Abstract
Background
Neurodegenerative movement disorders mainly include Parkinson’s disease (PD), atypical parkinsonisms, Huntington’s disease (HD), and Friedreich’s ataxia (FA). With mitochondrial dysfunction observed in these diseases, mitochondrial enhancement such as creatine, coenzyme Q10 (CoQ10) and its analogues (idebenone and mitoquinone) has been regarded as a potential treatment.
Aim
In this paper, we systematically analysed and summarized the efficacy of mitochondrial enhancement in improving motor and other symptoms in neurodegenerative movement disorders.
Methods
We searched the electronic databases PubMed, EMBASE, CINAHL, Cochrane Library and China National Knowledge Infrastructure until September 2013 for eligible randomized controlled trials (RCTs), as well as unpublished and ongoing trials. We calculated the mean differences for continuous data with 95 % confidence intervals and pooled the results using a fixed-effect model, if no significant statistical heterogeneity was found (I 2 < 50 %).
Results
We included 16 studies with 1,557 randomized patients, which compared creatine, CoQ10 or its analogues with placebo in motor and other symptoms. No significant improvements were found in the motor symptoms of PD, atypical parkinsonisms or HD patients, while only the high dose of idebenone seems to be promising for motor improvement in FA. Certain benefits are found in other symptoms.
Conclusions
There is insufficient evidence to support the use of mitochondrial enhancement in patients with neurodegenerative movement disorders. More well-designed RCTs with large samples are required for further confirmation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Neurodegenerative diseases (NDs) are generally acknowledged as a group of neurological disorders with degeneration and loss of neurons, and most are with pathological accumulations in the brain. Clinical movement disorders are the main symptoms in some NDs, such as Parkinson’s disease (PD), atypical parkinsonisms, Huntington’s disease (HD), and Friedreich’s ataxia (FA). Meanwhile, non-motor symptoms are also common in the course of NDs. Although neurodegeneration is one of the most important topics in neuroscience research, and huge resources have been devoted into this field, there is still a lack of efficient methods to completely prevent the progression of these diseases. The current aim in clinical therapy is mainly focused on relieving symptoms and life quality improvement.
Mitochondrial dysfunction has been suggested to be involved in the pathophysiological process of NDs. Creatine, coenzyme Q10 (CoQ10) and its analogues, idebenone and mitoquinone (or MitoQ), are believed to complement energy by improving mitochondrial function. Therefore, they were regarded as potential neuroprotective agents for NDs [1]. Moreover, it has been found that both CoQ10 and creatine can inhibit the loss of nigral dopaminergic neurons in animal PD models [2, 3]. The incidence of ’PD has been found to correlate with the enzyme NADH-quinone oxidoreductase (NQO1) genotype frequencies [4]. NQO1 was proved to be crucially involved in pharmacologically important benzoquinone redox-shuttling in the cellular environment [5]. Gene COQ2 is in charge of encoding parahydroxybenzoate polyprenyltransferase, which is important for the biosynthesis of CoQ10. A recent study demonstrated the impaired gene COQ2 is closely associated with multiple system atrophy (MSA), a type of atypical parkinsonism [6].
So far, some randomized controlled trials (RCTs) have already been conducted which focused on mitochondrial enhancement such as creatine, CoQ10 and its analogues in comparison with placebo for the treatment of cardiovascular diseases, mitochondrial disorders and NDs with well tolerance. Certain benefits have been found, especially with idebenone, for the treatment of FA [7]. Hereby, we systematically analysed and summarized the efficacy of mitochondrial enhancement in improving motor and other symptoms in neurodegenerative movement disorders.
2 Methods
We searched the electronic databases PubMed, EMBASE, CINAHL, Cochrane Library and China National Knowledge Infrastructure (CNKI) until September 2013 for all possible trials. For unpublished and ongoing trials, we searched the US National Institute of Health clinical trial site (http://www.clinicaltrials.gov/) and the WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/). We also used Science Citation Index Cited Reference Search for forward tracking of important articles, as well as reference lists of relevant reviews and retrieved articles. The key search terms included: (creatine OR CoQ10 OR Idebenone OR Mitoquinone) AND (Parkinson’s disease OR Multiple system atrophy OR Progressive supranuclear palsy OR Corticobasal degeneration OR Dementia with Lewy bodies OR Huntington’s disease OR ataxia) and their Chinese equivalents. There were no language limitations. We only included RCTs with either a parallel or crossover design. Two authors (JL, LW) independently evaluated and included the eligible trials. We calculated the mean differences (MD) for continuous data with 95 % confidence intervals (CIs) based on the same instrument. For studies with more than one experimental group, we combined all relevant experimental groups of the study into a single group. Concerning missing standard deviations for changes from baseline, we calculated these with CIs, standard errors, and t- or p-values, according to the principles provided in the Cochrane handbook [8]. We pooled the results using a fixed-effect model, if no significant statistical heterogeneity was found (I 2 < 50 %). When there was significant clinical heterogeneity, a descriptive summary of the results was given. We planned to use funnel plots to examine potential publication bias if more than ten trials were involved in the meta-analysis. Sensitivity analysis was undertaken where necessary.
3 Results
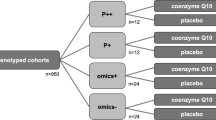
We identified a total of 796 references from the electronic database searches after excluding duplicates (Fig. 1). After screening of titles and abstracts, we obtained the full papers of 85 studies and assessed them for eligibility. According to the inclusion criteria, we included 16 studies with 1,557 randomized patients. Details of the included studies are provided in Table 1.
3.1 Efficacy in Motor Symptoms
As a result, seven RCTs with 802 randomized PD patients compared creatine, CoQ10 or its analogues with placebo [9–16]. An unpublished phase III trial on CoQ10 for PD compared placebo plus vitamin E (1,200 IU/day) versus CoQ10 at 1,200 mg/day plus vitamin E (1,200 IU/day) versus CoQ10 at 2,400 mg/day plus vitamin E (1,200 IU/day). No difference was seen among the groups when comparing the Unified Parkinson’s Disease Rating Scale (UPDRS) outcomes, and the study was halted as it was determined to be futile to continue. For atypical parkinsonisms, one RCT with 21 randomized progressive supranuclear palsy (PSP) patients reported improvement by PSP Rating Scale compared with placebo [17]. By meta-analysis, the change of motor score in UPDRS was MD −0.54, 95 % CI −1.08–0.00, p = 0.05; level of heterogeneity χ 2 = 5.14, degrees of freedom (df) = 5, p = 0.40, I 2 = 3 % (Fig. 2). By sensitivity analysis with random-effect model, no significance was found (MD −0.55, 95 % CI −1.11–0.02, p = 0.06; level of heterogeneity χ 2 = 5.14, df = 5, p = 0.40, I 2 = 3 %). In the forest plots, the larger boxes were correlated to the smaller standard deviation and meant more weight in the result of meta-analysis. For HD, two RCTs, respectively, with CoQ10 and creatine versus placebo found no difference in the changes of motor score or chorea score in the Unified Huntington’s Disease Rating Scale [18, 19], while one RCT with idebenone versus placebo found no difference in eye movement scale, chorea scale, and motor impairment scale [20]. Four RCTs on FA were measured by the International Cooperative Ataxia Rating Scale (ICARS). Three of these focused on idebenone versus placebo [21–23], and one RCT compared the efficacy between creatine and placebo [24]. No improvement of motor symptoms was found in creatine and low-dose idebenone [22–24]. However, the dose-related response of idebenone in FA was found, as well as the significant changes in ICARS in high-dose idebenone in comparison with placebo [21].
Changes of UPDRS motor score after mitochondrial enhancement in Parkinson’s disease and atypical parkinsonisms. As far as the changes of UPDRS motor score, no significant differences were found between mitochondrial enhancement and placebo. Creatine versus placebo for PD (Hass 2007 [10], The NINDS NET-PD 2006 [12]); CoQ10 versus placebo for PD (Müller 2003 [11], Shults 2002 [14], The NINDS NET-PD 2007 [13]); CoQ10 versus placebo for PSP (Stamelou 2008 [17]). CoQ10 coenzyme Q10, SD standard deviation, NINDS National Institute of Neurological Disorders and Stroke, NET-PD Neuroprotection Exploratory Trials in PD, PD Parkinson’s disease, PSP progressive supranuclear palsy, UPDRS Unified Parkinson’s Disease Rating Scale, df degrees of freedom
3.2 Efficacy in Other Symptoms
For PD and PSP patients, five RCTs reported changes in activities of daily living (ADL) score [10, 12–14, 17]. Significant difference was only found in the study by Shults et al. between CoQ10 and placebo for 16-month treatment in PD (MD −2.07, 95 % CI −3.72 to −0.42, p = 0.01) [14]. Other benefits were found, including improvements of 5 mg/day/kg nanoparticular CoQ10 for 6 weeks in PSP measured by the Frontal Assessment Battery, and cerebral energy metabolism on magnetic resonance spectroscopy [17]; improvements of 360 mg/day CoQ10 for 4 weeks in the Farnsworth-Munsell 100 Hue test in PD [11]; improvements of creatine with 20 g/day for 6 days, followed by 2 g/day for 6 months, and 4 g/day for remainder until 2 years in the UPDRS mentation, behaviour and mood in PD [9]; benefits of 600 mg/day CoQ10 for 30 months in the Brief Test of Attention and the Stroop test in HD [19]; and decrease in serum 8-hydroxy-2′-deoxyguanosine (8OHdG) by 8 g/day creatine for 16 weeks in HD, the indicator of oxidative injury to DNA [18]. Hypertrophic cardiomyopathy is the most common complication in FA patients. One RCT suggested that 5 mg/kg/day idebenone for 1 year could reduce interventricular septal thickness and left ventricular mass compared with placebo [23], while another study concluded idebenone could not decrease left ventricular hypertrophy or improve cardiac function [25].
4 Discussion
There were controversies surrounding the CoQ10 levels in PD patients. Earlier evidence from a Spanish group in 2000 did not demonstrate any statistically significant difference of CoQ10 levels in PD (with or without levodopa therapy) versus controls, even when normalizing for serum cholesterol levels [26]. Conversely, more recently a group evaluating levels of multiple nutritionally-derived antioxidants using a functional intracellular assay found that CoQ10 was more frequently functionally deficient in PD patients compared with controls [27]. So far, no evidence has suggested that ND patients were typically deficient in creatine, but poor permeability of the blood-brain barrier for creatine has been detected [28]. It is important to note that trials utilizing CoQ10 and creatine were not intended to replace patients with deficient levels, but rather to augment mitochondrial function, thereby enhancing neuronal bioenergetics. Therefore, selectively treating ND patients with deficiencies may still yield similar results.
Furthermore, no studies evaluated the efficacy of idebenone in PD or atypical parkinsonisms, although CoQ10 has been widely applied. Actually, the pharmacological differences in CoQ10 and its analogues may cause the different effects [29, 30]. Although the use of idebenone as a neuroprotective agent is a worthy idea, there was only an isolated in vitro study suggesting that idebenone induced apoptotic cell death in a commonly used cell line in the study of PD, human dopaminergic SHSY-5Y cells, which may explain why this has not been done to date [31]. Due to insufficient data, subgroup analyses focusing on the different populations of PD patients such as those with decreased complex I activity or parkin/PINK1 mutations, were not available. As far as atypical parkinsonisms are concerned, only one RCT evaluated the efficacy of CoQ10 in PSP patients, while other types such as MSA, dementia with Lewy body and corticobasal degeneration have still not been investigated in terms of any mitochondrial enhancement. In regard to positive association between the impaired gene COQ2 and MSA, CoQ10 and its analogues can be tested as potential interventions for patients with MSA in future RCTs.
Although none of the included studies reported positive findings in the UPDRS motor score, a borderline result was found from data synthesis. Therefore, we should carefully explain the negative results based on current human trials, which might be attributed to the study limitations. Duration of treatment for more than 2 years was only found in one RCT, which focused on HD. The shorter duration of trials, compared with typical duration of disease progression, might affect the effects. Moreover, it would be better to identify at-risk patients and start these treatments earlier. It is thought that the failure of these treatments may, in part, be due to difficulty reversing the considerable damage needed to cause clinically significant symptoms. The measurement of outcomes need to be more accurate. For instance, 8OHdG was thought to predict the progression of HD. However, a recent study suggested that 8OHdG was not the proper biomarker [32]. The limitations in animal models of NDs, especially for PD, can be a possible reason for the difference of therapy effects [33]. Finally, there was minimal evidence for therapeutic strategies to target atypical parkinsonian disorders due to difficulty with early diagnosis.
The potential limitations should also be considered. Although we strictly performed the search as described in the methods, and identified 16 studies, we cannot assert there are no other unpublished studies that we failed to identify. Concerning the uncertain data expressed by graphs, no additional information was available in contacting the related authors. It definitely affected the completeness of evidence. A total of six RCTs were included in the meta-analysis; therefore, publication bias could not be examined by funnel plots.
5 Conclusions
There are insufficient data to support the usage of creatine, CoQ10 or its analogues in improving motor symptoms in patients with PD, PSP or HD. High-dose idebenone seems to be promising for FA patients, as measured by ICARS score. Regarding other symptoms, the findings are controversial but certain benefits are detected in creatine, CoQ10 or idebenone compared with placebo. Meanwhile, all these positive results need to be further confirmed by future well-designed studies.
References
Chaturvedi RK, Beal MF. Mitochondrial approaches for neuroprotection. Ann N Y Acad Sci. 2008;1147:395–412.
Horvath TL, Diano S, Leranth C, Garcia-Segura LM, Cowley MA, Shanabrough M, et al. Coenzyme Q induces nigral mitochondrial uncoupling and prevents dopamine cell loss in a primate model of Parkinson’s disease. Endocrinology. 2003;144:2757–60.
Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, et al. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol. 1999;157:142–9.
Harada S, Fujii C, Hayashi A, Ohkoshi N. An association between idiopathic Parkinson’s disease and polymorphisms of phase II detoxification enzymes: glutathione S-transferase M1 and quinone oxidoreductase 1 and 2. Biochem Biophys Res Commun. 2001;288:887–92.
Giorgio V, Petronilli V, Ghelli A, Carelli V, Rugolo M, Lenaz G, et al. The effects of idebenone on mitochondrial bioenergetics. Biochim Biophys Acta. 2012;1817:363–9.
The Multiple-System Atrophy Research Collaboration. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369:233–44.
Tsou AY, Friedman LS, Wilson RB, Lynch DR. Pharmacotherapy for Friedreich ataxia. CNS Drugs. 2009;23:213–23.
Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://www.cochrane-handbook.org.
Bender A, Koch W, Elstner M, Schombacher Y, Bender J, Moeschl M, et al. Creatine supplementation in Parkinson disease: a placebo-controlled randomized pilot trial. Neurology. 2006;67:1262–4.
Hass CJ, Collins MA, Juncos JL. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trial. Neurorehabil Neural Repair. 2007;21:107–15.
Müller T, Buttner T, Gholipour AF, Kuhn W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci Lett. 2003;341:201–4.
The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–71.
The NINDS NET-PD Investigators. A randomized clinical trial of coenzyme Q10 and GPI-1485 in early Parkinson disease. Neurology. 2007;68:20–8.
Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–50.
Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25:1670–4.
Storch A, Jost WH, Vieregge P, Spiegel J, Greulich W, Durner J, et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64:938–44.
Stamelou M, Reuss A, Pilatus U, Magerkurth J, Niklowitz P, Eggert KM, et al. Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial. Mov Disord. 2008;23:942–9.
Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology. 2006;66:250–2.
The Huntington Study Group. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57:397–404.
Ranen NG, Peyser CE, Coyle JT, Bylsma FW, Sherr M, Day L, et al. A controlled trial of idebenone in Huntington’s disease. Mov Disord. 1996;11:549–54.
Di Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high-dose idebenone in patients with Friedreich’s ataxia: a randomized, placebo-controlled trial. Lancet Neurol. 2007;6:878–86.
Lynch DR, Perlman SL, Meier T. A phase 3, double-blind, placebo-controlled trial of idebenone in friedreich ataxia. Arch Neurol. 2010;67:941–7.
Mariotti C, Solari A, Torta D, Marano L, Fiorentini C, Di Donato S. Idebenone treatment in Friedreich patients: one-year-long randomized placebo-controlled trial. Neurology. 2003;60:1676–9.
Schöls L, Zange J, Abele M, Schillings M, Skipka G, Kuntz-Hehner S, et al. L-carnitine and creatine in Friedreich’s ataxia: a randomized, placebo-controlled crossover trial. J Neural Transm. 2005;112:789–96.
Lagedrost SJ, Sutton MS, Cohen MS, Satou GM, Kaufman BD, Perlman SL, et al. Idebenone in Friedreich ataxia cardiomyopathy: results from a 6-month phase III study (IONIA). Am Heart J. 2011;161(639–645):e1.
Jiménez-Jiménez FJ, Molina JA, de Bustos F, García-Redondo A, Gómez-Escalonilla C, Martínez-Salio A, et al. Serum levels of coenzyme Q10 in patients with Parkinson’s disease. J Neural Transm. 2000;107:177–81.
Mischley LK, Allen J, Bradley R. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J Neurol Sci. 2012;318:72–5.
Braissant O. Creatine and guanidinoacetate transport at blood-brain and blood-cerebrospinal fluid barriers. J Inherit Metab Dis. 2012;35:655–64.
James AM, Cochemé HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–312.
Esposti MD, Ngo A, Ghelli A, Benelli B, Carelli V, McLennan H, et al. The interaction of Q analogs, particularly hydroxydecyl benzoquinone (idebenone), with the respiratory complexes of heart mitochondria. Arch Biochem Biophys. 1996;330:395–400.
Tai KK, Pham L, Truong DD. Idebenone induces apoptotic cell death in the human dopaminergic neuroblastoma SHSY-5Y cells. Neurotox Res. 2011;20:321–8.
Borowsky B, Warner J, Leavitt BR, Tabrizi SJ, Roos RA, Durr A, et al. 8OHdG is not a biomarker for Huntington disease state or progression. Neurology. 2013;80:1934–41.
Meissner W, Hill MP, Tison F, Gross CE, Bezard E. Neuroprotective strategies for Parkinson’s disease: conceptual limits of animal models and clinical trials. Trends Pharmacol Sci. 2004;25:249–53.
Acknowledgments
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
Jia Liu and Lu-ning Wang declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Wang, Ln. Mitochondrial Enhancement for Neurodegenerative Movement Disorders: A Systematic Review of Trials Involving Creatine, Coenzyme Q10, Idebenone and Mitoquinone. CNS Drugs 28, 63–68 (2014). https://doi.org/10.1007/s40263-013-0124-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-013-0124-4