-Takaki GM (2006) Production and stability studies of the bioemulsifier obtained from a new strain of Candida glabrata UCP 1002. Electronic J Biotechnol 9:400–406
Shin KH, Kim KW, Seagren EA (2004) Combined effects of pH and biosurfactant addition on solubilization and biodegradation of phenanthrene. Appl Microbiol Biotechnol 65:336–343
Siemann M, Wagner F (1993) Prospects and limits for the production of biosurfactants using immobilized biocatalysts. Surfactant Sci Ser 48:99–133
Vipulanandan C, Ren X (2000) Enhanced solubility and biodegradation of naphthalene with biosurfactant. J Environ Eng 7:629–634
Webb C, Black GM, Atkinson B (1986) Process engineering aspects of immobilized cell systems. Institution of Chemical Engineers, Oxford, 320 pp
Wilson NG, Bradley G (1996) The effect of immobilization on rhamnolipid production by Pseudomonas fluorescens. J Appl Bacteriol 81(5):525–530
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abouseoud, M., Yataghene, A., Amrane, A. et al. Biosurfactant production by free and alginate entrapped cells of Pseudomonas fluorescens . J Ind Microbiol Biotechnol 35, 1303–1308 (2008). https://doi.org/10.1007/s10295-008-0411-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-008-0411-0