Abstract
This study tested the hypothesis that dietary l-arginine supplementation confers beneficial effects on growing pigs fed a mold-contaminated diet. The measured variables included: (1) the average daily weight gain and feed:gain ratio; (2) activities of total superoxide dismutase, glutathione peroxidase, diamine oxidase, as well as amino acid and d-lactate concentrations in serum; (3) intestinal morphology; (4) expression of the genes for SLC7A7 (amino acid transporter light chain, y+L system, family 7, member 7), SLC7A1 (cationic amino acid transporter, y+ system, family 7, member 1), SLC1A1 (neuronal/epithelial high affinity glutamate transporter, system XAG, member 1), SLC5A1 (sodium/glucose cotransporter, family 5, member 1) in the ileum and jejunum. Mycotoxins in feedstuffs resulted in an enlarged small intestine mass, oxidative injury in tissues, and reduced growth performance in pigs. Dietary arginine supplementation enhanced (P < 0.05) expression of jejunal SLC7A7 and ileal SLC7A1, in comparison with the control and mycotoxin groups. In addition, supplementing 1 % l-arginine to the mycotoxin-contaminated feed had the following beneficial effects (P < 0.05): (1) alleviating the imbalance of the antioxidant system in the body; (2) ameliorating intestinal abnormalities; and (3) attenuating whole-body growth depression, compared with the mycotoxin group without arginine treatment. Collectively, these results indicate that dietary supplementation with l-arginine exerts a protective role in pigs fed mold-contaminated foods. The findings may have important nutritional implications for humans and other mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycotoxins are secondary metabolites produced by a wide variety of fungal species (Rezaei et al. 2013a, b). They are commonly found in moldy feedstuffs and raw feed materials. Mycotoxins are considered to be the major toxic contaminants, including aflatoxin (AF), deoxynivalenol (DON), zearalenone (ZEA), ochratoxins (OCH), fumonisins (FB) and T-2 (Binder 2007; Richard et al. 2007). Many studies have shown that the ingestion of these mycotoxins may induce feed refusal, a decrease in animal productivity, organ damage, and increased incidence of disease due to immune suppression (Chaytor et al. 2011). Therefore, dietary mycotoxins can greatly affect the health, growth, and reproduction of humans and other animals, including pigs (Fokunang et al. 2006). Although many studies have reported the toxic effects of individual mycotoxins in various animal species, exposure to only one mycotoxin is unlikely to occur under normal feeding conditions in the livestock industry. Indeed, during the feed-manufacturing process, various batches of different raw materials are mixed together, leading to an increase in the risk for mycotoxin-mixed contamination (Binder et al. 2007). Thus, animals are usually exposed to synergistic interactions among multiple mycotoxins.
With regard to the alleviation of mycotoxicosis, most studies have focused on methods of physical and chemical degradation of mycotoxins (Park et al. 2007; Young et al. 1986), as well as the use of their adsorbents, such as aluminosilicates (Liu et al. 2011) and esterified glucomannan (Aravind et al. 2003). However, the results of some in vivo studies (Liu et al. 2011) have shown that these methods are not always effective against all chemically diverse mycotoxins. At present, little is known about the use of nutritional treatment to ameliorate intestinal abnormalities and growth retardation induced by the ingestion of mycotoxins.
l-Arginine is a conditionally essential amino acid for pigs (Wu 2010a, b). It is not only synthesized from glutamine, glutamate, and proline by enterocytes of the pig small intestine (Wu et al. 1994; Wu and Knabe 1995; Wu et al. 1996a), but is also degraded by both intestinal mucosal cells (Wu et al. 1996b) and luminal bacteria (Dai et al. 2012a, b, 2013a). In addition, concentrations of arginine in the blood circulation are reduced under stress conditions, including weaning (Wu et al. 2010), lactation (Lei et al. 2012), starvation (Wu and Morris 1998), and infection (Li et al. 2007). Results of recent studies have demonstrated that dietary supplementation with arginine can promote (1) intestinal cell proliferation (Tan et al. 2010), (2) reduction of white adipose tissue in obese rats (Wu et al. 2007a) and pigs (Tan et al. 2009), (3) expression of antioxidative genes (Jobgen et al. 2009), and (4) healing wounds (Stechmiller et al. 2005) via mechanisms involving the synthesis of nitric oxide (NO) (Wu et al. 2009). Furthermore, arginine can enhance growth performance (Kim and Wu 2004), improve immune function (Li et al. 2007; Ren et al. 2012), beneficially alter the metabolic profile in animals (Tan et al. 2012a), stimulate the mammalian (mechanistic) target of rapamycin (mTOR) signaling pathway and protein synthesis (Kong et al. 2012), affect intestinal morphology and production of inflammatory cytokines (Zhou et al. 2012), and up-regulate expression of vascular endothelial growth factor in the gut (Yao et al. 2011). Based on the foregoing, the present study was conducted to test the hypothesis that dietary supplementation with l-arginine may confer beneficial effects on growing pigs fed a mold-contaminated diet.
Materials and methods
Preparation of a mold-contaminated diet
A mold-contaminated diet was prepared as described by Liu et al. (2011). In brief, water was added to a non-contaminated basal diet until it reached 20 % moisture. The wet feed was then cultured under ambient conditions (temperature 23–28 °C, humidity 68–85 %) until mildew was clearly observed (Liu et al. 2011). Finally, the mold-contaminated diet was naturally air-dried, mixed, and sampled for detection of mycotoxins. Their content in the mold-contaminated diet was determined by liquid chromatography (Beijing Taileqi, Beijing, China) (Table 1).
Experimental design
The experiment was carried out in accordance with the Chinese guidelines for animal welfare. Experimental protocol and approved by the Animal Care and Use Committee of the Chinese Academy of Sciences.
Fifteen growing pigs (Landrace × Large White) (Zheng Hong Co., China) with a mean body weight (BW) of 55 kg were randomly assigned into one of three treatment groups (n = 5/group): (1) the control group in which pigs were fed the uncontaminated diet; (2) the mycotoxin group in which pigs were fed the mold-contaminated diet; and (3) the arginine group in which pigs were fed the mold-contaminated diet supplemented with 1.0 % l-arginine (purity >99 %, Beijing Chemclin Biotech, Beijing, China). The basal diet (Table 2) was prepared from corn, soybean meal, wheat bran, limestone, CaHPO4, NaCl, and additive premix to meet or exceed the nutritional requirements of growing pigs as recommended by the NRC (1998). This diet contained 0.99 % l-arginine, as determined by Li et al. (2011).
Pigs had free access to drinking water and their respective diets throughout the experimental period and were weighed individually at days 0 and 60. The feed consumption of pigs was measured weekly. The average daily weight gain (ADG; g/day) and feed:gain ratios of pigs were then calculated. All pigs were food deprived for 12 h at the end of the experimental period before blood samples were obtained from the jugular vein (Rezaei et al. 2013b) for the determination of total superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), diamine oxidase (DAO) activities, d-lactate, and amino acid levels in serum (Hou et al. 2012; Yao et al. 2012). After pigs were anesthetized by intravenous administration of sodium pentobarbital (50 mg/kg body weight) and then euthanized by exsanguination (Deng et al. 2009), the small intestine was obtained, its luminal content was carefully removed, and the lumen was washed three times with saline (Wang et al. 2008). Then, two samples were taken from both the mid-jejunum and mid-ileum. One of the intestinal samples (3 cm) was placed in 10 % neutral buffered formalin for morphological analysis (Wu et al. 1996a), and the other (approximately 2 g) was immediately frozen in liquid nitrogen and stored at −70 °C for subsequent gene expression analysis (Wang et al. 2009).
Analyses T-SOD, GSH-Px, and DAO activities, d-lactate, and amino acid profile
Serum activities of T-SOD and GSH-Px were measured using spectrophotometric kits in accordance with the manufacturer’s instructions (Nanjing Jiangcheng Biotechnology Institute, China). Serum d-lactate was determined using an assay kit in accordance with the manufacturer’s instructions (Biovision Inc., USA). DAO was measured according to a previous report (Hou et al. 2012, 2013). Amino acids in serum were determined by LC–MS/MS (HPLC Ultimate3000 and 3200 Q TRAP LC–MS/MS) using standards from Sigma Chemicals (St. Louis, MO, USA) (Ruan et al. 2013).
Determination of intestinal morphology
One piece of the jejunal and ileal segments (3 cm) was maintained in 4 % neutral buffered 10 % formalin, processed using routine histological methods, and mounted in paraffin blocks (Wang et al. 2008). Six-micrometer-thick sections were cut and stained with the Masson’s trichrome solution (Zhang et al. 2013b). All specimens were examined under a light microscope (Nikon, Japan). Villus height and crypt depth were measured using an image-analysis system.
Quantification of mRNA by real-time PCR analysis
Total RNA was isolated from liquid nitrogen-pulverized tissues with TRIzol regent (Invitrogen, USA) and then treated with DNase I (Invitrogen, USA) according to the manufacturer’s instructions (Liu et al. 2012). Synthesis of the first strand (cDNA) was performed with oligo (dT) 20 and Superscript II reverse transcriptase (Invitrogen, USA) (Zhang et al. 2013a). Primers were designed with Primer 5.0 according to the gene sequence of pig to produce an amplification product (Table 3). β-Actin was used as a housekeeping gene to normalize target gene transcript levels. Real-time PCR was performed as described by Ren et al. (2011). In brief, 1-μl cDNA template was added to a total volume of 10 μl containing 5 μl SYBR Green mix, 0.2 μl Rox, 3 μl H2O, and 0.4 μmol/l each of forward and reverse primers. The following protocol applied: (1) pre-denaturation (10 s at 95 °C); (2) amplification and quantification 40 cycles (5 s at 95 °C, 20 s at 60 °C); and (3) melting curve program (60–99 °C with a heating rate of 0.1 °C S-1 and fluorescence measurement). The relative levels of genes were expressed as a ratio of the target gene to the control gene using the formula 2−(ΔΔCt), where ΔΔCt = (Ct Target − Ct β-actin)treatment − (Ct Target − Ct β-actin)control (Fu et al. 2010). The relative expression of genes in the mycotoxin and arginine groups is given in comparison to those in the control group and is reported as the fold change from the control value (He et al. 2013a).
Statistical analysis
Statistical analyses were performed with the SPSS17.0 software (Chicago, IL, USA). The normality and constant variance for data were tested by the Levene’s test (Wei et al. 2012). Data were subjected to one-way analysis of variance followed by the Duncan’s multiple comparisons test. Values are expressed as the mean ± standard error of the mean (SEM).
Results
Growth performance
Data on pig growth performance are summarized in Table 4. Compared to the control group, ADG was 18.3 % lower (P < 0.05) and the feed:gain ratio was 13.7 % higher (P < 0.05) in the mycotoxin group. Interestingly, dietary supplementation with 1 % arginine to pigs fed the mold-contaminated feedstuffs improved (P < 0.05) feed efficiency (as indicated by a reduced feed:gain ratio) by 8.5 %, in comparison with the mycotoxin group.
Analysis of serum T-SOD and GSH-Px activity
The activity of serum total SOD in the mycotoxin group was 49.4 % lower (P < 0.05) than that in the control group (Fig. 1). However, there was no difference in total SOD activity between the mycotoxin and the arginine groups. The activity of serum glutathione peroxidase did not differ among the control and mycotoxin groups, but was slightly higher (P < 0.05) in the arginine group.
Effects of dietary supplementation with arginine on serum T-SOD and GSH-Px activities in growing pigs fed a mold-contaminated feed. Data are mean ± SEM, n = 5. Control group: pigs were fed an uncontaminated diet. Mycotoxin group: pigs were fed a mold-contaminated diet. Arginine group: pigs were fed a mold-contaminated diet supplemented with 1 % arginine. T-SOD superoxide dismutase (U/ml), GSH glutathione peroxidase (×10 μU/l). For enzymatic activity, 1 U/ml is defined as the amount of SOD in 1 ml plasma required for catalyzing the conversion of 1 μmol superoxide anion (O2 −) into its product per min at 25 °C. a, b For each variable, means with different letters differ (P < 0.05)
Serum d-lactate and DAO
After pigs were fed the mold-contaminated feed, the concentration of d-lactate in serum was increased (P < 0.05), in comparison with the control group (Fig. 2). However, there was no difference in serum d-lactate concentrations between the mycotoxin and the arginine groups. The activity of serum DAO did not differ among the three groups of pigs.
Effects of dietary supplementation with arginine on DAO activity (U/ml) and d-lactate levels (μmol/l) in the serum of growing pigs fed a mold-contaminated diet. Data are mean ± SEM, n = 5. See Fig. 1 for explanation of legends. DAO diamine oxidase. a, b For each variable, means with different letters differ (P < 0.05)
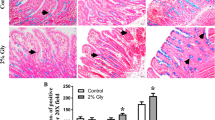
Intestinal morphology
No abnormal morphology was observed for the small intestine in the control group (Fig. 3). In contrast, villus height in both the jejunum and the ileum of the mycotoxin-challenged pigs showed strong scattering and desquamation. Interestingly, villus height in the jejunum and the ileum was decreased (P < 0.05) by dietary arginine supplementation, compared with pigs fed the mold-contaminated diet without arginine treatment. An increase in crypt depth was also observed (P < 0.05) in both the ileum and jejunum after exposure to the mold-contaminated diet (Table 5). Compared with the mycotoxin group, dietary supplementation with 1 % arginine decreased (P < 0.05) ileal crypt depth and tended to reduce (P > 0.05) jejunal crypt depth.
Effects of dietary supplementation with arginine on small intestinal morphology (HE ×400) in growing pigs fed a mold-contaminated diet. Pigs in the control group (Panels 1 and 2) were fed a uncontaminated diet. Pigs in the mycotoxin group (Panels 3 and 4) were fed a mold-contaminated diet. Pigs in the arginine group (Panels 5 and 6) were fed a mold-contaminated diet supplemented with 1 % l-arginine. There was no histological damage in the small intestine of the control pigs. In the mycotoxin group, villus was scattered and desquamated seriously in the jejunum and ileum. Higher villus height in the jejunum and ileum was observed in the arginine group, compared with the mycotoxin group
Concentrations of amino acids in serum
Concentrations of amino acids and related metabolites in serum were determined by LC–MS/MS. In response to mycotoxin challenge, most of the amino acids exhibited decreases in the blood circulation (Table 6). For example, serum concentrations of glycine, l-tyrosine, l-glutamine, 1-methyl-l-histidine, and l-proline were decreased (P < 0.05) by the mold-contaminated diet, compared to the control group. In contrast, l-citrulline showed an increase in the serum (P < 0.05), likely as a result of increased production of this amino acid by intestinal microbes and fungi. Dietary supplementation with 1 % l-arginine reduced (P < 0.05) the circulating levels of citrulline but increased (P < 0.05) those for l-histidine and 3-methyl-l-histidine in pigs fed the mycotoxin-treated diet. As expected from the pharmacokinetics of arginine in animals (Wu et al. 2007b), the concentration of arginine in the serum of 12-h fasted pigs did not differ among the three treatment groups (Table 6).
Expression of genes for intestinal amino acid transporters
Dietary arginine supplementation up-regulated SLC7A7 expression (P < 0.05) in the jejunum (the major site for absorption of amino acids), compared to the mycotoxin group without arginine treatment (Fig. 4). Similar results were obtained for the ileal SLC7A1 gene. mRNA levels for SLC7A7, SLC1A1, and SLC5A1 in the ileum of pigs fed the mycotoxin-contaminated diet without arginine supplementation did not differ from those in the control group (Fig. 4).
Effects of dietary supplementation with arginine on gene expression in the jejunum (a) and the ileum of growing pigs (b). See Fig. 1 for explanation of legends. Data are presented as mean ± SEM, n = 5. SLC7A1 solute carrier family 7 (cationic amino acid transporter, y+ system; member 1), SLC7A7 solute carrier family 7 (amino acid transporter light chain, y+L system, member 7), SLC1A1 solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system XAG, member 1), and SLC5A1 solute carrier family 5 (sodium/glucose cotransporter, member 1). a, b For each variable, means with different letters differ (P < 0.05)
Discussion
Among various mycotoxins, AF, DON, ZEA, OCH, FUM, and T-2 are often encountered in feedstuffs at high concentrations worldwide (Binder et al. 2007; Guan et al. 2011). Decreased feed intake and the subsequent suppression of growth are characteristics of mycotoxicosis (Andretta et al. 2012; Rezaei et al. 2013a; Swamy et al. 2003). Similar to previous reports (Rezaei et al. 2013a), results of the current study indicated that mold-contaminated feed substantially decreased growth performance in pigs. Importantly, dietary supplementation with arginine ameliorated intestinal abnormalities (Table 5) and growth depression (Table 4) in mycotoxin-challenged pigs.
Arginine regulates intestinal gene expression, growth and mucosal integrity, nutrient absorption, and metabolic pathways (Rhoads and Wu 2009; Wu et al. 2012, 2013a, b). Thus, arginine has been recognized as a conditionally essential amino acid for young mammals, including piglets (Wu 2013a). There is also growing interest in arginine in a functional amino acid in human and animal nutrition (Ren et al. 2012; Tan et al. 2012b; Wu 2013b). Notably, many reports have shown that dietary supplementation with arginine may play an important role in counteracting the suppressive effects of abnormal regulation of metabolic pathway and immunological challenges on animal growth (Yin et al. 2010). The feed:gain ratio (an indicator of the efficiency in utilization of dietary nutrients for growth) was reduced in arginine-supplemented pigs, as compared to pigs in the mycotoxin group (Table 4). This novel result suggests a potentially important role for arginine in mitigating adverse effects of food-borne mycotoxins on growing swine. Large-scale studies are required to examine this possibility under production conditions.
Antioxidant enzymes (such as SOD and GSH-Px) comprise the major defense system for preventing organ injury due to excessive quantities of reactive oxygen species that attack proteins, lipids, and DNA (Yin et al. 2013a, b). An increasing number of studies have demonstrated that some mycotoxins can contribute to oxidative stress in cells. For example, ZEA decreases the activities of SOD and GSH-Px with progressive liver and kidney injury (Jiang et al. 2011). In addition, DON induces an adapted response of the antioxidant defense system to oxidative stress in Hek-293 cells (Dinu et al. 2011). Furthermore, Mary et al. (2012) reported that the interaction of AFB1 and FB1 caused oxidative damage in spleen mononuclear cells. In this study, we found that the activity of total SOD in the pig serum was markedly decreased after exposure to mold-contaminated feedstuffs, indicating that mycotoxins resulted in oxidative stress in the whole body. Arginine stimulates NO production within physiological ranges in the small intestine and other tissues (Rhoads and Wu 2009; Wu amd Meininger 2002), and NO plays an important role in regulating the antioxidant defense system (Dai et al. 2013b). Thus, dietary supplementation with arginine helps scavenge the excess reactive oxygen species induced by moldy feeds, thereby improving the balance between the production of reactive oxygen species (e.g., superoxide anion, hydrogen peroxide, and hydroxyl radical) and the biological defense against the toxicity of these oxidants.
d-Lactate, a byproduct of bacterial metabolism, is neither produced nor metabolized by mammalian cells (He et al. 2012). Under normal conditions, production of d-lactate by the small intestine is insignificant, and thus blood has a very low or negligible level of d-lactate (Dai et al. 2011). However, if the intestinal segment is infected and the mucosal barrier of the gut is degraded, d-lactate can cross the mucosal barrier in large quantities (Packer et al. 2012). Therefore, abnormally high serum concentrations of d-lactate are considered to be indicators of intestinal injury. After pigs were exposed to mold-contaminated feeds, serum d-lactate concentrations were increased, indicating that mold-contaminated feeds resulted in d-lactate production and damage to the gut. Meanwhile, the results of the macroscopic observation and histological evaluation of the small intestine (e.g., an enlarged mucosa) further demonstrated that mycotoxins induced intestinal abnormalities (Rezaei et al. 2013b). Catabolism of amino acids (e.g., arginine and methionine) by intestinal microbes to generate polyamines and other metabolites may contribute to the enlargement of the intestinal mucosa in pigs fed mold-contaminated feedstuffs. Similarly, Yunus et al. (2011) and Applegate et al. (2009) found that the weight of the small intestine and crypt depth were affected by dietary AFB1. In addition, Awad et al. (2006) found that the small intestinal morphology in the duodenum and jejunum of broilers was altered by the feeding of DON-contaminated diets at 10 mg/kg. Taken together, these findings indicate that the small intestine is one of the most important targets of mycotoxins in animals. Notably, dietary supplementation with 1 % arginine had a significant effect on ileal crypt depth and protected villi from scattering and desquamation. While the mechanisms of these positive effects are still unclear, we postulate that arginine stimulates the intestinal production of NO to inhibit the growth of luminal bacteria and fungi (Koppelmann et al. 2012). NO is an important signaling molecule involved in neurotransmission, vascular homeostasis, immune regulation, and host defense (Anggard 1994; Tan et al. 2010; Wu et al. 2009). Furthermore, arginine and NO are also critical for the normal physiology of the gastrointestinal tract and for maintaining the mucosal integrity of the intestine by enhancing growth and development of enterocytes (Rhoads et al. 2008), stimulating intestinal cell migration and intestinal protein synthesis through a focal adhesion kinase-dependent mechanism (Yao et al. 2011), and regulating expression of the bcl-2 family of proteins (Koppelmann et al. 2012).
Amino acids play important roles as metabolic intermediates in nutrition, immune response, and growth performance (Wu 2009). In the current work, we observed that concentrations of glycine, l-tyrosine, and l-glutamine in serum were decreased by the exposure to mycotoxins in feedstuffs. It is likely that degradation of dietary glycine, glutamine, and proline by the small intestine is increased by a mold-contaminated food, resulting in their deficiencies in the animals. Emerging evidence shows that these three amino acids are very important for tissue protein synthesis and metabolic regulation (Wang et al. 2013; Wu et al. 2011a, b). It still remains to be determined whether dietary supplementation with glycine, glutamine, and proline could overcome adverse effects of mycotoxins on pigs.
The absorption of amino acids mainly depends on their transporters on the membrane of the enterocyte (Wu 2013a). Although there were no differences in mRNA levels for SLC7A7, SLC7A1, SLC1A1, and SLC5A1 between the control and mycotoxin groups, intestinal protein levels for these transporters need to be quantified using western blot techniques (Zhang et al. 2013a). Likewise, rates of amino acid transport by the small intestinal mucosa isolated from pigs treated with or without mycotoxins can be measured with the use of Ussing Chambers (He et al. 2013b). Indeed, Maresca et al. (2002) reported that mycotoxins modulate the activities of intestinal nutrient transporters in a concentration-dependent manner. Importantly, we found that arginine enhanced expression of jejunal SLC7A7 and ileal SLC7A1 in mycotoxin-challenged pigs (Fig. 4). An increase in the entry of amino acids (e.g., arginine, lysine, and histidine) from the lumen of the small intestine into the enterocyte can enhance tissue protein synthesis and improve the efficiency of utilization of dietary nutrients, including proteins (Table 4).
In conclusion, mycotoxins in feedstuffs result in an enlarged small intestine mass, oxidative injury in tissues, and reduced growth performance in pigs. Dietary supplementation with 1 % arginine enhances expression of jejunal SLC7A7 and ileal SLC7A1, augments whole-body antioxidant capacity, and ameliorates intestinal abnormalities and growth depression in swine fed mold-contaminated feedstuffs. Thus, arginine exerts a protective role against mycotoxicosis in pigs. These results may also have important nutritional implications for humans and other mammals.
References
Andretta I, Kipper M, Lehnen CR et al (2012) Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs. Animal 6:1476–1482
Anggard E (1994) Nitric oxide: mediator, murderer, and medicine. Lancet 343:1199–1206
Applegate TJ, Schatzmayr G, Prickel K et al (2009) Effect of aflatoxin culture on intestinal function and nutrient loss in laying hens. Poult Sci 88:1235–1241
Aravind KL, Patil VS, Devegowda G et al (2003) Efficacy of esterified glucomannan to counteract mycotoxicosis in naturally contaminated feed on performance and serum biochemical and hematological parameters in broilers. Poult Sci 82:571–576
Awad WA, Bohm J, Razzazi-Fazeli E et al (2006) Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult Sci 85:974–979
Binder EM (2007) Managing the risk of mycotoxins in modern feed production. Anim Feed Sci Technol 133:149–166
Binder EM, Tan LM, Chin LJ et al (2007) Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim Feed Sci Technol 137:265–282
Chaytor AC, Hansen JA, van Heugten E et al (2011) Occurrence and decontamination of mycotoxins in swine feed. Asian-Australas J Anim Sci 24:723–738
Dai ZL, Wu G, Zhu WY (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16:1768–1786
Dai ZL, Li XL, Xi PB et al (2012a) Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 42:1597–1608
Dai ZL, Li XL, Xi PB et al (2012b) Regulatory role for l-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 43:233–244
Dai ZL, Li XL, Xi PB et al (2013a) l-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 45:501–512
Dai ZL, Wu ZL, Yang Y et al (2013b) Nitric oxide and energy metabolism in mammals. BioFactors 39:383–391
Deng D, Yin YL, Chu WY et al (2009) Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. J Nutr Biochem 20:544–552
Dinu D, Bodea GO, Ceapa CD et al (2011) Adapted response of the antioxidant defense system to oxidative stress induced by deoxynivalenol in Hek-293 cells. Toxicon 57:1023–1032
Fokunang CN, Tembe-Fokunang EA, Tomkins P et al (2006) Global impact of mycotoxins on human and animal health management. Outlook Agric 35:247–253
Fu WJ, Stromberg AJ, Viele K et al (2010) Statistics and bioinformatics in nutritional sciences: analysis of complex data in the era of systems biology. J Nutr Biochem 21:561–572
Guan S, Gong M, Yin YL et al (2011) Occurrence of mycotoxins in feeds and feed ingredients in China. J Food Agric Environ 9:163–167
He QH, Ren PP, Kong XF et al (2012) Comparison of serum metabolite compositions between obese and lean growing pigs using an NMR-based metabonomic approach. J Nutr Biochem 23:133–139
He LQ, Yang HS, Li TJ et al (2013a) Effects of dietary l-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45:383–391
He LQ, Yin YL, Li TJ et al (2013b) Use of the Ussing chamber technique to study nutrient transport by epithelial tissues. Front Biosci 18:1266–1274
Hou YQ, Wang L, Zhang W et al (2012) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 43:1233–1242
Hou YQ, Wang L, Yi D et al (2013) N-Acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 45:513–522
Jiang SZ, Yang ZB, Yang WR et al (2011) Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J Anim Sci 89:3008–3015
Jobgen W, Fu WJ, Gao H et al (2009) High fat feeding and dietary l-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids 37:187–198
Kim SW, Wu G (2004) Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr 134:625–630
Kong X, Tan B, Yin Y et al (2012) l-arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem 23:1178–1183
Koppelmann T, Pollak Y, Mogilner J et al (2012) Dietary l-arginine supplementation reduces methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol 12:41
Lei J, Feng DY, Zhang YL et al (2012) Nutritional and regulatory role of branched-chain amino acids in lactation. Front Biosci 17:2725–2739
Li P, Yin YL, Li DF et al (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li XL, Rezaei R, Li P et al (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Liu YL, Meng GQ, Wang HR et al (2011) Effect of three mycotoxin adsorbents on growth performance, nutrient retention and meat quality in broilers fed on mould-contaminated feed. Br Poult Sci 52:255–263
Liu XD, Wu X, Yin YL et al (2012) Effects of dietary l-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids 42:2111–2119
Maresca M, Mahfoud R, Garmy N et al (2002) The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J Nutr 132:2723–2731
Mary VS, Theumer MG, Arias SL et al (2012) Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology 302:299–307
Packer RA, Moore GE, Chang CY et al (2012) Serum d-lactate concentrations in cats with gastrointestinal disease. J Vet Intern Med 26:905–910
Park BJ, Takatori K, Sugita-Konishi Y et al (2007) Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf Coat Technol 201:5733–5737
Ren W, Luo W, Wu M et al (2011) Dietary l-glutamine supplementation improves pregnancy outcome in mice infected with type-2 porcine circovirus. Amino Acids 45:479–488
Ren W, Yin YL, Liu G et al (2012) Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42:2089–2094
Rezaei R, Knabe DA, Wu G (2013a) Impact of aflatoxins on swine nutrition and possible measures of control and amelioration. Aflatoxin control: safeguarding animal feed with calcium smectite. In: Joe B. Dixon, Ana L. Barrientos Velázquez, Youjun Deng, (eds) American Society of Agronomy and Soil Science, Madison, pp 54–67. doi:10.2136/2013.aflatoxins.c6
Rezaei R, Knabe DA, Tekwe CD et al (2013b) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rhoads JM, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Rhoads JM, Liu Y, Niu X et al (2008) Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. J Nutr 138:1652–1657
Richard JL et al (2007) Some major mycotoxins and their mycotoxicoses—an overview. Int J Food Microbiol 119:3–10
Ruan Z, Lv YF, Fu XF et al (2013) Metabolomic analysis of amino acid metabolism in colitic rats supplemented with lactosucrose. Amino Acids 45:877–887
Stechmiller JK, Childress B, Cowan L et al (2005) Arginine supplementation and wound healing. Nutr Clin Pract 20:52–61
Swamy HVLN, Smith TK, MacDonald EJ et al (2003) Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on growth and immunological measurements of starter pigs, and the efficacy of a polymeric glucomannan mycotoxin adsorbent. J Anim Sci 81:2792–2803
Tan BE, Yin YL, Liu ZQ et al (2009) Dietary l-arginine supplementation increases muscle gain and reduces body fat mass in growing-finishing pigs. Amino Acids 37:169–175
Tan BE, Yin YL, Kong XF et al (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Tan B, Li X, Wu G et al (2012a) Dynamic changes in blood flow and oxygen consumption in the portal-drained viscera of growing pigs receiving acute administration of l-arginine. Amino Acids 43:2481–2489
Tan BE, Li XG, Yin YL et al (2012b) Regulatory roles for l-arginine in reducing white adipose tissue. Front Biosci 17:2237–2246
Wang JJ, Chen LX, Li P et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Wang XQ, Ou DY, Yin JD et al (2009) Proteomic analysis reveals altered expression of proteins related to glutathione metabolism and apoptosis in the small intestine of zinc oxide-supplemented piglets. Amino Acids 37:209–218
Wang WW, Wu ZL, Dai ZL et al (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010a) Recent advances in swine amino acid nutrition. J Anim Sci Biotechnol 1:49–61
Wu G (2010b) Functional amino acids in growth, reproduction and health. Adv Nutr 1:31–37
Wu G (2013a) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G (2013b) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton
Wu G, Knabe DA (1995) Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol Regul Integr Comp Physiol 269:R621–R629
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Knabe DA, Flynn NE (1994) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299:115–121
Wu G, Meier SA, Knabe DA (1996a) Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr 126:2578–2584
Wu G, Knabe DA, Flynn NE et al (1996b) Arginine degradation in developing porcine enterocytes. Am J Physiol Gastrointest Liver Physiol 271:G913–G919
Wu G, Collins JK, Perkins-Veazie P et al (2007a) Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr 137:2680–2685
Wu G, Bazer FW, Cudd TA et al (2007b) Pharmacokinetics and safety of arginine supplementation in animals. J Nutr 137:1673S–1680S
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu X, Ruan Z, Gao YL et al (2010) Dietary supplementation with l-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids 39:831–839
Wu G, Bazer FW, Johnson GA et al (2011a) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Bazer FW, Burghardt RC et al (2011b) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu X, Yin YL, Liu YQ et al (2012) Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows. Anim Reprod Sci 132:187–192
Wu G, Wu ZL, Dai ZL et al (2013a) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu G, Bazer FW, Satterfield MC et al (2013b) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Yao K, Guan S, Li T et al (2011) Dietary l-arginine supplementation enhances intestinal development and expression of vascular endothelial growth factor in weanling piglets. Br J Nutr 105:703–709
Yao K, Yin YL, Li XL et al (2012) Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids 42:2491–2500
Yin YL, Tan B et al (2010) Manipulation of dietary nitrogen, amino acids and phosphorus to reduce environmental impact of swine production and enhance animal health. J Food Agric Environ 8:447–462
Yin J, Ren W, Liu G et al (2013a) Birth oxidative stress and the development of an antioxidant system in newborn piglets. Free Radic Res 47:1027–1035
Yin J, Ren WK, Wu XS et al (2013b) Oxidative stress-mediated signaling pathways: a review. J Food Agric Environ 11:132–139
Young JC, Blackwell BA, Apsimon JW et al (1986) Alkaline-degradation of the mycotoxin 4-deoxynivalenol. Tetrahedron Lett 27:1019–1022
Yunus AW, Ghareeb K, Abd-El-Fattah AA et al (2011) Gross intestinal adaptations in relation to broiler performance during chronic aflatoxin exposure. Poult Sci 90:1683–1689
Zhang J, Yin YL, Shu XG et al (2013a) Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 45:1169–1177
Zhang SH, Qiao SY, Ren M et al (2013b) Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 45:1191–1205
Zhou XH, Wu X, Yin YL et al (2012) Preventive oral supplementation with glutamine and arginine has beneficial effects on the intestinal mucosa and inflammatory cytokines in endotoxemic rats. Amino Acids 43:813–821
Acknowledgments
The present work was supported by Grants from the 973 National Key Basic Research of China (No. 2013CB127301), the National Natural Science Foundation of China (No. 31272463, 31072042, and 31272450), Hunan Provincial Natural Science Foundation of China (No. K1307007-21), and Texas AgriLife Research Hatch Project (H-8200).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Yin and W. Ren contributed equally to the present study.
Rights and permissions
About this article
Cite this article
Yin, J., Ren, W., Duan, J. et al. Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids 46, 883–892 (2014). https://doi.org/10.1007/s00726-013-1643-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1643-5