Abstract
The effect of short-term creatine (Cr) supplementation upon content of skeletal muscle-derived-reactive oxygen species (ROS) was investigated. Wistar rats were supplemented with Cr (5 g/kg BW) or vehicle, by gavage, for 6 days. Soleus and extensor digitorum longus (EDL) muscles were removed and incubated for evaluation of ROS content using Amplex-UltraRed reagent. The analysis of expression and activity of antioxidant enzymes (superoxide dismutase 1 and 2, catalase and glutathione peroxidase) were performed. Direct scavenger action of Cr on superoxide radical and hydrogen peroxide was also investigated. Short-term Cr supplementation attenuated ROS content in both soleus and EDL muscles (by 41 and 33.7%, respectively). Cr supplementation did not change expression and activity of antioxidant enzymes. Basal TBARS content was not altered by Cr supplementation. In cell-free experiments, Cr showed a scavenger effect on superoxide radical in concentrations of 20 and 40 mM, but not on hydrogen peroxide. These results indicate that Cr supplementation decreases ROS content in skeletal muscle possibly due to a direct action of Cr molecule on superoxide radical.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Creatine (Cr) is synthesized in the kidney, pancreas and liver (especially in the later) from glycine, arginine and methionine (Persky et al. 2003). Its biosynthesis involves the action of two enzymes: arginine/glycine amidinotransferase (AGAT) and guanidinoacetate methyltransferase (GAMT). Daily, about 2 g of Cr are converted, through non-enzymatic reaction, in creatinine that freely cross cell membrane and is subsequently excreted by kidneys (Greenhaff 1997; Wyss and Kaddurah-Daouk 2000). The replenishment of Cr pool occurs both by endogenous synthesis as by food intake in a typical omnivorous diet.
Cr has important role in metabolism and contractile function in skeletal muscle. Total intracellular Cr content (both free and phosphorylated forms) is about 120–125 mmol/kg dry weight and 95 % of this amount is founded in skeletal muscle (Persky et al. 2003). Phosphocreatine (PCr) acts as an intracellular energy buffer, restoring ATP pool and also to buffer hydrogen ions associated with lactate production in fatiguing skeletal muscle (Stricker 1998; Feldman 1999). This energy buffer system is predominantly used at the beginning of muscle work, as well in efforts of very short duration and high intensity, when a faster ATP regeneration is required. Cr–PCr system is important to the strength production and relaxation capacity of skeletal muscle. The Cr depletion by β-guanidino-propionic acid (β-GPA) administration impairs contractile function in rat skeletal muscle (Petrofsky and Fitch 1980).
Apart from the use in sports, Cr supplementation has shown positive clinical effects in the treatment of some chronic diseases such as arthritis, congestive heart failure, muscular dystrophy, McArdle disease, mitochondrial diseases and neurological disorders (Persky et al. 2003). Most of these clinical disorders involve a dramatic increase in content of reactive oxygen species (ROS), such as superoxide anion radical (O ·−2 ), hydrogen peroxide (H2O2) and hydroxyl radical (OH·). Matthews et al. (1998) showed by the first time that oral Cr supplementation attenuates the generation of both hydroxyl radical and peroxynitrite in rats submitted to chronic administration of nitropropionic acid, an animal model of Huntington’s disease. This effect was similar to that observed when N-acetylcysteine (NAC), a potent unspecific antioxidant, was used in this model (Fontaine et al. 2000). The authors, however, did not attribute this protective effect to neither a direct ROS scavenger nor augmentation of antioxidant capacity in cells. Later, Lawler et al. (2002) utilizing non-cellular systems provided evidence that Cr acts as a radical scavenger, quenching diverse aqueous radical and reactive species. Moreover, Sestili et al. (2006) utilizing various cell lineages demonstrated similar results. However, the in vivo antioxidant effects of Cr supplementation in skeletal muscle tissue were not demonstrated yet.
The aim of this study was to evaluate the effects of short-term oral Cr supplementation on content of skeletal muscle-derived ROS as well in gene expression and activity of some antioxidant enzymes. This data help to explain in vivo effects of Cr supplementation, in terms of antioxidant potential, as well direct Cr effects upon ROS, in cell-free experiments. This data could contribute to explain in vivo effects of Cr supplementation, in terms of antioxidant potential, as well direct Cr effects upon ROS, in acellular systems.
Methods
Animals
Male Wistar rats (6–8 weeks and weighing 200–250 g) were obtained from the Institute of Biomedical Sciences of the University of São Paulo. The animals were maintained in groups of five with water and food ad libitum in a room with 12/12 h light–dark cycle at 22 °C. All experimental procedures were carried out in agreement with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Washington, DC). The experimental protocol was approved by the Ethical Committee for Animal Research (CEEA) of the Institute of Biomedical Sciences/University of São Paulo.
Creatine supplementation protocol
Creatine monohydrate supplementation (Sigma-Aldrich Corporation, St. Louis, MO, USA) was carried out by gavage, at the dose of 5 g/kg body weight/day, divided in 3 daily doses, for 6 days (creatine group). The control group received the same volume of vehicle (tap water) by the same route. This dose was chosen because it was capable of significantly increasing intracellular Cr pool (Op ’t Eijnde et al. 2001; Aoki et al. 2004) and induces functional effects on rat skeletal muscle (Bassit et al. 2010). According to Op ’t Eijnde et al. (2001) an increase in free Cr (30 %), phosphocreatine (15 %) and total Cr (20 %) content are observed after 5 days of supplementation. These effects were similar to the dose commonly used in humans (Casey et al. 1996; Greenhaff et al. 1994).
Measurement of muscle-derived ROS
The rats were supplemented with Cr to evaluate the effects on content of muscle-derived ROS.
The content of muscle-derived ROS was evaluated in incubated soleus and extensor digitorum longus (EDL) muscles. The measurement of ROS content during organ incubation was used to evaluate the impact of Cr supplementation on antioxidant capacity of skeletal muscle. Under anesthesia, muscles were quickly removed and immediately incubated in oxygenated (95 % O2–5 % CO2) Krebs solution in the presence of 50 μM Amplex® UltraRed reagent (Molecular Probes®, Eugene, OR, USA) and 0.1 U/mL horseradish (HRP) for 60 min. Amplex® dye has been used to measurement ROS in skeletal muscle preparations (Pinheiro et al. 2010, 2012). In the presence of peroxidase, the Amplex® reagent reacts with H2O2 in a 1:1 stoichiometry to produce the red-fluorescent oxidized product, resorufin (Zhou et al. 1997). HRP also catalyzes the decomposition of H2O2 to the hydroxyl radical, which is then reduced to water as a result of irreversible chemical oxidation of Amplex Red. Thus, this probe is specific for H2O2 detection. Amplex Red reagent is a highly sensitive and stable substrate for HRP with selectivity for H2O2. Resorufin is also stable and its long-wave spectrum results in reduced interference from autofluorescence in most biological samples. Amplex Red is widely used for the measurement of low levels of H2O2 in various biological samples and another advantage of this assay is that HRP is active over a wide pH range (Rhee et al. 2010). The fluorescence of resorufin was determined in 530 nm wavelength (excitation) and 590 nm wavelength (emission). Measurements of ROS were calibrated using a known dose of exogenous H2O2 (10 μM, positive control) in the presence or absence of CAT (300 U/mL), GPX (5 U/mL) and GSH (0.5 mM). All measurements were conducted in Krebs solution (1 mL), pH 7.4, at 37 °C. The content of H2O2 was calculated from the standard curve and the results were normalized in relation to muscle weight and expressed as percentage of control as described (Pinheiro et al. 2012).
Total mRNA extraction and real-time PCR
Total RNA was extracted from soleus and EDL muscles as previously described (Gerlinger-Romero et al. 2011). Two micrograms of total RNA were used to synthesize the first strand complementary DNA (cDNA) using oligo-dT primers and the M-MLV reverse transcriptase kit (Invitrogen Corp., Carlsbad, CA), according to the manufacturer’s recommendations. Reverse transcription reaction was performed at 70 °C for 10 min, followed by 37 °C for 60 min, and 10 min at 95 °C. Real-time quantitative polymerase chain reaction (PCR) amplification was performed using the SYBR® Green PCR master mix kit (Invitrogen Corp., Carlsbad, CA) and the primers for superoxide dismutase (Cu/Zn-SOD; forward: 5′-AAGCCTACAAAGTCAGCTCG-3′, reverse: 5′-GGTCTTGTTTCCTGCACTT-3′), mitochondrial manganese superoxide dismutase (Mn-SOD; forward: 5′-GGATTCATGTGCCAGGGTGG-3′, reverse: 5′-CACATGCTTGCCATCCAGCC-3′), catalase (Forward: 5′-GGATTCATGTGCCAGGGTGG-3′, reverse: 5′-CACATGCTTGCCATCCAGCC-3′), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; forward: 5′-GGATTCATGTGCCAGGGTGG-3′, reverse: 5′-CACATGCTTGCCATCCAGCC-3′) as internal control. The reaction conditions consisted of two steps at 50 °C for 2 min and 95 °C for 10 min, followed by 45 cycles of three steps: 20 s denaturation at 95 °C, 60 s annealing at 58 °C and 20 s at 72 °C, as described (Lawler et al. 2002). SYBR Green-based real-time PCR analysis was carried out with the Rotor-gene Q detector (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. RNA aliquots are routinely sham reverse transcribed (i.e., reverse transcriptase omitted) to ensure the absence of products other than those from the reverse transcribed mRNAs. The relative abundance of Cu/Zn-SOD, Mn-SOD, catalase and GAPDH mRNAs was calculated, using the 2−ΔΔCt method (Livak and Schmittgen 2001), and the results were expressed in arbitrary units (AU).
Analysis of antioxidant enzyme activities
The activities of catalase and glutathione peroxidase (GPX) were measured as previously described (Aebi 1984; Flohé and Günzler 1984). Soleus and EDL muscles were homogenized in 10 mM potassium phosphate buffer, pH 7.0, with a Polytron PT-3100 (Kinematica AG, Littau, Switzerland). The samples were centrifuged for 20 min at 13,000 rpm, 4 °C, and the supernatant were used for activity assays of both enzymes. Catalase activity was determined on spectrophotometer by measuring the breakdown of hydrogen peroxide at 30 °C and 240 nm (Aebi 1984). GPX activity was determined as described by Flohé and Günzler (1984), following the rate of NADPH oxidation, at 340 nm, 37 °C, in an assay medium containing 50 mM potassium phosphate buffer (pH 7.0), 0.15 mM NADPH, glutathione reductase (0.24 U/mL) and 1 mM reduced glutathione. The reaction was initiated by the addition of t-butyl hydroperoxide (1.2 mM).
Measurement of ROS scavenger action of Cr
The cell-free experiment consisted in analysis of quenching on H2O2 added to solution and superoxide generated by electrolysis of oxygenated Krebs solution. In these experiments, we evaluated the scavenger properties of Cr with no incubated organ producing ROS.
To evaluate the effects of Cr on H2O2, Amplex® Ultrared and HRP were utilized as describe above. Fluorescence was obtained in 4 conditions, in triplicates: (a) Amplex® + HRP (blank condition); (b) H2O2 (500 nM) + Amplex® + HRP (control condition); (c) creatine (10, 20 and 40 mM) + Amplex® + HRP (condition without H2O2); and creatine (10, 20 and 40 mM) + H2O2 (500 nM) + Amplex® + HRP. Results were expressed in absorbance (550 nm) normalized to blank condition. We use a Cr range (10–40 mM) that represents physiological levels of total Cr found within muscle cells (Kushmerick et al. 1992; McKenna et al. 1999; Lawler et al. 2002).
The effect of Cr on electrolysis-induced superoxide production was also evaluated. Krebs solution was previously oxygenated using 95 % O2–5 % CO2 gas mixture prior to electrical stimulation using 50 Hz of frequency, 0.5 ms pulse duration and 50 V for 10 min using silver electrodes in the presence of ferricytochrome c (Sigma-Aldrich Corporation, St. Louis, MO, USA). This condition leads to formation of ROS due to electrolysis, predominantly the hydroxyl radical and the superoxide anion radical at the side of the anode and cathode (Lecour et al. 1998). Superoxide production was monitored by ferricytochrome c reduction followed by sample read in a spectrophotometer at 550 nm using Krebs solution with no electrical stimulation as blank (Azzi et al. 1975).
Determination of muscle TBARS content
Thiobarbituric acid-reactive substances (TBARS) were measured as previously described (Winterbourn et al. 1985). Muscles were homogenized using scissor in saline buffer and the precipitated proteins were removed by centrifugation at 12,000g for 10 min. The supernatant (500 μL) was mixed with 500 μL thiobarbituric acid (1 % in 50 mM NaOH) and 500 μL of HCl at 25 %. The samples were then heated in a boiling water bath for 10 min and, after cooling, TBARS were extracted with 1.5 mL of n-butanol. The mixture was centrifuged at 12,000g for 10 min and the absorbance of the supernatant was determined at 532 nm. Thiobarbituric acid reacts with products of lipid peroxidation, mainly malondialdehyde, producing a colored compound. The results were expressed as micromoles of TBARS content relative to milligram of the total protein determined using Bradford’s method (Bradford 1976).
Statistical analysis
All data were expressed as mean ± SEM and the significance level was set at 5 % (P = 0.05) with 8–10 animals per group (n = 8–10). The statistical analysis was performed using an unpaired Student’s t test and one-way analysis of variance (ANOVA), when appropriate (Prism GraphPad, Version: 5.0; GraphPad Software Inc., San Diego, CA).
Results
Oral Cr supplementation during 6 days did not change muscle morphology: body weight and soleus and EDL wet weights/body weight (Table 1).
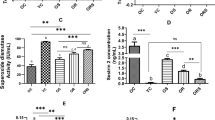
The hydrogen peroxide production by skeletal muscles was significantly lower in Cr-supplemented animals. This production was 59 and 76.3 % of the control values in soleus and EDL muscles, respectively (Fig. 1). In spite of this effect, supplementation did not change the levels of the antioxidant enzymes mRNA (Cu/Zn-SOD, Mn-SOD, and catalase) in either soleus or EDL muscles (Fig. 2), as well GPX and catalase activities (Table 2).
TBARS content was not affected by Cr supplementation in soleus (control: 0.95 ± 0.33; Cr: 0.90 ± 0.15 MDA eq.mg of protein−1) and EDL (control: 1.05 ± 0.24; Cr: 0.91 ± 0.28 MDA eq.mg of protein−1) muscles.
In cell-free experiments, Cr exerted a direct scavenger effect on superoxide radical in concentrations of 20 and 40 mM (Fig. 3a). This effect, however, was not verified on hydrogen peroxide in any concentration of Cr (Fig. 3b).
Scavenger effects of creatine on reactive oxygen species. Effect of creatine upon electrolysis-induced superoxide production in oxygenated Krebs solution (a); and lack of scavenger effect of creatine on hydrogen peroxide (500 nM) (b). *P < 0.05; **P < 0.01 versus control. Data are expressed as mean ± SEM
Discussion
Although the antioxidant properties of Cr have been previously demonstrated, in either cell-free or in vitro experiments, we reported herein that short-term Cr supplementation decreased ROS content in slow and fast-twitch skeletal muscles. It is the first demonstration of direct in vivo effects of Cr upon muscle redox state. The antioxidant effects of Cr could be due to modulation of expression and/or activities of antioxidant enzymes; a direct scavenger effect on ROS; or a modulation of ROS production in specific sites. We demonstrated herein that Cr supplementation did not change expression and activities of antioxidant enzymes. However, a direct scavenger effect of Cr on superoxide radical but not in hydrogen peroxide was observed.
The decline of ROS content in skeletal muscles after Cr supplementation may be explained by: a direct ROS scavenger action by Cr molecule; a stimulation of antioxidant enzymes expression and/or activities; or an effect upon ROS production through mitochondria or NADPH oxidase. Lawler et al. (2002) demonstrated that Cr molecule exerts a scavenger action on diverse radical and reactive species (superoxide anions and peroxynitrite, but not on hydrogen peroxide). These authors utilized cell-free systems and found a direct dose–response relationship between Cr concentration and antioxidant scavenger activity. Later, Sestili et al. (2006) tested the effects of Cr on the cytotoxicity caused by exogenous oxidants in human and murine myotubes. In spite of no alterations in the activities of catalase and GPX, Cr attenuated the cytotoxic effects of H2O2, butylhydroperoxide and peroxynitrite upon these cells corroborating with the results reported in the present study. This work provided information on the role of Cr as an antioxidant, indicating that the mechanism is dependent on a direct scavenging effect on free radicals. Our results also confirm this mechanism of Cr antioxidant action in skeletal muscle.
Skeletal muscles with predominance of oxidative fibers exhibit higher activities of antioxidant enzymes compared to glycolytic fibers (Oh-Ishi et al. 1995). Our results support these findings. Muscle fibers with higher oxidative capacity, such as predominantly in soleus, also have higher ROS production and this is an important stimulus to expression of antioxidant enzymes (see Dröge 2002 for review). Despite differences among oxidative and glycolytic skeletal muscles neither the soleus nor the EDL exhibits altered expression and activities of antioxidant enzymes in response to Cr supplementation, indicating that its redox action may occur by other mechanism. In the presence of Cr, the content of reduced cytochrome c was low indicating a direct scavenger effect of Cr on superoxide radical. Low content of superoxide results in low content of its ROS derivatives such as H2O2 (Dröge 2002). This effect of Cr on superoxide radical supports the proposition that the decrease of ROS content in skeletal muscles from supplemented animals may be due to an increase in intracellular Cr content.
Previous works of our group have demonstrated that Cr supplementation promotes significant attenuation of acute muscle fatigue similar to that observed when the antioxidant NAC was administered to adult rats prior to intense contractile activity (Bassit et al. 2010; Pinheiro et al. 2012). In both, the intervention decreased the markers of contraction-induced muscle injury. These results point to a possible antioxidant action of Cr in skeletal muscle tissue in addition to the metabolic and/or hormonal alterations already demonstrated (Greenhaff et al. 1994; Deldicque et al. 2005; Olsen et al. 2006). The decrease in ROS production was not enough to reduce the TBARS content. We believed that in a specific condition where TBARS is substantially elevated characterizing a chronic oxidative stress, Cr supplementation may exert an effect in this parameter.
Conclusion
We reported herein in vivo effect of short-term Cr supplementation on ROS content in skeletal muscle. In spite of no alterations in expression and activities of antioxidant enzymes, Cr appears to act directly as an ROS scavenger. These results support the proposition that Cr has antioxidant properties in skeletal muscle. ROS production has an important role in muscle wasting and contractile dysfunction observed in chronic diseases as diabetes, muscular dystrophy, chronic heart failure and obstructive pulmonary disease (for review see Moylan and Reid 2007). Thus, the decrease in ROS content in skeletal muscle in response to Cr supplementation suggests a possible therapeutic application in neuromuscular chronic diseases and stimulates further investigations focusing this issue.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Aoki MS, Lima WP, Miyabara EH, Gouveia CH, Moriscot AS (2004) Deleterious effects of immobilization upon rat skeletal muscle: role of creatine supplementation. Clin Nutr 23:1176–1183
Azzi A, Montecucco C, Richter C (1975) The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem Biophys Res Commun 65:597–603
Bassit RA, Pinheiro CH, Vitzel KF, Sproesser AJ, Silveira LR, Curi R (2010) Effect of short-term creatine supplementation on markers of skeletal muscle damage after strenuous contractile activity. Eur J Appl Physiol 108:945–955
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff PL (1996) Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Am J Physiol 271:E31–E37
Deldicque L, Louis M, Theisen D, Nielens H, Dehoux M, Thissen JP, Rennie MJ, Francaux M (2005) Increased IGF mRNA in human skeletal muscle after creatine supplementation. Med Sci Sports Exerc 37:731–736
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Feldman EB (1999) Creatine: a dietary supplement and ergogenic aid. Nutr Rev 57:45–50
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–121
Fontaine MA, Geddes JW, Banks A, Butterfield DA (2000) Effect of exogenous and endogenous antioxidants on 3-nitropionic acid-induced in vivo oxidative stress and striatal lesions: insights into Huntington’s disease. J Neurochem 75:1709–1715
Gerlinger-Romero F, Guimarães-Ferreira L, Giannocco G, Nunes MT (2011) Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMβ) increases the activity of the GH/IGF-I axis and induces hyperinsulinemia in rats. Growth Horm IGF Res 21:57–62
Greenhaff Pl (1997) The nutritional biochemistry of creatine. J Nutr Biochem 8:610–618
Greenhaff PL, Bodin K, Soderlund K, Hultman E (1994) Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Am J Physiol 266:E725–E730
Kushmerick MJ, Moerland TS, Wiseman RW (1992) Mammalian skeletal muscle fibers distinguished by contents of phosphocreatine, ATP, and Pi. Proc Natl Acad Sci USA 89:7521–7525
Lawler JM, Barnes WS, Wu G, Song W, Demaree S (2002) Direct antioxidant properties of creatine. Biochem Biophys Res Commun 290:47–52
Lecour S, Baouali AB, Maupoil V, Chahine R, Abadie C, Javouhey-Donzel A, Rochette L, Nadeau R (1998) Demonstration of the production of oxygen-centered free radicals during electrolysis using E. S. R. spintrapping techniques: effects on cardiac function in the isolated rat heart. Free Radic Biol Med 24:573–579
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2(−delta delta C(T)) method. Methods 25:402–408
Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF (1998) Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. J Neurosci 18:156–163
McKenna MJ, Morton J, Selig SE, Snow RJ (1999) Creatine supplementation increases muscle total creatine but not maximal intermittent exercise performance. J Appl Physiol 87:2244–2252
Moylan JS, Reid MB (2007) Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35:411–429
Oh-Ishi S, Kizaki T, Yamashita H, Nagata N, Suzuki K, Taniguchi N, Ohno H (1995) Alterations of superoxide dismutase iso-enzyme activity, content, and mRNA expression with aging in rat skeletal muscle. Mech Ageing Dev 84:65–76
Olsen S, Aagaard P, Kadi F, Tufekovic G, Verney J, Olesen JL, Suetta C, Kjaer M (2006) Creatine supplementation augments the increase in satellite cell and myonuclei number in human skeletal muscle induced by strength training. J Physiol 573:525–534
Op ‘t Eijnde B, Richter EA, Henquin JC, Kiens B, Hespel P (2001) Effect of creatine supplementation on creatine and glycogen content in rat skeletal muscle. Acta Physiol Scand 171:169–176
Persky AM, Brazeau GA, Hochhaus G (2003) Pharmacokinetics of the dietary supplement creatine. Clin Pharmacokinet 42:557–574
Petrofsky JS, Fitch CD (1980) Contractile characteristics of skeletal muscles depleted of phosphocreatine. Pflugers Arch 384:123–129
Pinheiro CH, Silveira LR, Nachbar RT, Vitzel KF, Curi R (2010) Regulation of glycolysis and expression of glucose metabolism-related genes by reactive oxygen species in contracting skeletal muscle cells. Free Radic Biol Med 48:953–960
Pinheiro CH, Vitzel KF, Curi R (2012) Effect of N-acetylcysteine on markers of skeletal muscle injury after fatiguing contractile activity. Scand J Med Sci Sports 22:24–33
Rhee SG, Chang TS, Jeong W, Kang D (2010) Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells 29:539–549
Sestili P, Martinelli C, Bravi G, Piccoli G, Curci R, Battistelli M, Falcieri E, Agostini D, Gioacchini AM, Stocchi V (2006) Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic Biol Med 40:837–849
Stricker PR (1998) Other ergogenic agents. Clin Sports Med 17:283–297
Winterbourn CC, Gutteridge JM, Halliwell B (1985) Doxorubicin-dependent lipid peroxidation at low partial pressures of O2. Free Radic Biol Med 1:43–49
Wyss M, Kaddurah-Daouk R (2000) Creatine and creatinine metabolism. Physiol Rev 80:1107–1213
Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP (1997) A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem 253:162–168
Acknowledgments
We are thankful to Leonice Lourenço Poyares for technical support. L.G-F., F.G-R., K.F.V. and R.T.N. are recipients of fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). C.H.J.P. is recipient of fellowship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). M.T.N. and R.C. are recipients of fellowships from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Michael Lindinger.
L. Guimarães-Ferreira and C.H.J. Pinheiro contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guimarães-Ferreira, L., Pinheiro, C.H.J., Gerlinger-Romero, F. et al. Short-term creatine supplementation decreases reactive oxygen species content with no changes in expression and activity of antioxidant enzymes in skeletal muscle. Eur J Appl Physiol 112, 3905–3911 (2012). https://doi.org/10.1007/s00421-012-2378-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-012-2378-9