Abstract
A growing body of scientific reports indicates that the role of creatine (Cr) in cellular biochemistry and physiology goes beyond its contribution to cell energy. Indeed Cr has been shown to exert multiple effects promoting a wide range of physiological responses in vitro as well as in vivo. Included in these, Cr promotes in vitro neuron and muscle cell differentiation, viability and survival under normal or adverse conditions; anabolic, protective and pro-differentiative effects have also been observed in vivo. For example Cr has been shown to accelerate in vitro differentiation of cultured C2C12 myoblasts into myotubes, where it also induces a slight but significant hypertrophic effect as compared to unsupplemented cultures; Cr also prevents the anti-differentiation effects caused by oxidative stress in the same cells. In trained adults, Cr increases the mRNA expression of relevant myogemic factors, protein synthesis, muscle strength and size, in cooperation with physical exercise. As to neurons and central nervous system, Cr favors the electrophysiological maturation of chick neuroblasts in vitro and protects them from oxidative stress-caused killing; similarly, Cr promotes the survival and differentiation of GABA-ergic neurons in fetal spinal cord cultures in vitro; in vivo, maternal Cr supplementation promotes the morpho-functional development of hippocampal neurons in rat offsprings. This article, which presents also some new experimental data, focuses on the trophic, pro-survival and pro-differentiation effects of Cr and examines the ensuing preventive and therapeutic potential in pathological muscle and brain conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Creatine (Cr) is widely used in the sports’ industry as a supplement to increase skeletal muscle (SM) mass and performance. Cr—along with creatine kinase (CK) and phospho-creatine (PCr)—plays a fundamental role in cellular energy storage, spatial transfer and supply, especially in high energy demanding tissues and organs such as SM, brain, kidney, testis and small intestine (Wallimann 2015; Wallimann et al. 2011). The human brain in particular, although constituting only about 2 % of the body mass, may spend up to 20 % of the total energy consumed in the body. In myofibres and neurons the Cr/PCr/CK system provides an adequate fueling for ATP-dependent processes and, particularly, for those specific to their own functions, namely motor proteins’ activity, maintenance of electrical membrane potentials, signaling activities and cycling of neurotransmitters.
Cr deficiency syndromes (Nasrallah et al. 2010; Schulze 2013) represent a group of inborn errors of Cr synthesis [arginine:glycine amidinotransferase (AGAT) deficiency and guanidinoacetate methyltransferase (GAMT) deficiency] and transport [Cr transporter (CrT) deficiency] characterized by a prevalence of neurological symptoms due to both its importance for brain energetics and the low permeability of the blood–brain barrier (BBB) to circulating Cr, which renders central nervous system (CNS) almost totally dependent on the endogenously synthesized Cr (Braissant et al. 2007). The majority of these syndromes can be prevented and/or treated by supplementation with high, chronic intakes of exogenous Cr (Schulze 2013); the benefits of additional Cr are thought to depend mostly on the amelioration of the cellular energy handling. According to this view, Cr supplementation has also been shown to be effective as an adjuvant treatment in a number of myopathies and neuromuscular diseases characterized by perturbation of cellular bioenergetics, although independent of Cr metabolism (Wyss et al. 2007; Tarnopolsky 2007).
Interestingly, over the last two decades, Cr has been shown to exert biologically relevant activities unrelated to its paramount role in cellular energetics, and the interest for the non-ergogenic effects and their potential exploitation has rapidly grown (Wallimann et al. 2011; Sestili et al. 2011). These activities include direct and indirect antioxidant and protective effects against different and physio- or pathophysiologically relevant stressors such as reactive oxygen (ROS) and nitrogen species (Sestili et al. 2006, 2011; Young et al. 2010), UV light (Shamban 2009), hyperosmolarity (Alfieri et al. 2006); protection of mitochondrial genome from UV and oxidative attack (Berneburg et al. 2005; Guidi et al. 2008; Lenz et al. 2005); direct anti-apoptotic activity through prevention or delay of mitochondrial permeability transition pore opening (Dolder et al. 2003; Wallimann et al. 2011); improvement of cerebral blood flow after stroke secondary to the preservation of vascular endothelial cell function (Prass et al. 2007); anti inflammatory-like effects on vascular endothelial cells (Moraes et al. 2014; Nomura et al. 2003).
The consequence of these findings is twofold. Firstly, the therapeutic and health promoting potential of Cr received greater attention and strengthened the rationale for its use in the treatment of neurological and muscular diseases where its efficacy has in some cases been confirmed in randomized clinical trials (Gualano et al. 2012; Tarnopolsky 2007). Secondly, today Cr is no longer considered to function solely as an ergogenic compound, but also as a molecule acting through pleiotropic mechanisms which converge to maintain cell homeostasis with amelioration of cell viability and function (Sestili et al. 2011; Sestili et al. 2015; Wallimann et al. 2011).
Cr has also been shown to favour the differentiation of myoblasts and neuroblasts in vitro, either under normal or adverse conditions, and additionally to improve muscle growth and neuron maturation in vivo (see Table 1). These pro-differentiation and trophic effects, in turn, are likely mediated by the pleiotropic nature of Cr. The present article aims to review the studies on this topic and to critically discuss their implication and clinical prospects in brain and muscle.
Creatine in muscle differentiation and growth
Creatine activates signaling cascades that enhance muscle differentiation and growth
Adult mammalian SM is a dynamically remodeled and regenerating tissue where the successful differentiation of myogenic cells into integrated/functioning myotubes, corresponding to multi-nucleated syncytia, is a fundamental requisite for its structural and functional homeostasis (Forcales and Puri 2005). Toxic and pathological conditions, as well as aging, can affect the efficiency of physiological muscle differentiation (Barbieri and Sestili 2012). Satellite cells represent the SM myogenic stem cell lineage (Charge and Rudnicki 2004; Collins et al. 2005): upon activation they start proliferating and differentiating to build up new muscle fibers and/or fuse with existing ones (Holterman and Rudnicki 2005; Partridge et al. 1978). According to a widely accepted, basic view, the initiation of muscle differentiation requires the coordinated silencing of non-muscle genes and the expression of muscle-specific ones, orchestrated by a very complex signaling cascade in which muscle regulatory factors (MRFs) play a central role. The MRFs (which include Myo-D, Myf-5, MRF-4, and myogenin) act as transcription activators by virtue of their intrinsic properties as DNA binding proteins (Sabourin and Rudnicki 2000); MRFs initiate transcription and regulate expression level of some muscle specific genes, such as myosin heavy chain (MHC) and myosin light chain, actin, or CK (Lowe et al. 1998). Other co-factors such as IGF-1 and -2 are also known to contribute to this process (Deldicque et al. 2005; Louis et al. 2004): indeed IGF-1 and MRFs are involved in the hypertrophic response of SM, in regulating net muscle protein synthesis and the addition of myonuclei from recruited satellite cells to existing, terminally differentiated myofibers, respectively (Holterman and Rudnicki 2005; Jacquemin et al. 2007; Sabourin and Rudnicki 2000); IGF-1 promotes hypertrophism via multiple mechanisms, involving the phosphatidyl-inositol 3-kinase-Akt/PKB-mTOR, the mitogen-activated protein kinase/extracellular signal-regulated receptor kinase (MAPK/ERK), and the calcineurin pathways (Deldicque et al. 2007; Louis et al. 2004).
The involvement of Cr in muscle growth and differentiation was first described in two studies indicating that it promotes an increased synthesis of muscle-specific proteins in cultured skeletal myotubes and cardiomyocytes (Ingwall 1976; Ingwall et al. 1972). More recently when studying the effect of oral Cr supplementation and resistance training in untrained adults, it was reported that Cr, in cooperation with physical exercise, increased muscle strength and size, MHC synthesis, myogenin and MRF-4 mRNA and protein expression (Willoughby and Rosene 2001, 2003). A further study (Hespel et al. 2001) dealing with the effect of oral Cr supplementation on MRFs expression during human leg immobilization and rehabilitation showed that the expression of MRF-4, but not of myogenin, was stimulated by Cr. Shortly after it was shown (Vierck et al. 2003) that addition of Cr to differentiating satellite cells in vitro, significantly enhanced their fusion and the formation of myotubes. Louis et al. (Louis et al. 2004) reported that addition of Cr to differentiating C2C12 cultured myoblasts caused the formation of hypertrophic mature myotubes, an effect correlating with the increased expression level of Myo-D, Myf-5, MRF-4, myogenin and IGF-1 mRNAs. IGF-1. Expression of IGF-1, which increased constantly and significantly over the entire differentiation stage, was the most sensitive to Cr supplementation. Hence, these authors suggested IGF-1 as the key target for the effect of Cr. Subsequently our group confirmed the increased expression of IGF-1 and MRFs in Cr-supplemented C2C12 differentiating cells (Sestili et al. 2009). Other investigators (Deldicque et al. 2007), although confirming the pro-differentiation and anabolic effects of Cr in C2C12 cells, found an early up-regulation of protein kinase B (Akt/PKB) phosphorylation and, at later times, also of p38-MAPK. Hence, they proposed the p38-MAPK and the Akt/PKB pathways as more likely mediators of the Cr action.
A very recent study, (Mobley et al. 2014) showed that Cr counteracts myostatin-induced muscle fiber atrophy by significantly increasing the pro-myogenic factor Akirin-1/Mighty mRNA expression level, suggesting that this effect could be mechanistically related to SM hypertrophy in vivo. This hypothesis needs to be further explored, but it would also be important to ascertain whether the increased expression of MRFs/IGF-1 and the decreased efficiency of myostatin-linked signaling routes act in a cooperative fashion contributing to Cr activity. Finally, Cr seems to favour a better and faster assembly of elongated mitochondrial networks in differentiating C2C12 myoblasts (Barbieri et al. 2013a), an effect which, given the importance of mitochondria and mitochondriogenesis in myogenesis (Herzberg et al. 1993; Sestili et al. 2015), could be mechanistically associated with the Cr-related hypertrophic response in SM.
Human studies confirmed that supplemental Cr increases the expression of MRFs, IGF-1 or other myogenic cofactors (Burke et al. 2008; Deldicque et al. 2005; Safdar et al. 2008). Indeed Cr increased the expression of IGF-1 mRNA, with a corresponding elevation in the phosphorylation state of 4E-BP1 (a downstream effector of the PI3K-Akt/PKB-mTOR pathway triggered by IGF-1) (Deldicque et al. 2005); short-term Cr supplementation augmented mRNA expression of sphingosine kinase-1, protein and mRNA content of PKBα, p38 MAPK and extracellular-regulated mitogen-activated protein kinase-6 (Safdar et al. 2008), which are necessary for myoblast differentiation; supplemental Cr further increased resistance training-induced intramuscular IGF-1 accumulation in healthy men and women (Burke et al. 2008).
Creatine antagonizes oxidative stress
Studies from our group suggested that Cr might also be important in regulating the crossroad between muscle differentiation and oxidative stress. Oxidative stress is known to impair myogenesis (Zaccagnini et al. 2007) while Cr is known to exert antioxidant activity through direct and indirect mechanisms (Sestili et al. 2011).
ROS may be generated at multiple sites either under normal or pathological conditions and act in a typical hormetic fashion in SM (Barbieri and Sestili 2012): low and transient levels of ROS elicit physiological/adaptive responses, while high and durable levels lead to loss of function and cell death. In SM cells, excessive ROS pressure (i.e. oxidative stress) is known to inhibit myogenic cell proliferation and differentiation (Ardite et al. 2004; Barbieri et al. 2011; Hansen et al. 2007; Langen et al. 2002; Ribas et al. 2014; Sestili et al. 2009; Singh et al. 2014) and to promote wasting of myofibers (Buck and Chojkier 1996; Zaccagnini et al. 2007).
Further data in the literature indicate that oxidative stress inhibits the differentiation capacity of myogenic cultures, such as L6 (Zaccagnini et al. 2007) and C2C12 myoblasts (Sestili et al. 2009). In particular we showed that a mildly toxic oxidative stress (0.3 mM H2O2 for 1 h given 24 h after committing myoblasts to differentiate), besides causing 20/30 % cell death, impeded the execution of differentiation in surviving C2C12 cells for up to 5 days post challenge (Sestili et al. 2009). From a molecular, biochemical and morphological point of view oxidatively-intoxicated cells showed reduction of MyoD, myogenin, MRF4 and IGF-1 transcription; depletion of GSH levels; consumption of ATP and PCr stores; extensive damage to mitochondria with swelling of the matrix, loss of mitochondrial cristae and organelle demise. Notably, these effects are known to contribute to the impairment of myogenic potential, and, more interestingly, all of them were attenuated by Cr supplementation which significantly reduced cell death and prevented the loss of the normal differentiative capacity of C2C12 cells. In parallel, comparing the activity of Cr with that of the reference antioxidant Trolox, we found that the latter, although promoting a lower cell demise in intoxicated cells, was unable to restore even partially, their myogenic potential. It was concluded that the effects of Cr did not exclusively depend on its capacity to reduce the extent of oxidative damage. Indeed Trolox prevented GSH and ATP depletion, but did not augment Cr and PCr levels or significantly protect from decreases in MRFs and IGF-1 mRNA expression and did not mitigate ultrastructural mitochondrial damage. As to this latter effect, it is important considering that the prevention of mitochondrial damage may also derive from a mitochondrially-directed antioxidant activity which, in the case of Cr, but not of Trolox, would be guaranteed by its high intramitochondrial localization (Saks et al. 2007).
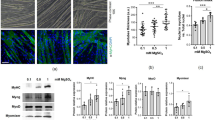
Since Cr is known to protect mitochondria from oxidative injury in C2C12 cells and from mtDNA damage in human endothelial cells, and it is well known that mitochondrial integrity, function and biogenesis are fundamental in SM differentiation (Barbieri et al. 2011; Hamai et al. 1997; Rochard et al. 2000), we recently focused on these organelles to estimate their involvement in this toxicity/rescue experimental setting. To this end we used confocal microscopy/image analysis-based methods to observe mitochondrial single unit integrity, morphology and networking (Barbieri et al. 2013a). Over the first 48 h of differentiation, mitochondria formed a typically elongated and diffused network paralleling the major cellular axis in control, C2C12 cells. This network was disrupted (round shaped and perinuclear-located mitochondria) by oxidative stress while Cr supplementation preserved to some extent the mitochondrial integrity and morphology (see Fig. 1 for representative micrographs).
Creatine reduces the fragmentation of the mitochondrial network inflicted by oxidative stress in differentiating C2C12 murine myoblasts. Representative confocal micrographs of C2C12 myoblasts committed to differentiate by serum starvation, incubated for 24 h in the absence (b) or presence of 3 mM Cr (d) and then treated with 300 µM H2O2; medium was changed and the cells cultured for further 24 h. Finally myoblasts were incubated with Mitotracker 1 to stain mitochondria and then observed with a confocal microscope. Also shown: A untreated cultures; C Cr-supplemented only cells. Bars 50 µm
Furthermore, a high amount of autophagic vacuoles colocalized with mitochondria in oxidant-injured C2C12 cells, suggesting the occurrence of intense mitophagy which, not surprisingly, could be prevented by Cr pre-loading (Barbieri et al. 2013a).
The efficacy of Cr to prevent mitochondrial damage was also confirmed by the lower extent of H2O2-inflicted cardiolipin peroxidation, a reliable marker of mitochondrial oxidative damage (Li et al. 2015). Thus, all these effects of Cr are likely to converge toward the protection and stabilization of mitochondrial function and homeostasis. Accordingly, Cr significantly attenuated the H2O2-instigated fall of mitochondrial membrane potential (∆Ψ) per mitochondrial unit (Sestili et al. unpublished). Notably Cr supplementation per se, as compared to control cells, stimulated mitochondrial activity. Hence, taken together, these findings lend support to the notion that Cr reduces H2O2-induced mito-dysfunction and that this activity is important in the protection from oxidative stress-related myogenic arrest.
Some of the beneficial effects of Cr described so far on muscle mitochondria are directly related to the enhancement of cellular energetics (Lenz et al. 2005; Meyer et al. 2006; Sestili et al. 2009, 2011; Wallimann et al. 2011), such as the effects on ∆Ψ or the reduction of mitochondrial ROS generation through an ADP-recycling mechanism. To this regard, it was only recently that Cr was shown to activate—in muscle cells and SM tissue—AMP-activated protein kinase (AMPK), which is a pivotal energy sensor playing a central role in linking mitochondrial regulation (mitochondrial biogenesis, turnover and function) to cellular metabolic demands (Alves et al. 2012; Barbieri et al. 2013b; Ceddia and Sweeney 2004; Handschin and Spiegelman 2006; Jager et al. 2007).
NF-kB, TNF-α, glutathione- (GSH) stores, fibroblast growth factor 21, cycloglobin, p66shc, IGF-1 represent sensitive ROS targets/triggers (Ardite et al. 2004; Barbieri et al. 2013a; Hansen et al. 2007; Langen et al. 2002; Ribas et al. 2014; Sestili et al. 2009; Singh et al. 2014; Zaccagnini et al. 2007) contributing to the arrest of differentiation and to the development of muscle inflammation. Persisting inflammation, in turn, impairs muscle regeneration (Buck and Chojkier 1996; Pizza et al. 2005; Zaccagnini et al. 2007) and is involved in a variety of multifactorial muscular pathologies characterized by proliferation/differentiation imbalance (Messina et al. 2006; Tidball 2005; Toscano et al. 2005). Hence, any antioxidant intervention should in principle counteract the myogenic impairment caused by oxidative stress.
Cr—through its direct and indirect antioxidant capacity and the additional advantage of its high muscular tropism, ergogenic and anabolic effects—may represent an ideal candidate for antagonizing SM oxidative stress.
Creatine activates AMP-activated protein kinase
Currently the role of AMPK in the myogenic process—positive (Alves et al. 2012; Fu et al. 2013) or negative (Williamson et al. 2009)—is not yet completely understood. In this regard, in agreement with Ceddia and Sweeney (2004) we found that Cr induces a rapid and transient increase of AMPK phosphorylation (Sestili et al. unpublished) in differentiating C2C12 myoblasts, i.e. the same condition where Cr promotes differentiation and myotube hypertrophy. This finding would suggest that Cr-induced AMPK activation, be it by direct or indirect means, might represent an adjunctive mechanism in Cr pro-differentiation effects, providing an enhanced adaptive mechanism to better regulate cellular energy metabolism under highly demanding conditions such as the task of differentiation. In respect of this, Cr-induced AMPK activation and its contribution to energy regulation might also play a role in protecting C2C12 myoblasts from the H2O2-induced differentiative arrest, a topic which is currently under investigation in our laboratory. To this regard, our preliminary results (unpublished) suggest that the Cr-induced increase in AMPK phosphorylation promotes the up-regulation of PGC-1α activation, with a peak after 3 days of differentiation. PGC-1α has a key role in the antioxidant enzymes biosynthesis and its genetic deletion has shown an inhibitory effect toward superoxide dismutase 2 and catalase expression levels (Lu et al. 2010): conversely, its activation might concur to render cells less sensitive to the oxidative insult via an amelioration of the cellular antioxidant defenses.
The characterization of AMPK-downstrem effectors recruited upon Cr activation will be very important in that it is widely accepted that the pharmacological activation of AMPK in resting muscle—as in the case of metformin and other drugs or bioactive compounds, such as 5-aminoimidazole-4-carboxamide riboside, lipoic acid, berberine, resveratrol, rooibos, thiazolidinediones, glucagon-like peptide-1, dipeptidyl peptidase-4 (Coughlan et al. 2014; Gwinn et al. 2008; Mazibuko et al. 2013; Pold et al. 2005)—has fundamental and potentially physiologically valuable effects on glucose, lipid and protein metabolisms and on the expression of a great number of genes involved in dysmetabolic disorders. Thus, the identification of these Cr-activated downstream effectors of AMPK signaling might provide additional targets for restoring cellular and tissue homeostasis in the above diseases.
Creatine and neuron maturation/brain development
Brain development and functions need the Cr/PCr/CK system to maintain high energy levels for regeneration and buffering of ATP levels (Andres et al. 2008). However, and in analogy with SM and other cell types/tissues, the energetic function is not the only one exerted by Cr in CNS. For example Cr also exerts antioxidant activity, as shown above with muscle, an effect which has been demonstrated also in rat brains in vivo and in CNS cells in vitro (Ireland et al. 2011; Sartini et al. 2012); Cr acts as a compatible osmolyte in vitro (Alfieri et al. 2006) and it is thought to be active in this role in vivo (Bothwell et al. 2002; Braissant et al. 2011); Cr, acting on the cerebral vasculature, leads to improved brain blood circulation (Prass et al. 2007); Cr exerts anti-apoptotic activity through prevention or delay of mitochondrial permeability transition pore opening (Dolder et al. 2003), an effect due to the converging action of direct/indirect antioxidant and ergogenic activities (Sestili et al. 2011). Hence, in general, the neuroprotective activity of Cr is a consequence of the simultaneous occurrence of multiple mechanisms conferring on Cr a pleiotropic nature (Andres et al. 2008; Sestili et al. 2011; Wallimann et al. 2011). Finally, Cr acts at central GABA-A receptors as a partial agonist, and in so doing mimics a neuromodulator released in an action potential-dependent manner (Almeida et al. 2006) and, notably, has pro-differentiation/trophic actions on maturing neuroblasts. In particular, in cultured human and rodent neurons, both chronic or short-term Cr exposure promote a significant increase of GABA-immunoreactive neuron density, without affecting total neuronal cell density (Ducray et al. 2007a, b).
To meet Cr requirements the mature brain produces its own Cr via a complex, endogenous biosynthetic process identified by Braissant et al. (2007, 2005). Cr brain synthesis relies on the differential, cell type-specific expression of AGAT and GAMT, as well as of the CrT, a Na/Cl–Cr-co-transporter (SLC6A8) belonging to the family of solute transporter family (Christie 2007). This complicated route is likely to derive from the necessity of overcoming the import of Cr from periphery through the mature BBB, which has a limited permeability for Cr because of the absence of the SLC6A8 CrT in astrocytes and particularly in astrocyte feet lining microcapillary endothelial cells (Braissant et al. 2011). On the contrary, fetal needs are mainly supported by the maternal source of Cr transferred via the placental unit, since endogenous synthesis of Cr (including brain synthesis) is not operative at least up to the end of fetal life (Ireland et al. 2009) and the immature, “leacky” fetal BBB is still permeable to circulating Cr.
Thus, considering the importance of Cr for CNS, its neuroprotective effects (see Table 1) and that fetus relies on maternal Cr supply for much of gestation, Cr supplementation has been proposed as a preventive measure to protect the embryo during pregnancy (Wallimann et al. 2011) whenever oxidative stress arises such as in feto-placental hypoxia, fetal growth restriction, premature birth, mother malnutrition or when parturition is delayed or complicated by oxygen deprivation of the newborn (Dickinson et al. 2014; Ireland et al. 2011).
In a previous work from our group (Sartini et al. 2012), we addressed the question of whether Cr given to maturing neuroblasts could induce effects similar to those observed in cultured, differentiating C2C12 muscle cells (see above). The study was carried out on primary cultures of neuroblasts (obtained from the spinal cords of 7-day old chick embryos) loaded with Cr for 24 h and then allowed to differentiate for further 3 days under normal conditions or after a challenge with a brief oxidative stress (20 µM H2O2 for 40 min). Notably, oxidative stress is an event which is known to be involved in the aetiology of a number of brain pathological conditions such as, for example, perinatal hypoxia–ischemia (Dickinson et al. 2014; Ireland et al. 2011). Neuroblast viability, neurite length and voltage-gated ion currents (investigated by whole-cell patch-clamp) were monitored and taken as indexes of morphological and functional differentiation.
As to oxidatively stressed cells and similarly to differentiating myoblasts, Cr supplementation was generally protective and mitigated the deleterious effects induced by H2O2. In particular Cr significantly hampered the decrease of viability, the fall of GSH stores and the arrest of neurite development. Cr protection was likely to depend on direct/indirect radical scavenging and a better cellular energy charge sustained by the Cr/PCr system (Sartini et al. 2012).
Interestingly, we also found that in non-oxidatively stressed cells, Cr supplementation induced a significant and dose-dependent increase of Na+ and K+ voltage-gated currents during the period of in vitro neuron network building (Sartini et al. 2012). This effect was likely to derive from an increased channel density in the cell membrane, compatible with either an enhanced translocation of this channel to the plasma membrane or enhanced expression of the same. Consistently with this finding, an increased firing ability, in terms of spike number following a depolarizing step, was also revealed (Sartini et al. 2012). All these effects were dependent on the cytosolic fraction of Cr, as they could be prevented by guanidino-propionic acid, a potent CrT blocker (Otten et al. 1986).
More recently, we set out a study (Sartini et al. 2015) to see whether Cr is capable to affect in vivo neuron development, network formation and functioning. To this end, Cr supplementation was carried out during pregnancy (10 days, up to 24 h before delivery) and offspring hippocampal pyramidal neurons were analyzed between 14 and 21 postnatal days. The hippocampus was chosen as the target region of our investigation because its neurons are involved in learning processes and exhibit functional and structural synaptic plasticity varying their intrinsic excitability. Pups’ brains and CA1 neurons from hippocampal slices were then subjected to biochemical and morphological/electrophysiological determinations, respectively.
Our results show (Sartini et al. 2015), at birth, a significant increase of whole brain Cr- and PCr-levels in pups from Cr-supplemented dams and, according to the direct/indirect antioxidant Cr activity, a higher content of brain GSH. Morphologically, maternal Cr supplementation promoted overgrowth and more complex branching of the CA1 neurite trees. Figure 2 shows representative micrographs of CA1 hyppocampal neurons from control (A) and Cr-group (B) and the Scholl analysis of their branching (C); the mean neurite length in 16–18 days old pups was 5,092,168 ± 430,654 µm and 329,771 ± 30,878 µm in Cr versus control group, respectively. Electrophysiological measurements demonstrated an increased excitability of CA1 pyramidal neurons, a higher efficiency of synapses on CA1 (a likely result of the combination of an enhanced release probability of neurotransmitter and the more complex and extended dendritic tree), and a greater maximum firing frequency of CA1 neurons following a depolarizing step. On the whole, these responses would suggest a higher hippocampal output in the pups under maternal Cr supplementation conditions. Consistently, we found an increased capacity to maintain long-term potentiation (LTP) in the Cr group. Taken collectively, these results would then suggest that maternal Cr supplementation promotes a faster functional maturation of offspring CA1 neurons, a finding which extends to an in vivo situation the Cr-pro-maturation effects observed in vitro (Ducray et al. 2007a, b; Sartini et al. 2012).
Creatine maternal supplementation affects neuronal morphology. Representative confocal micrographs of biocityn (a neuroanatomical tracer taken up by axons and dendrites) stained hippocampal CA1 neurons from eighteen days old pups born from Cr-unsupplemented (a) or -supplemented (b) mothers. Also shown in (c) Scholl analysis plot showing numbers of dendritic crossings along the Scholl rings as a function of distance from soma. Distribution of dendritic crossings results in significant difference between the two groups. Mixed-factor ANOVA for basal dendrites: F(1,26) = 4.652, p = 0.040; *Bonferroni’s post hoc: p < 0.01. Mixed factor ANOVA for apical dendrites: F(1,26) = 6.560, p = 0.016. CTRL n = 10, TREAT n = 18. Pregnant rats were supplemented with 1 % w/v (Ipsiroglu et al. 2001) Cr in drinking tap water from the eleventh to the second-to-last day of pregnancy. Bars 50 µm
At this stage, we can only make some tempting hypothesis on the mechanisms underlying the observed in vivo effects of Cr on neuron differentiation. These could involve its energetic function, which is likely to contribute to the enhanced dendritic growth and arborization; in this view, the increased brain Cr/PCr availability would provide more substrate for CK to replenish ATP consumed for cytoskeleton polymerization, dendrite formation and elongation. In addition to the ergogenic activity, changes in expression pattern of membrane ion channels involved in neuron electrical activity could account for the electrophysiological effects of Cr. Indeed membrane ion channel expression mutates during development in terms of type and subunit isoforms, moving toward the mature ion channel configuration. To this regard, Cr was shown to influence gene expression: in particular, hippocampal brain-derived neurotrophic factor expression appears to be affected by the presence of Cr (Allen et al. 2015). Notably the brain-derived neurotrophic factor/TrKb pathway was shown to regulate ion channel trafficking in membranes via the phosphoinositide 3-kinase (PI3 K)-protein kinase B (PKB/Akt) cascade (Duan et al. 2012). Moreover, ion channel phosphorylation could also alter ion channel kinetics, changing the electrical properties of neuronal cell membrane; the extent of phosphorylation may in turn be affected, in addition to activation of signaling pathways, by changes in ATP availability.
In analogy with the effects promoted by Cr in other situations, it is likely that those observed in pups from dams receiving gestational Cr supplementation are the result, again, of Cr pleiotropism, i.e. of its multiple activities converging toward a faster maturation.
The Cr-induced faster neuron maturation as well as the enhanced LTP (which underlies learning and memory processes) might represent a positive effect with a potential benefit to learning ability and cognition, although at this stage this is mere speculation. Thus, taken collectively, our data lend further support to the emerging idea of giving Cr during the last period of pregnancy as a preventive intervention (i.e. the third trimester in humans) to protect a number of major organs against the challenge of the transition from fetal to newborn life. This idea, proposed by Wallimann et al. (2011) and Dickinson et al. (2014), is grounded on the notion that the ‘pleiotropic’ properties of Cr might afford benefits to “many fetal tissues where vasoconstriction, oxidative stress, or glutamate toxicity may arise, in addition to its main function of maintaining mitochondrial function and ATP turnover”. Not secondarily, the trophic and pro-differentiation effects observed in our experimental setting might also contribute to enhance the capacity of maturing brain to counteract stressing and harmful situations.
Nonetheless, from a different point of view one could merely speculate that the overall highest excitability we observed, in conjunction with an incomplete development of the inhibitory synaptic system and other inducing/facilitating factors such as high body temperature, could imply an increased risk of seizure and excitotoxic effects. However, this hypothesis seems unlikely in that Cr has rather been reported—although in experimental settings not directly comparable with our own—to be neuroprotective and to prevent seizures. Indeed various studies reported (Cannata et al. 2010; Ellery et al. 2013; Ireland et al. 2008, 2011) that maternal Cr protects the fetal brain, diaphragm, and kidney against hypoxic insult at term; Cr has been shown, in immature rats, to suppress seizures caused by hypoxia (Holtzman et al. 1998) and protect against ischemic/anoxic brain damage (Adcock et al. 2002) the most common case of clinical seizures in the newborn period and, to the best of our knowledge, there is no report indicating an association of the increased risk of seizure with increased brain Cr levels. Therefore, although the influence on morpho-functional neuron development promoted by Cr might be regarded as a bifaceted phenomenon deserving further investigation, the notion that Cr might represent a promising and inexpensive tool to reduce the perinatal morbidity and mortality seems more likely.
Clinical applications and future perspectives
Data discussed so far would extend the perspective of the use of Cr in brain and muscle conditions (see Table 1 for the last 5 years reviews on the clinical effects of Cr). With regard to brain for example, the use of Cr as a new strategy to reduce fetal and neonatal morbidity and prevent mortality in high-risk human pregnancy (Dickinson et al. 2014) seems to be a very promising and important field, and research aimed at investigating whether oral Cr might represent a recommended, multi-purpose nutrient for fetal brain development during late pregnancy, i.e. the third trimester, should be encouraged.
The anabolic, pro-survival and pro-differentiation effects of Cr supplementation support its use also in pathologies/situations causing weakness and muscle mass waste such as corticoid therapy, long-term bed rest/inactivity, muscle rehabilitation, malnutrition, and cachexia. Skeletal muscle aging is known to reduce myogenesis and to cause a variety of disturbances ranging from weakness to sarcopenia. Notably, decreased muscle integrity and function is also detrimental to the progression of metabolic disorders such as hyperlipidemias and diabetes (Sestili et al. 2015). The effect of Cr supplementation has been studied in each of these conditions, associated or not to physical training, and a tendency to a beneficial effect has been identified. As to sarcopenia, although recent data suggest that Cr supplementation is likely to be beneficial (Candow 2011; Candow et al. 2014; Moon et al. 2013; Smith et al. 2014), its long-term efficacy to the elderly population needs to be further investigated before including its supplementation as an established recommended strategy.
In general, the effects of Cr discussed so far, in terms of a single cell’s fate mean higher viability, higher capacity to survive stressful situations and to conserve the ability to differentiate into the mature cellular phenotype. It is important to note that Cr has been shown to exert these effects not only in muscle cells and neurons, but also in other cell types such as osteoblasts (Gerber et al. 2005), as well as in endothelial and promonocytic cells (Sestili et al. 2006). Indeed, although Cr activity might be stronger in high energy demanding tissues/cell types such as muscle, kidney and brain cells, by virtue of its fundamental role in cellular biochemistry and pleiotropic activity, Cr might be beneficial to a broader range of cell types facing adverse conditions.
Cell replacement therapy offers great promise to the future of regenerative medicine in its bid to treat a wide range of pathologies, such as neurological, musculoskeletal, ostheoarticular, dermatological, ophtalmological, pulmonary and cardiovascular diseases or for replacing cells in infarcted/damaged organs that hardly have any intrinsic renewal capacity. However, existing strategies are restricted by low cell survival which limits either the in vitro expansion of cell populations or their engraftment/homing in receiving organs and tissues. A strategy to circumvent this limitation consists in triggering survival and adaptive signaling cascades or identifying prosurvival factors to expand and pre-condition autologous stem cells (Ding and Schultz 2005; Pourrajab et al. 2014). As to cell replacement in the infarcted heart, for example, It has been reported that overexpression of IGF-1 in mesenchymal stem cells improved their survival and engraftment and promoted stem cell incorporation through paracrine release of stromal cell-derived factor-1α (Tang et al. 2011). Incidentally Cr is known to increase the expression level of IGF-1 in SM cells (Louis et al. 2004; Sestili et al. 2009) and might promote effects similar to those reported by Tang et al. (2011). Furthermore, Cr has recently been shown to enhance the metabolic state of murine and human neuronal stem cells and to promote expansion, migration and neuronal induction (Andres et al. unpublished).
Cr could then represent a rational candidate as an effective pro-survival, small molecule (Ding and Schultz 2005) in that it promotes cell viability, growth, differentiation and function—as for brain and muscle cells—with virtually no limitation with regard to other cell types. Indeed Cr has a cell type-independent biological relevance: all the cells and their mitochondria use it for energy purposes and accumulate Cr via CrTs in the intracellular fraction where it is trapped and can exert its long lasting, beneficial and pleiotropic activity.
Thus the inclusion of millimolar Cr as a routine, pro-survival component of growth media for ex vivo culture and expansion of autologous stem cells deserves consideration and might represent a simple, costless and promising option to reduce some of the problems afflicting cell replacement therapies.
Conclusions
An increasing body of evidence accumulated over the last two decades (see Table 1) suggests that Cr has trophic and pro-differentiation activities in brain and muscle cells, in vitro and in vivo settings, and protective effects against different stressors which reduce their viability and/or differentiation capacity. Apart from the established importance as a sport dietary supplement, this emerging scenario not only strengthens the rationale for using Cr as adjunctive/causative treatment in specific brain, muscular and neuromuscular pathological situations (Beal 2011; Gualano et al. 2012; Rae and Broer 2015; Tarnopolsky 2007), but also paves the road to the utilization of Cr in other clinical directions whose exploitation deserves consideration.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- AGAT:
-
Arginine:glycine amidinotransferase
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- Cr:
-
Creatine
- PCr:
-
Phospho-creatine
- CK:
-
Creatine kinase
- CrT:
-
Creatine transporter
- GSH:
-
Glutathione
- GAMT:
-
Guanidinoacetate methyltransferase
- LTP:
-
Long-term potentiation
- ROS:
-
Reactive oxygen species
- SM:
-
Skeletal muscle
References
Adcock KH, Nedelcu J, Loenneker T, Martin E, Wallimann T, Wagner BP (2002) Neuroprotection of creatine supplementation in neonatal rats with transient cerebral hypoxia-ischemia. Dev Neurosci 24(5):382–388
Alfieri RR, Bonelli MA, Cavazzoni A, Brigotti M, Fumarola C, Sestili P, Mozzoni P, De Palma G, Mutti A, Carnicelli D, Vacondio F, Silva C, Borghetti AF, Wheeler KP, Petronini PG (2006) Creatine as a compatible osmolyte in muscle cells exposed to hypertonic stress. J Physiol 576(Pt 2):391–401
Allahyar R, Iqbal F (2014) Creatine monohydrate supplemented diets affect serum inflammatory cytokine levels (IL-6 and IL-18) in female albino mice following neonatal hypoxic ischemic encephalopathy. Pak J Zool 46(5):1311–1315
Allahyar R, Akbar A, Iqbal F (2015) Creatine monohydrate supplementation for 10 weeks mediates neuroprotection and improves learning/memory following neonatal hypoxia ischemia encephalopathy in female albino mice. Brain Res 1595:92–100
Allen PJ (2012) Creatine metabolism and psychiatric disorders: does creatine supplementation have therapeutic value? Neurosci Biobehav Rev 36(5):1442–1462
Allen PJ, DeBold JF, Rios M, Kanarek RB (2015) Chronic high-dose creatine has opposing effects on depression-related gene expression and behavior in intact and sex hormone-treated gonadectomized male and female rats. Pharmacol Biochem Behav 130:22–33
Almeida LS, Salomons GS, Hogenboom F, Jakobs C, Schoffelmeer AN (2006) Exocytotic release of creatine in rat brain. Synapse 60(2):118–123
Alves CR, Ferreira JC, de Siqueira-Filho MA, Carvalho CR, Lancha AH Jr, Gualano B (2012) Creatine-induced glucose uptake in type 2 diabetes: a role for AMPK-alpha? Amino Acids 43(4):1803–1807
Andres R, Ducray A, Huber A, Pérez-Bouza A, Krebs S, Schlattner U, Seiler R, Wallimann T, Widmer HR (2005) Effects of creatine treatment on survival and differentiation of GABA-ergic neurons in cultured striatal tissue. J Neurochem 95(1):33–45
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR (2008) Functions and effects of creatine in the central nervous system. Brain Res Bull 76(4):329–343
Ardite E, Barbera JA, Roca J, Fernandez-Checa JC (2004) Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation. Am J Pathol 165(3):719–728
Barbieri E, Sestili P (2012) Reactive oxygen species in skeletal muscle signaling. J Signal Transduct 2012:982794
Barbieri E, Battistelli M, Casadei L, Vallorani L, Piccoli G, Guescini M, Gioacchini AM, Polidori E, Zeppa S, Ceccaroli P, Stocchi L, Stocchi V, Falcieri E (2011) Morphofunctional and biochemical approaches for studying mitochondrial changes during myoblasts differentiation. J Aging Res 2011:845379
Barbieri E, Calcabrini C, Guescini M, Vallorani L, Luchetti F, Canonico B, Ciacci C, Casadei L, Gioacchini AM, Battistelli M, Falcieri E, Stocchi V, Sandri M, Sestili P (2013a) Creatine supplementation enhances the mitochondrial function in oxidatively injured myoblasts. Eur J Transl Myol Basic Appl Myol 23:130–131
Barbieri E, Sestili P, Vallorani L, Guescini M, Calcabrini C, Gioacchini AM, Annibalini G, Lucertini F, Piccoli G, Stocchi V (2013b) Mitohormesis in muscle cells: a morphological, molecular, and proteomic approach. Muscles Ligaments Tendons J 3(4):254–266
Beal MF (2011) Neuroprotective effects of creatine. Amino Acids 40(5):1305–1313
Bender A, Beckers J, Schneider I, Holter SM, Haack T, Ruthsatz T, Vogt-Weisenhorn DM, Becker L, Genius J, Rujescu D, Irmler M, Mijalski T, Mader M, Quintanilla-Martinez L, Fuchs H, Gailus-Durner V, de Angelis MH, Wurst W, Schmidt J, Klopstock T (2008) Creatine improves health and survival of mice. Neurobiol Aging 29(9):1404–1411
Berneburg M, Gremmel T, Kurten V, Schroeder P, Hertel I, von Mikecz A, Wild S, Chen M, Declercq L, Matsui M, Ruzicka T, Krutmann J (2005) Creatine supplementation normalizes mutagenesis of mitochondrial DNA as well as functional consequences. J Invest Dermatol 125(2):213–220
Berti SL, Nasi GM, Garcia C, Castro FL, Nunes ML, Rojas DB, Moraes TB, Dutra-Filho CS, Wannmacher CM (2012) Pyruvate and creatine prevent oxidative stress and behavioral alterations caused by phenylalanine administration into hippocampus of rats. Metab Brain Dis 27(1):79–89
Bortoluzzi VT, de Franceschi ID, Rieger E, Wannmacher CM (2014) Co-administration of creatine plus pyruvate prevents the effects of phenylalanine administration to female rats during pregnancy and lactation on enzymes activity of energy metabolism in cerebral cortex and hippocampus of the offspring. Neurochem Res 39(8):1594–1602
Bothwell JH, Styles P, Bhakoo KK (2002) Swelling-activated taurine and creatine effluxes from rat cortical astrocytes are pharmacologically distinct. J Membr Biol 185(2):157–164
Braissant O, Henry H, Villard AM, Speer O, Wallimann T, Bachmann C (2005) Creatine synthesis and transport during rat embryogenesis: spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev Biol 5:9
Braissant O, Bachmann C, Henry H (2007) Expression and function of AGAT, GAMT and CT1 in the mammalian brain. Subcell Biochem 46:67–81
Braissant O, Henry H, Beard E, Uldry J (2011) Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 40(5):1315–1324
Buck M, Chojkier M (1996) Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J 15(8):1753–1765
Burke DG, Candow DG, Chilibeck PD, MacNeil LG, Roy BD, Tarnopolsky MA, Ziegenfuss T (2008) Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int J Sport Nutr Exerc Metab 18(4):389–398
Candow DG (2011) Sarcopenia: current theories and the potential beneficial effect of creatine application strategies. Biogerontology 12(4):273–281
Candow DG, Chilibeck PD, Forbes SC (2014) Creatine supplementation and aging musculoskeletal health. Endocrine 45(3):354–361
Cannata DJ, Ireland Z, Dickinson H, Snow RJ, Russell AP, West JM, Walker DW (2010) Maternal creatine supplementation from mid-pregnancy protects the diaphragm of the newborn spiny mouse from intrapartum hypoxia-induced damage. Pediatr Res 68(5):393–398
Caretti A, Bianciardi P, Marini M, Abruzzo PM, Bolotta A, Terruzzi C, Lucchina F, Samaja M (2013) Supplementation of creatine and ribose prevents apoptosis and right ventricle hypertrophy in hypoxic hearts. Curr Pharm Des 19(39):6873–6879
Ceddia RB, Sweeney G (2004) Creatine supplementation increases glucose oxidation and AMPK phosphorylation and reduces lactate production in L6 rat skeletal muscle cells. J Physiol 555(Pt 2):409–421
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84(1):209–238
Christie DL (2007) Functional insights into the creatine transporter. Subcell Biochem 46:99–118
Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122(2):289–301
Coughlan KA, Valentine RJ, Ruderman NB, Saha AK (2014) AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes 7:241–253
Cunha MP, Martin-de-Saavedra MD, Romero A, Parada E, Egea J, Del Barrio L, Rodrigues AL, Lopez MG (2013) Protective effect of creatine against 6-hydroxydopamine-induced cell death in human neuroblastoma SH-SY5Y cells: involvement of intracellular signaling pathways. Neuroscience 238:185–194
Darabi S, Tiraihi T, Delshad A, Sadeghizadeh M (2013) A new multistep induction protocol for the transdifferentiation of bone marrow stromal stem cells into GABAergic neuron-like cells. Iran Biomed J 17(1):8–14
Deldicque L, Louis M, Theisen D, Nielens H, Dehoux M, Thissen JP, Rennie MJ, Francaux M (2005) Increased IGF mRNA in human skeletal muscle after creatine supplementation. Med Sci Sports Exerc 37(5):731–736
Deldicque L, Theisen D, Bertrand L, Hespel P, Hue L, Francaux M (2007) Creatine enhances differentiation of myogenic C2C12 cells by activating both p38 and Akt/PKB pathways. Am J Physiol Cell Physiol 293(4):C1263–C1271
Deminice R, Jordao A (2012) Creatine supplementation reduces oxidative stress biomarkers after acute exercise in rats. Amino Acids 43(2):709–715
Di Santo S, Mina A, Ducray A, Widmer HR, Senn P (2014) Creatine supports propagation and promotes neuronal differentiation of inner ear progenitor cells. Neuroreport 25(7):446–451
Dickinson H, Ellery S, Ireland Z, LaRosa D, Snow R, Walker DW (2014) Creatine supplementation during pregnancy: summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in high-risk human pregnancy. BMC Pregnancy Childbirth 14:150
Ding S, Schultz PG (2005) Small molecules and future regenerative medicine. Curr Top Med Chem 5(4):383–395
Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T (2003) Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem 278(20):17760–17766
Duan B, Liu DS, Huang Y, Zeng WZ, Wang X, Yu H, Zhu MX, Chen ZY, Xu TL (2012) PI3-kinase/Akt pathway-regulated membrane insertion of acid-sensing ion channel 1a underlies BDNF-induced pain hypersensitivity. J Neurosci 32(18):6351–6363
Ducray A, Kipfer S, Huber AW, Andres RH, Seiler RW, Schlattner U, Wallimann T, Widmer HR (2006) Creatine and neurotrophin-4/5 promote survival of nitric oxide synthase-expressing interneurons in striatal cultures. Neurosci Lett 395(1):57–62
Ducray AD, Qualls R, Schlattner U, Andres RH, Dreher E, Seiler RW, Wallimann T, Widmer HR (2007a) Creatine promotes the GABAergic phenotype in human fetal spinal cord cultures. Brain Res 1137(1):50–57
Ducray AD, Schlappi JA, Qualls R, Andres RH, Seiler RW, Schlattner U, Wallimann T, Widmer HR (2007b) Creatine treatment promotes differentiation of GABA-ergic neuronal precursors in cultured fetal rat spinal cord. J Neurosci Res 85(9):1863–1875
Ellery SJ, Ireland Z, Kett MM, Snow R, Walker DW, Dickinson H (2013) Creatine pretreatment prevents birth asphyxia-induced injury of the newborn spiny mouse kidney. Pediatr Res 73(2):201–208
Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF (2000) Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci 20(12):4389–4397
Fimognari C, Sestili P, Lenzi M, Cantelli-Forti G, Hrelia P (2009) Protective effect of creatine against RNA damage. Mutat Res 670(1–2):59–67
Forcales SV, Puri PL (2005) Signaling to the chromatin during skeletal myogenesis: novel targets for pharmacological modulation of gene expression. Semin Cell Dev Biol 16(4–5):596–611
Fu X, Zhao JX, Zhu MJ, Foretz M, Viollet B, Dodson MV, Du M (2013) AMP-activated protein kinase alpha1 but not alpha2 catalytic subunit potentiates myogenin expression and myogenesis. Mol Cell Biol 33(22):4517–4525
Gerber I, ap Gwynn I, Alini M, Wallimann T (2005) Stimulatory effects of creatine on metabolic activity, differentiation and mineralization of primary osteoblast-like cells in monolayer and micromass cell cultures. Eur Cell Mater 10:8–22
Gualano B, Roschel H, Lancha-Jr AH, Brightbill CE, Rawson ES (2012) In sickness and in health: the widespread application of creatine supplementation. Amino Acids 43(2):519–529
Guidi C, Potenza L, Sestili P, Martinelli C, Guescini M, Stocchi L, Zeppa S, Polidori E, Annibalini G, Stocchi V (2008) Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochim Biophys Acta 1780(1):16–26
Guimaraes-Ferreira L, Pinheiro CH, Gerlinger-Romero F, Vitzel KF, Nachbar RT, Curi R, Nunes MT (2012) Short-term creatine supplementation decreases reactive oxygen species content with no changes in expression and activity of antioxidant enzymes in skeletal muscle. Eur J Appl Physiol 112(11):3905–3911
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30(2):214–226
Hamai N, Nakamura M, Asano A (1997) Inhibition of mitochondrial protein synthesis impaired C2C12 myoblast differentiation. Cell Struct Funct 22(4):421–431
Handschin C, Spiegelman BM (2006) Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27(7):728–735
Hansen JM, Klass M, Harris C, Csete M (2007) A reducing redox environment promotes C2C12 myogenesis: implications for regeneration in aged muscle. Cell Biol Int 31(6):546–553
Herzberg NH, Zwart R, Wolterman RA, Ruiter JP, Wanders RJ, Bolhuis PA, van den Bogert C (1993) Differentiation and proliferation of respiration-deficient human myoblasts. Biochim Biophys Acta 1181(1):63–67
Hespel P, Op’t Eijnde B, Van Leemputte M, Urso B, Greenhaff PL, Labarque V, Dymarkowski S, Van Hecke P, Richter EA (2001) Oral creatine supplementation facilitates the rehabilitation of disuse atrophy and alters the expression of muscle myogenic factors in humans. J Physiol 536(Pt 2):625–633
Holterman CE, Rudnicki MA (2005) Molecular regulation of satellite cell function. Semin Cell Dev Biol 16(4–5):575–584
Holtzman D, Togliatti A, Khait I, Jensen F (1998) Creatine increases survival and suppresses seizures in the hypoxic immature rat. Pediatr Res 44(3):410–414
Hosamani R, Ramesh SR, Muralidhara (2010) Attenuation of rotenone-induced mitochondrial oxidative damage and neurotoxicty in Drosophila melanogaster supplemented with creatine. Neurochem Res 35(9):1402–1412
Ingwall JS (1976) Creatine and the control of muscle-specific protein synthesis in cardiac and skeletal muscle. Circ Res 38(5 Suppl 1):I115–I123
Ingwall JS, Morales MF, Stockdale FE (1972) Creatine and the control of myosin synthesis in differentiating skeletal muscle. Proc Natl Acad Sci USA 69(8):2250–2253
Ipsiroglu OS, Stromberger C, Ilas J, Hoger H, Muhl A, Stockler-Ipsiroglu S (2001) Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci 69(15):1805–1815
Ireland Z, Dickinson H, Snow R, Walker DW (2008) Maternal creatine: does it reach the fetus and improve survival after an acute hypoxic episode in the spiny mouse (Acomys cahirinus)? Am J Obstet Gynecol 198(4):e431–e436
Ireland Z, Russell AP, Wallimann T, Walker DW, Snow R (2009) Developmental changes in the expression of creatine synthesizing enzymes and creatine transporter in a precocial rodent, the spiny mouse. BMC Dev Biol 9:39
Ireland Z, Castillo-Melendez M, Dickinson H, Snow R, Walker DW (2011) A maternal diet supplemented with creatine from mid-pregnancy protects the newborn spiny mouse brain from birth hypoxia. Neuroscience 194:372–379
Jacquemin V, Butler-Browne GS, Furling D, Mouly V (2007) IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci 120(Pt 4):670–681
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 104(29):12017–12022
Kley RA, Tarnopolsky MA, Vorgerd M (2011) Creatine for treating muscle disorders. Cochrane Database Syst Rev 6:CD004760
Langen RC, Schols AM, Kelders MC, Van Der Velden JL, Wouters EF, Janssen-Heininger YM (2002) Tumor necrosis factor-alpha inhibits myogenesis through redox-dependent and -independent pathways. Am J Physiol Cell Physiol 283(3):C714–C721
Lenz H, Schmidt M, Welge V, Schlattner U, Wallimann T, Elsasser HP, Wittern KP, Wenck H, Stab F, Blatt T (2005) The creatine kinase system in human skin: protective effects of creatine against oxidative and UV damage in vitro and in vivo. J Invest Dermatol 124(2):443–452
Li XX, Tsoi B, Li YF, Kurihara H, He RR (2015) Cardiolipin and its different properties in mitophagy and apoptosis. J Histochem Cytochem 63(5):301–311
Liu J, Wang LN (2014) Mitochondrial enhancement for neurodegenerative movement disorders: a systematic review of trials involving creatine, coenzyme Q10, idebenone and mitoquinone. CNS Drugs 28(1):63–68
Louis M, Van Beneden R, Dehoux M, Thissen JP, Francaux M (2004) Creatine increases IGF-I and myogenic regulatory factor mRNA in C2C12 cells. FEBS Lett 557(1–3):243–247
Lowe DA, Lund T, Alway SE (1998) Hypertrophy-stimulated myogenic regulatory factor mRNA increases are attenuated in fast muscle of aged quails. Am J Physiol 275(1 Pt 1):C155–C162
Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, Chen Y (2010) PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal 13(7):1011–1022
Malcon C, Kaddurah-Daouk R, Beal MF (2000) Neuroprotective effects of creatine administration against NMDA and malonate toxicity. Brain Res 860(1):195–198
Mazibuko SE, Muller CJ, Joubert E, de Beer D, Johnson R, Opoku AR, Louw J (2013) Amelioration of palmitate-induced insulin resistance in C(2)C(1)(2) muscle cells by rooibos (Aspalathus linearis). Phytomedicine 20(10):813–819
Messina S, Bitto A, Aguennouz M, Minutoli L, Monici MC, Altavilla D, Squadrito F, Vita G (2006) Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp Neurol 198(1):234–241
Meyer LE, Machado LB, Santiago AP, da-Silva WS, De Felice FG, Holub O, Oliveira MF, Galina A (2006) Mitochondrial creatine kinase activity prevents reactive oxygen species generation: antioxidant role of mitochondrial kinase-dependent ADP re-cycling activity. J Biol Chem 281(49):37361–37371
Mobley CB, Fox CD, Ferguson BS, Amin RH, Dalbo VJ, Baier S, Rathmacher JA, Wilson JM, Roberts MD (2014) L-leucine, beta-hydroxy-beta-methylbutyric acid (HMB) and creatine monohydrate prevent myostatin-induced Akirin-1/Mighty mRNA down-regulation and myotube atrophy. J Int Soc Sports Nutr 11:38
Mohammad-Gharibani P, Tiraihi T, Mesbah-Namin SA, Arabkheradmand J, Kazemi H (2012) Induction of bone marrow stromal cells into GABAergic neuronal phenotype using creatine as inducer. Restor Neurol Neurosci 30(6):511–525
Moon A, Heywood L, Rutherford S, Cobbold C (2013) Creatine supplementation: can it improve quality of life in the elderly without associated resistance training? Curr Aging Sci 6(3):251–257
Moraes R, Van Bavel D, Moraes BS, Tibirica E (2014) Effects of dietary creatine supplementation on systemic microvascular density and reactivity in healthy young adults. Nutr J 13(1):115
Naia L, Ribeiro MJ, Rego AC (2011) Mitochondrial and metabolic-based protective strategies in Huntington’s disease: the case of creatine and coenzyme Q. Rev Neurosci 23(1):13–28
Nasrallah F, Feki M, Kaabachi N (2010) Creatine and creatine deficiency syndromes: biochemical and clinical aspects. Pediatr Neurol 42(3):163–171
Nomura A, Zhang M, Sakamoto T, Ishii Y, Morishima Y, Mochizuki M, Kimura T, Uchida Y, Sekizawa K (2003) Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br J Pharmacol 139(4):715–720
Ohira Y, Matsuoka Y, Kawano F, Ogura A, Higo Y, Ohira T, Terada M, Oke Y, Nakai N (2011) Effects of creatine and its analog, beta-guanidinopropionic acid, on the differentiation of and nucleoli in myoblasts. Biosci Biotechnol Biochem 75(6):1085–1089
Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M (2006) Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol 573(Pt 2):525–534
Otten JV, Fitch CD, Wheatley JB, Fischer VW (1986) Thyrotoxic myopathy in mice: accentuation by a creatine transport inhibitor. Metabolism 35(6):481–484
Owen L, Sunram-Lea SI (2011) Metabolic agents that enhance ATP can improve cognitive functioning: a review of the evidence for glucose, oxygen, pyruvate, creatine, and L-carnitine. Nutrients 3(8):735–755
Partridge TA, Grounds M, Sloper JC (1978) Evidence of fusion between host and donor myoblasts in skeletal muscle grafts. Nature 273(5660):306–308
Passaquin AC, Renard M, Kay L, Challet C, Mokhtarian A, Wallimann T, Ruegg UT (2002) Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscul Disord: NMD 12(2):174–182
Pena-Altamira E, Crochemore C, Virgili M, Contestabile A (2005) Contestabile A (2005) Neurochemical correlates of differential neuroprotection by long-term dietary creatine supplementation. Brain Res 1058(1–2):183–188
Perasso L, Spallarossa P, Gandolfo C, Ruggeri P, Balestrino M (2013) Therapeutic use of creatine in brain or heart ischemia: available data and future perspectives. Med Res Rev 33(2):336–363
Pizza FX, Peterson JM, Baas JH, Koh TJ (2005) Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol 562(Pt 3):899–913
Pold R, Jensen LS, Jessen N, Buhl ES, Schmitz O, Flyvbjerg A, Fujii N, Goodyear LJ, Gotfredsen CF, Brand CL, Lund S (2005) Long-term AICAR administration and exercise prevents diabetes in ZDF rats. Diabetes 54(4):928–934
Pourrajab F, Babaei Zarch M, Baghi Yazdi M, Rahimi Zarchi A, Vakili Zarch A (2014) Application of stem cell/growth factor system, as a multimodal therapy approach in regenerative medicine to improve cell therapy yields. Int J Cardiol 173(1):12–19
Prass K, Royl G, Lindauer U, Freyer D, Megow D, Dirnagl U, Stockler-Ipsiroglu G, Wallimann T, Priller J (2007) Improved reperfusion and neuroprotection by creatine in a mouse model of stroke. J Cereb Blood Flow Metab 27(3):452–459
Rae CD, Broer S (2015) Creatine as a booster for human brain function. How might it work? Neurochem Int 89:249–259
Rahimi R (2011) Creatine supplementation decreases oxidative DNA damage and lipid peroxidation induced by a single bout of resistance exercise. J Strength Cond Res 25(12):3448–3455
Rambo L, Ribeiro L, Della-Pace I, Stamm D, da Rosa GR, Prigol M, Pinton S, Nogueira C, Furian A, Oliveira M, Fighera M, Royes L (2013) Acute creatine administration improves mitochondrial membrane potential and protects against pentylenetetrazol-induced seizures. Amino Acids 44(3):857–868
Ribas F, Villarroya J, Hondares E, Giralt M, Villarroya F (2014) FGF21 expression and release in muscle cells: involvement of MyoD and regulation by mitochondria-driven signalling. Biochem J 463(2):191–199
Rochard P, Rodier A, Casas F, Cassar-Malek I, Marchal-Victorion S, Daury L, Wrutniak C, Cabello G (2000) Mitochondrial activity is involved in the regulation of myoblast differentiation through myogenin expression and activity of myogenic factors. J Biol Chem 275(4):2733–2744
Sabourin LA, Rudnicki MA (2000) The molecular regulation of myogenesis. Clin Genet 57(1):16–25
Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA (2008) Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics 32(2):219–228
Saks V, Kaambre T, Guzun R, Anmann T, Sikk P, Schlattner U, Wallimann T, Aliev M, Vendelin M (2007) The creatine kinase phosphotransfer network: thermodynamic and kinetic considerations, the impact of the mitochondrial outer membrane and modelling approaches. Subcell Biochem 46:27–65
Santiago AP, Chaves EA, Oliveira MF, Galina A (2008) Reactive oxygen species generation is modulated by mitochondrial kinases: correlation with mitochondrial antioxidant peroxidases in rat tissues. Biochimie 90(10):1566–1577
Saraiva AL, Ferreira AP, Silva LF, Hoffmann MS, Dutra FD, Furian AF, Oliveira MS, Fighera MR, Royes LF (2012) Creatine reduces oxidative stress markers but does not protect against seizure susceptibility after severe traumatic brain injury. Brain Res Bull 87(2–3):180–186
Sartini S, Sestili P, Colombo E, Martinelli C, Bartolini F, Ciuffoli S, Lattanzi D, Sisti D, Cuppini R (2012) Creatine affects in vitro electrophysiological maturation of neuroblasts and protects them from oxidative stress. J Neurosci Res 90(2):435–446
Sartini S, Lattanzi D, Ambrogini P, Di Palma M, Galati C, Savelli D, Polidori E, Calcabrini C, Rocchi MB, Sestili P, Cuppini R (2015) Maternal creatine supplementation affects the morpho-functional development of hippocampal neurons in rat offspring. Neuroscience 312:120–129
Schulze A (2013) Creatine deficiency syndromes. Handb Clin Neurol 113:1837–1843
Sestili P, Martinelli C, Bravi G, Piccoli G, Curci R, Battistelli M, Falcieri E, Agostini D, Gioacchini AM, Stocchi V (2006) Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med 40(5):837–849
Sestili P, Barbieri E, Martinelli C, Battistelli M, Guescini M, Vallorani L, Casadei L, D’Emilio A, Falcieri E, Piccoli G, Agostini D, Annibalini G, Paolillo M, Gioacchini AM, Stocchi V (2009) Creatine supplementation prevents the inhibition of myogenic differentiation in oxidatively injured C2C12 murine myoblasts. Mol Nutr Food Res 53(9):1187–1204
Sestili P, Martinelli C, Colombo E, Barbieri E, Potenza L, Sartini S, Fimognari C (2011) Creatine as an antioxidant. Amino Acids 40(5):1385–1396
Sestili P, Barbieri E, Stocchi V (2015) Effects of creatine in skeletal muscle cells and in myoblasts differentiating under normal or oxidatively stressing conditions. Mini Rev Med Chem 16(1):4–11
Shamban AT (2009) Current and new treatments of photodamaged skin. Facial Plast Surg 25(5):337–346
Singh S, Canseco DC, Manda SM, Shelton JM, Chirumamilla RR, Goetsch SC, Ye Q, Gerard RD, Schneider JW, Richardson JA, Rothermel BA, Mammen PP (2014) Cytoglobin modulates myogenic progenitor cell viability and muscle regeneration. Proc Natl Acad Sci USA 111(1):E129–E138
Smith RN, Agharkar AS, Gonzales EB (2014) A review of creatine supplementation in age-related diseases: more than a supplement for athletes. F1000Res 3:222
Stevens PR, Gawryluk JW, Hui L, Chen X, Geiger JD (2014) Creatine protects against mitochondrial dysfunction associated with HIV-1 Tat-induced neuronal injury. Curr HIV Res 12(6):378–387
Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY (2011) VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res 91(3):402–411
Tarnopolsky MA (2007) Clinical use of creatine in neuromuscular and neurometabolic disorders. Subcell Biochem 46:183–204
Tarnopolsky MA (2011) Creatine as a therapeutic strategy for myopathies. Amino Acids 40(5):1397–1407
Tidball JG (2005) Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288(2):R345–R353
Toscano A, Messina S, Campo GM, Di Leo R, Musumeci O, Rodolico C, Aguennouz M, Annesi G, Messina C, Vita G (2005) Oxidative stress in myotonic dystrophy type 1. Free Radic Res 39(7):771–776
Vierck JL, Icenoggle DL, Bucci L, Dodson MV (2003) The effects of ergogenic compounds on myogenic satellite cells. Med Sci Sports Exerc 35(5):769–776
Wallimann T (2015) The extended, dynamic mitochondrial reticulum in skeletal muscle and the creatine kinase (CK)/phosphocreatine (PCr) shuttle are working hand in hand for optimal energy provision. J Muscle Res Cell Motil 36(4):297–300
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40(5):1271–1296
Williamson DL, Butler DC, Alway SE (2009) AMPK inhibits myoblast differentiation through a PGC-1alpha-dependent mechanism. Am J Physiol Endocrinol Metab 297(2):E304–E314
Willoughby DS, Rosene J (2001) Effects of oral creatine and resistance training on myosin heavy chain expression. Med Sci Sports Exerc 33(10):1674–1681
Willoughby DS, Rosene JM (2003) Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med Sci Sports Exerc 35(6):923–929
Wyss M, Braissant O, Pischel I, Salomons GS, Schulze A, Stockler S, Wallimann T (2007) Creatine and creatine kinase in health and disease–a bright future ahead? Subcell Biochem 46:309–334
Young JF, Larsen LB, Malmendal A, Nielsen NC, Straadt IK, Oksbjerg N, Bertram HC (2010) Creatine-induced activation of antioxidative defence in myotube cultures revealed by explorative NMR-based metabonomics and proteomics. J Int Soc Sports Nutr 7(1):7–9
Zaccagnini G, Martelli F, Magenta A, Cencioni C, Fasanaro P, Nicoletti C, Biglioli P, Pelicci PG, Capogrossi MC (2007) p66(ShcA) and oxidative stress modulate myogenic differentiation and skeletal muscle regeneration after hind limb ischemia. J Biol Chem 282(43):31453–31459
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: T. Wallimann and R. Harris.
P. Ambrogini, E. Barbieri and S. Sartini have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sestili, P., Ambrogini, P., Barbieri, E. et al. New insights into the trophic and cytoprotective effects of creatine in in vitro and in vivo models of cell maturation. Amino Acids 48, 1897–1911 (2016). https://doi.org/10.1007/s00726-015-2161-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-2161-4