Abstract
There is an extensive and still growing body of the literature supporting the efficacy of creatine (Cr) supplementation. In sports, creatine has been recognized as the most effective nutritional supplement in enhancing exercise tolerance, muscle strength and lean body mass. From a clinical perspective, the application of Cr supplementation is indeed exciting. Evidences of benefits from this supplement have been reported in a broad range of diseases, including myopathies, neurodegenerative disorders, cancer, rheumatic diseases, and type 2 diabetes. In addition, after hundreds of published studies and millions of exposures creatine supplementation maintains an excellent safety profile. Thus, we contend that the widespread application of this supplement may benefit athletes, elderly people and various patient populations. In this narrative review, we aimed to summarize both the ergogenic and therapeutic effects of Cr supplementation. Furthermore, we reviewed the impact of Cr supplementation on kidney function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the publication of the seminal paper by Professor Roger Harris and et al. (1992) pointing out that oral creatine (Cr) intake can augment its intramuscular content, the interest in Cr as a nutritional supplement has dramatically increased. Over the past two decades, the main focus of research has been on the ergogenic value of Cr. In fact, the consistent body of knowledge accumulated since then led some authors to consider Cr as the most effective nutritional supplement in enhancing exercise tolerance, muscle strength and lean body mass (Terjung et al. 2000; Buford et al. 2007). More recently, the clinical application of Cr supplementation has emerged as an exciting field of investigation. In this scenario, Cr has been recognized as a promising adjunct treatment in a broad spectrum of diseases, including those characterized by muscle wasting, low bone mass, joint syndromes, central nervous disturbances, and metabolic disturbances (Gualano et al. 2010a).
As a result of its efficacy in both clinical and sports settings, Cr supplementation has become one of the most popular nutritional supplements not only among amateur and professional athletes (Rawson and Clarkson 2004) but also among elderly and diseased people (Gualano et al. 2010a). However, there has been an empirical concern that long-term Cr supplementation might provoke adverse effects, particularly kidney dysfunction (Kuehl et al. 1998; Pritchard and Kalra 1998; Thorsteinsdottir et al. 2006). This has led some regulatory agencies (e.g., French Sanitary Agency and Brazilian National Agency for Sanitary Vigilance) to restrict the sale of Cr-based nutritional supplements to healthy adults, despite the well-known benefits of this nutrient for the elderly. In the light of the current scientific evidence, the position of such agencies can be challenged.
In this narrative review, we aimed to summarize both the ergogenic and therapeutic effects of Cr supplementation. In addition, the impact of Cr supplementation on kidney function was reviewed.
A brief overview of cr metabolism and its mechanisms of action
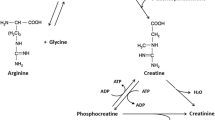
In addition to being present in the omnivore diet (approximately 1–5 grams per day), Cr is endogenously synthesized in the liver, kidney and pancreas by a two-step mechanism involving l-arginine: glycine amidinotransferase (AGAT) and S-adenosyl-l-methionine: N-guanidinoacetate methyltransferase (GAMT). Cellular Cr uptake is controlled by the specific transporter protein Creat-T. This moves Cr into the cell against a concentration gradient which is sodium and chloride dependent. Once inside the cell, Cr exists in a free and phosphorylated form (phosphorylcreatine, [PCr]). Both Cr and PCr are spontaneously and irreversibly degraded into creatinine, which is excreted via the kidneys in a rate of about 2 g per day (Wallimann et al. 2011).
Cr–PCr system exerts a crucial role in energy metabolism. Classically, it has been postulated that PCr acts a “temporal energy buffer” at sites of high energy translocation which operates when the rate of ATP utilization outstrips the rate of production by mitochondrial respiration, maintaining ATP homeostasis at specific sites of high energy turnover (e.g., skeletal muscle, brain, bone, heart) (Greenhalff 2001; Wallimann et al. 2011). A second role proposed for the Cr–PCr system is that of a cellular energy transport system (the “Cr–PCr shuttle”) from mitochondria to citosol, especially in tissues whose energetic demand is high, such as skeletal muscle and brain. This function is only possible due to different existent creatine kinase (CK) isoforms, which link the sites of ATP generation (i.e., mitochondria; Mt-CK) to those of ATP consumption (i.e., skeletal muscle and brain; MM-CK and BB-CK, respectively). This arrangement enables the Cr/PCr system to function as a “spatial energy buffer”. PCr and Cr have higher diffusion rates than ATP or ADP and, therefore, are more efficient as energy shuttles. At the sarcomere, where large amounts of ATP are hydrolyzed during repetitive contractions, the MM-CK allows for the immediate phosphorylation of ADP. This maintains a low ADP concentration, thus reducing the ADP-mediated leak of Ca2+ from the sarcoplasmic reticulum (SR), which would reduce the releasable Ca2+ and, hence, impair the force output of the muscle (Sahlin and Harris 2011). It also reduces the free inorganic phosphate (Pi), slowing the entry of Pi into the SR where it precipitates the Ca2+ to cause a failure of Ca2+ release, reducing the force produced by the muscle. Conversely, phosphorylation of Cr by Mi-CK keeps mitochondrial levels of ADP high, which stimulates the respiration rate and reduces the free energy required for ATP synthesis (Sahlin and Harris 2011; Greenhalff 2001). Moreover, it has been speculated that the coupling of Cr with ATP into the mitochondria could also attenuate the formation of reactive oxygen species. In fact, Cr posses a direct role Cr as a direct scavenger of radical species in an acellular setting (Sestili et al. 2011). The direct and indirect antioxidant effect of Cr may partially explain the therapeutic effects of Cr ingestion in a broad range of conditions, especially in neurodegenerative diseases (Beal 2011; Klopstock et al. 2011).
The ingestion of 20 g/day of Cr for 5 days can lead to more than 20% increase in muscle Cr, of which ~20% is in the form of PCr. However, it is important to mention that human muscle appears to have an upper limit of Cr storage of 150–160 mmol/kg dm, which, once achieved, with diet or supplementation, cannot be exceeded (Greenhalff 1995), meaning that Cr supplementation may produce larger effects in subjects who ingest lower Cr in diet (e.g., vegetarians) and consequently display lower tissue Cr (Harris et al. 1992). With respect to exercise performance, Cr supplementation may be ergogenic through several different mechanisms [reviewed in (Rawson and Persky 2007)], including: (i) increased pre-exercise PCr and glycogen, (ii) faster PCr resynthesis, (iii) increased expression of growth factors, (iv) educed muscle damage and inflammation, and (v) subsequently increased training volume. Eventually, Cr has also been reported to increase the Ca2+ sensitivity of the contractile proteins, by virtue of the reduction in ionic strength that occurs when water follows Cr osmotically into the muscle fiber (Head et al. 2011; Murphy et al. 2004). The ability to produce more force for a given intracellular concentration of Ca2+ would enhance muscle performance and alleviate some of the force loss resulting from reduced SR Ca2+ release during fatigue. In fact, osmotic effects offer an explanation for the ergogenic effect of Cr supplementation that is observed even before intramuscular PCr content has increased substantially. After longer-term Cr supplementation, when both the PCr/Cr ratio and the ionic strength have returned toward the pre-supplementation values, a beneficial effect on maximal force production persists (Murphy et al. 2004). Although the benefit of Cr supplementation on muscle performance is believed to be due to an increased ability to re-synthesize ATP via increased PCr concentration, human studies have showed a reduction in the PCr/Cr ratio after the supplementation, which would ultimately impair the ability of CK to rephosphorylate ADP to ATP. Therefore, a Cr-induced reduction in ionic strength as a consequence of water retention may be an alternative mechanism to explain the effects of Cr supplementation on muscle strength (Murphy et al. 2004).
The readers interested in deepen their knowledge on Cr-PCr system as well as in the bioenergetics and pleiotropic effects of Cr is referred to the special issue recently published in this journal (Amino Acids, vol. 40, 2011), namely to the papers by Sahlin and Harris (2011) and Wallimann et al. (2011).
Ergogenic effects of cr supplementation
There are several hundred published investigations of the effects of oral Cr supplementation, and the majority of studies with exercise performance outcomes support an ergogenic effect of Cr. Traditional supplementation protocols include a brief (about 5 d) high-dose (about 20 g/d or 0.3 g/kg/d) supplementation protocol (Harris et al. 1992) or a longer-duration (about 4–6 week) low-dose (about 3 g/d or 0.03 g/kg/d) supplementation protocol (Hultman et al. 1996). Unlike drugs, which can act rapidly, the content of nutrients like Cr must increase in skeletal muscle in order to have an effect. In this respect, “Cr loading” is much like “carbohydrate loading,” a well established, safe, and effective dietary practice. Both the short-duration and high-dose and the longer-duration low-dose Cr supplementation methods have been proven effective at increasing muscle Cr content by about 20%, and both methods have been shown to improve exercise performance [high-dose: reviewed in Branch (2003) and Bemben and Lamont (2005); low dose: Rawson et al. (2010)]. In particular, the performance of brief (<30 s) high-intensity exercise is improved following Cr supplementation, although some studies indicate performance enhancing effects in longer-duration exercise tasks (Table 1).
Resistance exercise performance
In a narrative review article based on 22 relevant studies, Rawson and Volek (2003) determined that the combination of Cr and resistance training increased maximal strength and number of repetitions at percent of maximal strength more than resistance training alone (8 and 14%, respectively). The beneficial effects of Cr supplementation on resistance training performance are well documented and are a consistent finding (Bemben and Lamont 2005; Hespel and Derave 2007). One could hypothesize that the benefits of Cr supplementation on resistance training performance are attributed to chronically increased training loads subsequent to increased pre-exercise muscle PCr and accelerated PCr resynthesis between sets/exercises. However, improvements in resistance training performance are not only noted in longer-term Cr supplementation plus resistance training studies [e.g., (Vandenberghe et al. 1997; Volek et al. 1999)] but also in shorter-term studies as well (Rossouw et al. 2000; Law et al. 2009). For instance, increased deadlift lifting volume in trained power-lifters ingesting Cr (25 g/d for 5 d) (Rossouw et al. 2000), and increased maximal squat strength after 5 days of Cr loading (20 g/d for 5 d) (Law et al. 2009) have been reported. Among the multiple metabolic, molecular, and physiological effects of Cr supplementation described above, Cr supplementation plus resistance training also increases muscle fiber size more than resistance training alone (Volek et al. 1999; Burke et al. 2008).
Sports performance
Cycling
The beneficial effects of Cr supplementation on sprint cycling performance in a laboratory setting have been well documented in numerous studies [reviewed in (Branch 2003; Bemben and Lamont 2005)]. In fact, Branch (2003) concluded that Cr supplementation was most effective at improving exercise performance during repeated bouts of brief (<30 s) high-intensity exercise. This is sensible when considering the large contribution of PCr to ATP production during brief, intermittent, high-intensity exercise. Although one would not expect Cr supplementation to improve endurance cycling performance, there is evidence that when sprints are imbedded during, or at the end of a cycling endurance challenge, as in real competitive cycling, Cr supplementation improves sprint cycling performance (Engelhardt et al. 1998; Vandebuerie et al. 1998).
Running
There are few studies of the effects of Cr supplementation on sprint running performance, but the available data suggest an ergogenic effect. For instance, Skare et al. (2001) reported that Cr supplementation (20 g/d) reduced 100 m sprint time by 0.9 s and 6 × 60 m total sprint time by 0.41 s. As with many sports, these improvements may seem minor in absolute magnitude, but might be the difference between winning and losing in competition. Given that some individuals experience a small increase in body mass following Cr ingestion, it is could be theorized that Cr supplementation might alter running mechanics and consequently be ergolytic in weight bearing activities. In fact, Schedel et al. (2000) reported that Cr supplementation (20 g/d for 7 d) increased body mass (0.8 kg), running speed, and stride frequency, but not stride length. However, Cox et al. (2002) reported improved 20 m sprint time and agility run performance despite an increase in body mass (0.8 kg). More work needs to be done in this area to determine if the small weight gain can offset the metabolic advantage and improved running performance associated with Cr supplementation.
Swimming
Seventeen studies of the effects of Cr supplementation on swim performance have been published, with the majority showing an ergogenic effect, particularly in those activities lasting ≤30 s (Branch 2003). In their review, Hopwood et al. (2006) noted that creatine supplementation benefits the performance of repeated (2–15 bouts) swim performance (improved performance in 8 of 9 studies; distance 25–100 m), but not single bouts of swimming (no effect of Cr in 7 of 8 studies; distance 25–400 m). This is in agreement with the literature on sprint cycling performance (i.e., beneficial effects of creatine more likely during repeated bouts than following a single bout). The magnitude of the improvement in swim performance subsequent to Cr ingestion may be small, but can be meaningful in competitive sport. As an example, Juhasz et al. (2009) recently reported about a 1.8 s decrease in swim time in two successive 100 m swims following Cr supplementation (20 g/d for 5 d) (n = 16 males). In the 2008 Beijing Olympic Games, the difference between 1st place and 10th place in the men’s 100 m freestyle swim was 1.07 s. Cr supplementation most likely influences swim performance through the mechanisms previously described, though Silva et al. (2007) recently showed that Cr supplementation reduces active drag force and hydrodynamic coefficient in swimmers, which reveals a beneficial effect of Cr on gross/propelling efficiency.
Other sports
The majority of studies on the ergogenic effects of Cr involved well-controlled laboratory performance tests, and, with the exception of cycling, running, and swimming performance, few studies are available that have described the effects of Cr on performance using sport-specific field tests. As such, it is difficult to develop a conclusion. Op ‘t Eijnde et al. (2001b)were unable to demonstrate a benefit of Cr supplementation (20 g for 5 d) on stroke precision, power, or error rates between the first and second services and on stroke precision, stroke velocity, and error rates during simulated competition in highly trained tennis players. (Pluim et al. 2006) were also unable to demonstrate a beneficial effect of Cr supplementation (0.3 g/kg for 6 d; followed by 0.03 g/kg for 28 d) on serving, forehand, or backhand velocity. It is possible that the tennis performance tests used in these studies were more a function of skill than energy availability, and so any performance enhancement from Cr supplementation would be difficult to detect. In soccer players, Cr supplementation (30 g/d for 7 d) has been shown to significantly improve dribbling test times (Ostojic 2004). Although there are few available data to describe the effects of Cr supplementation on sport-specific performance, many athletes use Cr to support their resistance training programs, with the expectation that improved performance in the weight room will translate into improved performance on the playing field/court. There is much work that can be done in the study of the effects of Cr supplementation on sports specific performance.
Therapeutic effects of cr supplementation
Muscle disorders
Due to its effects on muscle strength and lean mass (Terjung et al. 2000), Cr supplementation has been successfully used as an important adjuvant treatment in several myopathies, including muscle dystrophies, cytopathies, inflammatory myopathies, and peripheral neuropathy disorders (Tarnopolsky et al. 1997; Tarnopolsky and Martin 1999; Louis et al. 2003; Chung et al. 2007). Kley et al. (2008) reviewed the literature and concluded that Cr supplementation is able to promote both strength and lean mass gains in patients with some muscular dystrophies. Notably, patients with dystrophinopathies and myotonic dystrophy benefit the most from Cr treatment, whereas those with myotonic dystrophy type-1 and facioscapulohumeral dystrophy seem to be unresponsive. A recent randomized controlled trial (RCT) showed that Cr supplementation can enhance the effects of exercise training on muscle function in patients with dermatomyositis and polymyositis who were clinically weak after conventional pharmacological treatment (Chung et al. 2007). Given that the conventional drugs used in the treatment of myopathies (e.g., corticoids) may provoke serious adverse effects, non-inferiority trials should compare the efficacy and safety of Cr supplementation versus other current pharmacological treatments.
Bone and cartilage disorders
In vitro and animal studies have revealed an interesting potential of Cr supplementation in stimulating the development and differentiation of bone and cartilage cells (Funanage et al. 1992; Gerber et al. 2005). This has led some researchers to speculate that Cr could be used in the treatment of osteoporosis and osteoarthritis. However, evidence supporting this assumption is still scarce. Louis et al. (2003) reported increased bone mineral density by 3% and reduced the urinary concentrations of cross-linked N-telopeptides of type I collagen (NTx) (i.e., a marker of bone resorption) by 30% in Cr-supplemented patients with Duchenne dystrophy. Moreover, Candow et al. (2008) demonstrated that Cr supplementation attenuates urinary NTx concentrations by 27% in older males undergoing resistance training. Currently, our group and others have investigated whether long-term Cr supplementation can inhibit bone loss in elderly people with low bone mass (for details, see http://www.clinicaltrials.gov: NCT01163370 and NCT01057680).
We have recently demonstrated that a 3-month Cr supplementation protocol improves physical function, lower limb lean mass and quality of life in postmenopausal women with knee OA undergoing strengthening exercises (Neves et al. 2011a). Importantly, we attributed these outcomes to a possible effect of Cr on training quality rather than a direct effect on cartilage cells, since Cr-induced cartilage repair would be unlikely to occur within 3 months. Clearly, further RCTs must be conducted and the mechanisms by which Cr might act on bone and cartilage repair must be addressed.
Brain disorders
There is enough evidence to conclude that Cr exerts a vital role in cerebral energetic provision, corroborated by (i) the presence of creatine kinase (PCK) isoforms in both the brain and spinal cord (Kaldis et al. 1996); (ii) the association between brain Cr depletion (as seen in patients with Cr deficiency syndromes) and mental retardation, autism, speech delay, and brain atrophy (Salomons et al. 2003); and (iii) the reversal of these symptoms as a result of oral Cr administration (Stockler et al. 1996). In addition to the previously discussed role of Cr-PCr on energy system, Cr also possesses anti-apoptotic, anti-excitotoxic, and direct antioxidative pleiotropic effects both in vitro and in vivo (Klopstock et al. 2011). Furthermore, there is evidence that Cr can penetrate blood–brain barrier (Stockler et al. 1996) and cause no deleterious effects (see the last topic of this review). Altogether, these characteristics confer to Cr a therapeutic potential in neurodegenerative diseases.
Interestingly, Cr supplementation can improve cognitive performance in young subjects (Watanabe et al. 2002) as well as in elderly people (McMorris et al. 2007a). Furthermore, Cr intake can also alleviate mental fatigue induced by stressor stimulus such as mathematical calculus (Watanabe et al. 2002) and sleep deprivation (McMorris et al. 2007b). A recent study showed that in vegetarians rather than in those who consume meat, Cr supplementation improves memory (Benton and Donohoe 2010). Moreover, both vegetarians and meat eaters experienced decreased variability in the responses to a choice reaction-time task (Benton and Donohoe 2010). The mechanisms underlying these findings remain to be elucidated. In addition, the possible benefits of Cr supplementation on cognition in children as well as in patients with dementia need to be explored.
Cr supplementation has also been applied to several psychiatric and neurodegenerative diseases. In this respect, Cr seems to be effective in relieving symptoms, attenuating depression, and improving the quality of life in patients suffering from posttraumatic stress and fibromyalgia (Amital et al. 2006a, b, c). Inborn or acquired diseases characterized by progressive loss of nervous-system cells such as Alzheimer’s, Huntington’s, Charcot-Marie-Tooth’s and Parkinson’s disease and amyotrophic lateral sclerosis (ALS) could be also potentially treated by Cr administration (Brewer and Wallimann 2000; Mazzini et al. 2001; Tabrizi et al. 2003; NET-PDN 2006; Smith et al. 2006). These neurodegenerative disorders share common characteristics, including cerebral energy depletion (e.g., low brain Cr content), exacerbated oxidative stress and mitochondrial dysfunction. Interestingly, Cr supplementation might alleviate all of these abnormalities (Andres et al. 2008). In general, animal models have provided the most exciting evidence concerning the therapeutic effects of Cr in neurodegenerative diseases, while RCTs have produced less enthusiastic results. One case in point is ALS, where Cr supplementation clearly exerted a neuroprotective effects in the G93A SOD1 mouse model of this disease (Andreassen et al. 2001), but did not affect disease progression, strength, fatigue, and respiratory status in patients (Groeneveld et al. 2003). Klopstock et al. (2011) believe that such a dissonance between animal and human outcomes is due to methodological issues. First, the validity of the standard murine models for mimicking the complexity of neurodegenerative diseases has been debatable. Second, it is well known that animal models and humans respond differentially to Cr supplementation in several aspects (Harris et al. 1992; Tarnopolsky et al. 2003; Gualano et al. 2010a, b, c, d). In fact, the timing of treatment relative to disease onset and relative to age and the dosage of Cr supplementation have been excessively discordant between animal and human studies (Klopstock et al. 2011). Moreover, the few existing RTCs may have been underpowered, precluding the detection of statistical significant effects (Klopstock et al. 2011).
Perhaps one exception where in vitro, animal and human studies have pointed to the same direction concerns Parkinson`s disease. In vitro studies mimicking Parkinson’s disease revealed that Cr has protective effects against neurotoxic insults (Andres et al. 2005). A large RCT corroborated such promising observations, indicating that Cr supplementation is not futile in Parkinson’s patients. A RCT involving about 1,700 patients is currently being conducted and will help to clarify this issue (for details, see http://www.clinicaltrials.gov: NCT00449865). Further RCTs are indeed imperative to provide a fully appreciation of the role of Cr supplementation in neurodegenerative diseases. For further details on this topic, the reader is encouraged to see the broad review by Andres et al. (2008).
Other therapeutic applications
Remarkably, Cr supplementation has been used as an adjuvant treatment in several diseases other than those previously approached in this review. Studies have shown that Cr can offset the decline in total muscle GLUT-4 content in healthy subjects submitted to leg immobilization (Op ‘t Eijnde et al. 2001a). Moreover, there is evidence indicating that Cr supplementation combined with aerobic training promotes greater improvement in glucose tolerance than aerobic training alone in physically inactive males, suggesting that this nutritional supplement could be useful in metabolic disorders characterized by insulin resistance (Gualano et al. 2008). Confirming this concept, our group recently demonstrated that Cr supplementation improves glycemic control and insulin sensitivity in type-2 diabetic undergoing exercise training (Gualano et al. 2010b). The mechanism underlying these findings seems to be related to an increase in GLUT-4 translocation to the sarcolemma rather than in total muscle GLUT-4 content. The therapeutic application of Cr supplementation in diabetic people is indeed very promising.
Irrespective of their etiology, diseases characterized by physical deconditioning, muscle weakness and atrophy may also be potentially benefited by Cr supplementation. One case in point is chronic obstructive pulmonary disease (COPD), for which muscle atrophy is a strong predictor of mortality. Fuld et al. (2005) demonstrated that patients with CPOD supplemented with Cr have improved muscle mass, strength, aerobic capacity, and clinical condition compared with non-supplemented patients. Similar benefits from Cr supplementation have also been reported in patients with congenital heart failure (Andrews et al. 1998) and acute lymphoblastic leukemia (Bourgeois et al. 2008). The therapeutic application of Cr supplementation in patients suffering from long-term bed rest, physical inactive lifestyle, malnutrition and other types of cancer remain to be investigated. Figure 1 illustrates the state of art on the therapeutic application of Cr supplementation.
Diseases and conditions in which creatine supplementation would be “most likely beneficial” (green; “positive” results from one or more high-quality randomized controlled trial with large sample); “possibly beneficial” (yellow; limited evidence from in vitro, animal and non-human studies with low sample size or randomized human studies pointing to contradictory outcomes); or “unlikely beneficial” (red; “negative” results from one or more high-quality randomized controlled trial with large sample
Effects of cr supplementation on kidney function: is that a real concern?
The safety of Cr supplementation has been highly argued between health professionals since the publication of the case report by Pritchard and Kalra (1998), in which Cr intake was associated with kidney function deterioration in a 25-year-old man with focal segmental glomerulosclerosis and nephrotic relapses. Even though a few studies with rodents (Edmunds et al. 2001; Ferreira et al. 2005) and other case reports (Kuehl et al. 1998; Koshy et al. 1999; Thorsteinsdottir et al. 2006) have also reported some deleterious effects of Cr on kidney function, longitudinal studies (illustrated in Table 2) have consistently demonstrated that short-, medium- and long-term Cr supplementation does not affect kidney function in healthy humans (Poortmans et al. 1997; Poortmans and Francaux 1999; Kreider et al. 2003; Poortmans et al. 2005; Gualano et al. 2010a, b; Neves-Jr. et al. 2011b). More recently, our group has extended this concept to individuals with or at risk for kidney dysfunction, such as elderly people (Neves-Jr. et al. 2011b) and type 2 diabetic patients (Gualano et al. 2010b). In a case study, we also showed that a 30-d Cr supplementation protocol does not impair kidney function in a young man with a single kidney, leading us to conclude that there is no reason to suspect that Cr would affect kidney function in healthy individuals (Gualano et al. 2010a).
Still, one could argue that some conflicting results in the literature actually exist, especially from case reports and animal studies. First, case studies (summarized in Table 3) present retrospective design and, as such, do not provide control for Cr dosage and purity as well as subjects’ clinical background. These factors might clearly lead to misinterpretation. For instance, Revai et al. (2003) reported a case of diffuse membranoproliferative glomerulonephritis type I in “a 22-year-old man who had been continuously taking the anabolic-androgenic steroid methandion in a large quantity and 200 grams of Cr daily”. Obviously, the concomitant use of steroids as well as the abusive dose of Cr (about 100 times the recommended maintenance dose) would preclude any sort of conclusion. There are perhaps many nutrients for which 100 times the recommended dose would exceed the upper tolerable limit (e.g., vitamin C, vitamin E, calcium, etc.). Nonetheless, the authors surprisingly concluded their study stressing the “role of the continuously consumed Cr in the renal failure”. Second, data obtained in animals often cannot be replied in humans, particularly in Cr studies. In fact, a large inter-species variability is expected following Cr intake. One case in point is the study by Tarnopolsky et al. (2003) where Cr-induced hepatitis in SOD1 G93A transgenic mice (mutation in CU/Zn superoxide dismutase gene causing increased oxidative stress) but not in rats, suggesting that even close species may respond differently to Cr supplementation.
There are a few studies with healthy humans showing that short-term (1–10 d) and high-dose Cr supplementation (~20 g/d) provokes the production of an excess of urinary methylamine and formaldehyde (Yu and Deng 2000; Poortmans et al. 2005; Sale et al. 2009), which are cytotoxic compounds often associated with nephropathy and formed as a result of the conversion of Cr into sarcosine. However, the Cr-induced increased levels of these cytotoxic agents did not reach the normal upper limit values from healthy humans (Poortmans et al. 2005). Furthermore, it has been showed that lower and more frequent doses of Cr (i.e., 20 × 1 g/d in comparison to 4 × 5 g/d) appear to attenuate the formation of methylamine (Sale et al. 2009). Based on these findings, it has been recommended that the spread of the Cr dose evenly throughout the day or the use of an appropriate slow release formulation may be useful strategies in preventing Cr-induced accumulation of cytotoxic agents in long-term Cr users (Kim et al. 2011).
A critical analysis of the literature reveals that Cr supplementation does not affect kidney function in a short- and medium-term basis. The long-term studies are scarce but also point in the same direction. Further investigations involving children, adolescents, pregnant women, and athletes under high-protein diet are still necessary. However, as creatine is a nutrient commonly consumed in the diet, it would be unexpected if recommended doses of creatine supplementation induced renal dysfunction in any population with normal kidney function.
Concluding remarks
There is an extensive and still growing body of the literature regarding the efficacy of Cr supplementation. In sports, Cr supplementation has been recognized as the most effective ergogenic nutritional supplement currently available to athletes in terms of increasing high-intensity exercise capacity and lean body mass during training. Early on, athletes appear to have determined that if Cr does not improve the performance of their sport per se, it might offer benefits in the gym. Thus, some athletes ingest Cr as a sports performance aid, while others ingest it chronically as a training aid in the weight room. Overall, improved exercise performance and training adaptations subsequent to Cr supplementation are widely reported in the scientific literature. From a clinical perspective, the application of Cr supplementation is indeed exciting. Evidence of benefits from Cr has been reported in a broad range of diseases, including myopathies, neurodegenerative disorders, rheumatic diseases, and type 2 diabetes. In addition, after hundreds of published studies and millions of exposures Cr supplementation maintains an excellent safety profile (Persky and Rawson 2007). Cr is an effective, inexpensive, and safe dietary supplement. As such, its widespread application may benefit athletes, elderly people and patient populations.
References
Amital D, Fostick L, Polliack ML, Segev S, Zohar J, Rubinow A, Amital H (2006a) Posttraumatic stress disorder, tenderness, and fibromyalgia syndrome: are they different entities? J Psychosom Res 61(5):663–669. doi:10.1016/j.jpsychores.2006.07.003
Amital D, Vishne T, Roitman S, Kotler M, Levine J (2006b) Open study of creatine monohydrate in treatment-resistant posttraumatic stress disorder. J Clin Psychiatry 67(5):836–837
Amital D, Vishne T, Rubinow A, Levine J (2006c) Observed effects of creatine monohydrate in a patient with depression and fibromyalgia. Am J Psychiatry 163(10):1840–1841. doi:10.1176/appi.ajp.163.10.1840-b
Andreassen OA, Jenkins BG, Dedeoglu A, Ferrante KL, Bogdanov MB, Kaddurah-Daouk R, Beal MF (2001) Increases in cortical glutamate concentrations in transgenic amyotrophic lateral sclerosis mice are attenuated by creatine supplementation. J Neurochem 77(2):383–390
Andres RH, Ducray AD, Huber AW, Perez-Bouza A, Krebs SH, Schlattner U, Seiler RW, Wallimann T, Widmer HR (2005) Effects of creatine treatment on survival and differentiation of GABA-ergic neurons in cultured striatal tissue. J Neurochem 95(1):33–45
Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR (2008) Functions and effects of creatine in the central nervous system. Brain Res Bull 76(4):329–343. doi:10.1016/j.brainresbull.2008.02.035
Andrews R, Greenhaff P, Curtis S, Perry A, Cowley AJ (1998) The effect of dietary creatine supplementation on skeletal muscle metabolism in congestive heart failure. Eur Heart J 19(4):617–622 (pii:S0195668X97907673)
Barisic N, Bernert G, Ipsiroglu O, Stromberger C, Müller T, Gruber S, Prayer D, Moser E, Bittner RE, Stöckler-Ipsiroglu S (2002) Effects of oral creatine supplementation in a patient with MELAS phenotype and associated nephropathy.Neuropediatrics 33(3):157–161
Beal F (2011) Neuroprotective effects of creatine. Amino Acids 40(5):1305–1313
Bemben MG, Lamont HS (2005) Creatine supplementation and exercise performance: recent findings. Sports Med 35(2):107–125 (pii:3522)
Benton D, Donohoe R (2010) The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br J Nutr:1-6. doi:10.1017/S0007114510004733
Bourgeois JM, Nagel K, Pearce E, Wright M, Barr RD, Tarnopolsky MA (2008) Creatine monohydrate attenuates body fat accumulation in children with acute lymphoblastic leukemia during maintenance chemotherapy. Pediatr Blood Cancer 51(2):183–187. doi:10.1002/pbc.21571
Branch JD (2003) Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab 13(2):198–226
Brewer GJ, Wallimann TW (2000) Protective effect of the energy precursor creatine against toxicity of glutamate and beta-amyloid in rat hippocampal neurons. J Neurochem 74(5):1968–1978
Buford TW, Kreider RB, Stout JR, Greenwood M, Campbell B, Spano M, Ziegenfuss T, Lopez H, Landis J, Antonio J (2007) International society of sports nutrition position stand: creatine supplementation and exercise. J Int Soc Sports Nutr 4:6. doi:10.1186/1550-2783-4-6
Burke DG, Candow DG, Chilibeck PD, MacNeil LG, Roy BD, Tarnopolsky MA, Ziegenfuss T (2008) Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int J Sport Nutr Exerc Metab 18(4):389–398
Candow DG, Little JP, Chilibeck PD, Abeysekara S, Zello GA, Kazachkov M, Cornish SM, Yu PH (2008) Low-dose creatine combined with protein during resistance training in older men. Med Sci Sports Exerc 40(9):1645–1652. doi:10.1249/MSS.0b013e318176b310
Chung YL, Alexanderson H, Pipitone N, Morrison C, Dastmalchi M, Stahl-Hallengren C, Richards S, Thomas EL, Hamilton G, Bell JD, Lundberg IE, Scott DL (2007) Creatine supplements in patients with idiopathic inflammatory myopathies who are clinically weak after conventional pharmacologic treatment: six-month, double-blind, randomized, placebo-controlled trial. Arthr Rheum 57(4):694–702. doi:10.1002/art.22687
Cox G, Mujika I, Tumilty D, Burke L (2002) Acute creatine supplementation and performance during a field test simulating match play in elite female soccer players. Int J Sport Nutr Exerc Metab 12(1):33–46
DN NET-P (2006) A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 66(5):664–671. doi:10.1212/01.wnl.0000201252.57661.e1
Edmunds JW, Jayapalan S, DiMarco NM, Saboorian MH, Aukema HM (2001) Creatine supplementation increases renal disease progression in Han:SPRD-cy rats. Am J Kidney Dis 37(1):73–78
Engelhardt M, Neumann G, Berbalk A, Reuter I (1998) Creatine supplementation in endurance sports. Med Sci Sports Exerc 30(7):1123–1129
Ferreira LG, De Toledo Bergamaschi C, Lazaretti-Castro M, Heilberg IP (2005) Effects of creatine supplementation on body composition and renal function in rats. Med Sci Sports Exerc 37(9):1525–1529
Fuld JP, Kilduff LP, Neder JA, Pitsiladis Y, Lean ME, Ward SA, Cotton MM (2005) Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 60(7):531–537. doi:10.1136/thx.2004.030452
Funanage VL, Carango P, Shapiro IM, Tokuoka T, Tuan RS (1992) Creatine kinase activity is required for mineral deposition and matrix synthesis in endochondral growth cartilage. Bone Miner 17(2):228–236
Gerber I, ap Gwynn I, Alini M, Wallimann T (2005) Stimulatory effects of creatine on metabolic activity, differentiation and mineralization of primary osteoblast-like cells in monolayer and micromass cell cultures. Eur Cell Mater 10:8–22 (pii:vol010a02)
Greenhalff P (1995) Creatine and its application as an ergogenic aid. Int J Sport Nutr 5:S100–S110
Greenhalff P (2001) The creatine–phosphocreatine system: there’s more than one song in its repertoire. J Physiol 537(Pt 3):657
Groeneveld GJ, Veldink JH, van der Tweel I, Kalmijn S, Beijer C, de Visser M, Wokke JH, Franssen H, van den Berg LH (2003) A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann Neurol 53(4):437–445. doi:10.1002/ana.10554
Gualano B, Novaes RB, Artioli GG, Freire TO, Coelho DF, Scagliusi FB, Rogeri PS, Roschel H, Ugrinowitsch C, Lancha AH Jr (2008) Effects of creatine supplementation on glucose tolerance and insulin sensitivity in sedentary healthy males undergoing aerobic training. Amino Acids 34(2):245–250. doi:10.1007/s00726-007-0508-1
Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH (2010a) Exploring the therapeutic role of creatine supplementation. Amino Acids 38(1):31–44. doi:10.1007/s00726-009-0263-6
Gualano B, Ferreira DC, Sapienza MT, Seguro AC, Lancha AH Jr (2010b) Effect of short-term high-dose creatine supplementation on measured GFR in a young man with a single kidney. Am J Kidney Dis 55(3):e7–e9. doi:10.1053/j.ajkd.2009.10.053
Gualano B, de Salles Painelli V, Roschel H, Lugaresi R, Dorea E, Artioli GG, Lima FR, da Silva ME, Cunha MR, Seguro AC, Shimizu MH, Otaduy MC, Sapienza MT, da Costa Leite C, Bonfa E, Lancha Junior AH (2010c) Creatine supplementation does not impair kidney function in type 2 diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Eur J Appl Physiol. doi:10.1007/s00421-010-1676-3
Gualano B, de Salles Painneli V, Roschel H, Artioli GG, Junior MN, Lucia de Sa Pinto A, Rossi da Silva ME, Cunha MR, Otaduy MC, da Costa Leite C, Ferreira JC, Pereira RM, Brum PC, Bonfa E, Lancha AHJ (2010d) Creatine in type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Med Sci Sports Exerc. doi:10.1249/MSS.0b013e3181fcee7d
Harris RC, Soderlund K, Hultman E (1992) Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond) 83(3):367–374
Head SI, Greenaway B, Chan S (2011) Incubating isolated mouse EDL muscles with creatine improves force production and twitch kinetics in fatigue due to reduction in ionic strength. PLoS ONE 6(8): e22742. doi:10.1371/journal.pone.0022742
Hespel P, Derave W (2007) Ergogenic effects of creatine in sports and rehabilitation. Subcell Biochem 46:245–259
Hopwood MJ, Graham K, Rooney KB (2006) Creatine supplementation and swim performance: a brief review. J Sports Sci Med Sport 5:10–24
Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL (1996) Muscle creatine loading in men. J Appl Physiol 81(1):232–237
Juhasz I, Gyore I, Csende Z, Racz L, Tihanyi J (2009) Creatine supplementation improves the anaerobic performance of elite junior fin swimmers. Acta Physiol Hung 96(3):325–336. doi:10.1556/APhysiol.96.2009.3.6
Kaldis P, Hemmer W, Zanolla E, Holtzman D, Wallimann T (1996) ‘Hot spots’ of creatine kinase localization in brain: cerebellum, hippocampus and choroid plexus. Dev Neurosci 18(5–6):542–554
Kim JH, Kim CK, Carpentier A, Poortmans JR (2011) Studies on the safety of creatine supplementation. Amino Acids 40:1409–1418
Kley RA, Tarnopolsky MA, Vorgerd M (2008) Creatine treatment in muscle disorders: a meta-analysis of randomised controlled trials. J neurol, neurosurg, psychiatry 79(4):366–367. doi:10.1136/jnnp.2007.127571
Klopstock T, Elstner M, Bender A (2011) Creatine in mouse models of neurodegeneration and aging. Amino Acids. 40(5):1297–1303
Koshy KM, Griswold E, Schneeberger EE (1999) Interstitial nephritis in a patient taking creatine. N Engl J Med 340(10):814–815
Kreider RB, Melton C, Rasmussen CJ, Greenwood M, Lancaster S, Cantler EC, Milnor P, Almada AL (2003) Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol Cell Biochem 244(1–2):95–104
Kuehl K, Goldberg L, Elliot D (1998) Renal insufficiency after creatine supplementation in a college football athlete. Med Sci Sports Exerc 30:S235
Law YL, Ong WS, GillianYap TL, Lim SC, Von Chia E (2009) Effects of two and five days of creatine loading on muscular strength and anaerobic power in trained athletes. J Strength Cond Res 23(3):906–914. doi:10.1519/JSC.0b013e3181a06c59
Louis M, Lebacq J, Poortmans JR, Belpaire-Dethiou MC, Devogelaer JP, Van Hecke P, Goubel F, Francaux M (2003) Beneficial effects of creatine supplementation in dystrophic patients. Muscle Nerve 27(5):604–610. doi:10.1002/mus.10355
Mazzini L, Balzarini C, Colombo R, Mora G, Pastore I, De Ambrogio R, Caligari M (2001) Effects of creatine supplementation on exercise performance and muscular strength in amyotrophic lateral sclerosis: preliminary results. J Neurol Sci 191(1–2):139–144 (pii:S0022510X01006116)
McMorris T, Harris RC, Howard AN, Langridge G, Hall B, Corbett J, Dicks M, Hodgson C (2007a) Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav 90(1):21–28. doi:10.1016/j.physbeh.2006.08.024
McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A (2007b) Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14(5):517–528. doi:10.1080/13825580600788100
Murphy RM, Stephenson DG, Lamb GD (2004) Effect of creatine on contractile force and sensitivity in mechanically skinned single fibers from rat skeletal muscle. Am J Physiol Cell Physiol 287:C1589–C1595
Neves M Jr, Gualano B, Roschel H, Fuller R, Benatti FB, Pinto AL, Lima FR, Pereira RM, Lancha AH Jr, Bonfá E (2011a) Beneficial effect of creatine supplementation in knee osteoarthritis. Med Sci Sports Exerc.43(8):1538-43
Neves M Jr, Gualano B, Roschel H, Lima FR, Lúcia de Sá-Pinto A, Seguro AC, Shimizu MH, Sapienza MT, Fuller R, Lancha AH Jr, Bonfá E (2011b) Effect of Creatine Supplementation on measured glomerular filtration rate in postmenopausal women. Appl Physiol Nutr Metab. 36(3):419-22.
Op ‘t Eijnde B, Urso B, Richter EA, Greenhaff PL, Hespel P (2001a) Effect of oral creatine supplementation on human muscle GLUT4 protein content after immobilization. Diabetes 50(1):18–23
Op ‘t Eijnde B, Vergauwen L, Hespel P (2001b) Creatine loading does not impact on stroke performance in tennis. Int J Sports Med 22(1):76–80. doi:10.1055/s-2001-11334
Ostojic SM (2004) Creatine supplementation in young soccer players. Int J Sport Nutr Exerc Metab 14(1):95–103
Persky AM, Rawson ES (2007) Safety of creatine supplementation. Subcell Biochem 46:275–289
Pluim BM, Ferrauti A, Broekhof F, Deutekom M, Gotzmann A, Kuipers H, Weber K (2006) The effects of creatine supplementation on selected factors of tennis specific training. Br J Sports Med 40 (6):507–511 (discussion 511–502). doi:10.1136/bjsm.2005.022558
Poortmans JR, Francaux M (1999) Long-term oral creatine supplementation does not impair renal function in healthy athletes. Med Sci Sports Exerc 31(8):1108–1110
Poortmans JR, Auquier H, Renaut V, Durussel A, Saugy M, Brisson GR (1997) Effect of short-term creatine supplementation on renal responses in men. Eur J Appl Physiol Occup Physiol 76(6):566–567
Poortmans JR, Kumps A, Duez P, Fofonka A, Carpentier A, Francaux M (2005) Effect of oral creatine supplementation on urinary methylamine, formaldehyde, and formate. Med Sci Sports Exerc 37(10):1717–1720
Pritchard NR, Kalra PA (1998) Renal dysfunction accompanying oral creatine supplements. Lancet 351(9111):1252–1253
Rawson E, Clarkson P (2004) Scientifically debatable: is creatine worth its weight? GSSE 16:1–6
Rawson E, Persky A (2007) Mechanisms of muscular adaptations to creatine. Int Sport Med J 8:43–53
Rawson ES, Volek JS (2003) Effects of creatine supplementation and resistance training on muscle strength and weightlifting performance. J Strength Cond Res 17(4):822–831 (pii:R-13753)
Rawson ES, Stec MJ, Frederickson SJ, Miles MP (2010) Low-dose creatine supplementation enhances fatigue resistance in the absence of weight gain. Nutrition. doi:10.1016/j.nut.2010.04.001
Revai T, Sapi Z, Benedek S, Kovacs A, Kaszas I, Viranyi M, Winkler G (2003) Severe nephrotic syndrome in a young man taking anabolic steroid and creatine long term. Orv Hetil 144(49):2425–2427
Robinson TM, Sewell DA, Casey A, Steenge G, Greenhaff PL (2011) Dietary creatine supplementation does not affect some haematological indices, or indices of muscle damage and hepatic and renal function. Br J Sports Med 34(4):284–288
Rossouw F, Kruger P, Rossouw J (2000) The effect of creatine monohydrate loading on maximal intermittent exercise and sport-specific strength in well trained power-lifters. Nutr Res 20:505–514
Sahlin K, Harris RC (2011) The creatine kinase reaction: a simple reaction with functional complexity. Amino Acids 40(5):1363–1367
Sale C, Harris RC, Florance J, Kumps A, Sanvura R, Poortmans JR (2009) Urinary creatine and methylamine excretion following 4 9 5 g day−1 or 20 g 9 1 g day−1 of creatine monohydrate for 5 days. J Sports Sci 27:759–766
Salomons GS, van Dooren SJ, Verhoeven NM, Marsden D, Schwartz C, Cecil KM, DeGrauw TJ, Jakobs C (2003) X-linked creatine transporter defect: an overview. J Inherit Metab Dis 26(2–3):309–318
Schedel JM, Terrier P, Schutz Y (2000) The biomechanic origin of sprint performance enhancement after one-week creatine supplementation. Jpn J Physiol 50(2):273–276
Sestili P, Martinelli C, Colombo E, Barbieri E, Potenza L, Sartini S, Fimognari C (2011) Creatine as an antioxidant 40:1385–1396
Silva AJ, Machado Reis V, Guidetti L, Bessone Alves F, Mota P, Freitas J, Baldari C (2007) Effect of creatine on swimming velocity, body composition and hydrodynamic variables. J Sports Med Phys Fit 47(1):58–64
Skare OC, Skadberg, Wisnes AR (2001) Creatine supplementation improves sprint performance in male sprinters. Scand J Med Sci Sports 11 (2):96-102. (pii:sms110205)
Smith CA, Chetlin RD, Gutmann L, Yeater RA, Alway SE (2006) Effects of exercise and creatine on myosin heavy chain isoform composition in patients with Charcot-Marie-Tooth disease. Muscle Nerve 34(5):586–594. doi:10.1002/mus.20621
Stockler S, Hanefeld F, Frahm J (1996) Creatine replacement therapy in guanidinoacetate methyltransferase deficiency, a novel inborn error of metabolism. Lancet 348(9030):789–790 (pii:S0140673696041165)
Tabrizi SJ, Blamire AM, Manners DN, Rajagopalan B, Styles P, Schapira AH, Warner TT (2003) Creatine therapy for Huntington’s disease: clinical and MRS findings in a 1-year pilot study. Neurology 61(1):141–142
Tarnopolsky M, Martin J (1999) Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 52(4):854–857
Tarnopolsky MA, Roy BD, MacDonald JR (1997) A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle Nerve 20(12):1502–1509. doi:10.1002/(SICI)1097-4598(199712)20:12<1502:AID-MUS4>3.0.CO;2-C
Tarnopolsky MA, Bourgeois JM, Snow R, Keys S, Roy BD, Kwiecien JM, Turnbull J (2003) Histological assessment of intermediate- and long-term creatine monohydrate supplementation in mice and rats. Am J Physiol Regul Integr Comp Physiol 285(4):R762–R769. doi:10.1152/ajpregu.00270.2003
Terjung RL, Clarkson P, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, Kraemer WJ, Meyer RA, Spriet LL, Tarnopolsky MA, Wagenmakers AJ, Williams MH (2000) American college of sports medicine roundtable. The physiological and health effects of oral creatine supplementation. Med Sci Sports Exerc 32(3):706–717
Thorsteinsdottir B, Grande JP, Garovic VD (2006) Acute renal failure in a young weight lifter taking multiple food supplements, including creatine monohydrate. J Ren Nutr 16(4):341–345
Vandebuerie F, Vanden Eynde B, Vandenberghe K, Hespel P (1998) Effect of creatine loading on endurance capacity and sprint power in cyclists. Int J Sports Med 19(7):490–495. doi:10.1055/s-2007-971950
Vandenberghe K, Goris M, Van Hecke P, Van Leeputte M, Vangerven L, Hespel P (1997) Long-term creatine intake is beneficial to muscle performance during resistance training. J Appl Physiol 83(6):2055–2063
Volek JS, Duncan ND, Mazzetti SA, Staron RS, Putukian M, Gomez AL, Pearson DR, Fink WJ, Kraemer WJ (1999) Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med Sci Sports Exerc 31(8):1147–1156
Wallimann T, Tokarska-Schlattner M, Schlattner U (2011) The creatine kinase system and pleiotropic effects of creatine. Amino Acids 40(5):1271–1296
Watanabe A, Kato N, Kato T (2002) Effects of creatine on mental fatigue and cerebral hemoglobin oxygenation. Neurosci Res 42(4):279–285 (pii:S016801020200007X)
Yu PH, Deng Y (2000) Potential cytotoxic effect of chronic administration of creatine, a nutrition supplement to augment athletic performance. Med Hypotheses 54(5):726–728
Acknowledgments
We are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for supporting our studies with creatine supplementation (process 2010/18708-1). The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gualano, B., Roschel, H., Lancha-Jr., A.H. et al. In sickness and in health: the widespread application of creatine supplementation. Amino Acids 43, 519–529 (2012). https://doi.org/10.1007/s00726-011-1132-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1132-7