Abstract
This study determined the effects of dietary branched-chain amino acids (AA) (BCAA) on growth performance, expression of jejunal AA and peptide transporters, and the colonic microflora of weanling piglets fed a low-protein (LP) diet. One hundred and eight Large White × Landrace × Duroc piglets (weaned at 28 days of age) were fed a normal protein diet (NP, 20.9 % crude protein), an LP diet (LP, 17.1 % crude protein), or an LP diet supplemented with BCAA (LP + BCAA, 17.9 % crude protein) for 14 days. Dietary protein restriction reduced piglet growth performance and small-intestinal villous height, which were restored by BCAA supplementation to the LP diet to values for the NP diet. Serum concentrations of BCAA were reduced in piglets fed the LP diet while those in piglets fed the LP + BCAA diet were similar to values for the NP group. mRNA levels for Na+-neutral AA exchanger-2, cationic AA transporter-1, b0,+ AA transporter, and 4F2 heavy chain were more abundant in piglets fed the LP + BCAA diet than the LP diet. However, mRNA and protein levels for peptide transporter-1 were lower in piglets fed the LP + BCAA diet as compared to the LP diet. The colonic microflora did not differ among the three groups of pigs. In conclusion, growth performance, intestinal development, and intestinal expression of AA transporters in weanling piglets are enhanced by BCAA supplementation to LP diets. Our findings provide a new molecular basis for further understanding of BCAA as functional AA in animal nutrition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diarrhea in neonatal and early weaned piglets caused high mortality and morbidity and impaired the growth performance and growth potential of survived piglets, which hampered swine industry and resulted in huge economic losses in swine production (Armstrong and Cline 1976; Rezaei et al. 2013a, b). Reducing dietary protein level is a useful strategy to control the frequency and severity of diarrhea in weanling piglets (Opapeju et al. 2009; Wu et al. 2010). However, it may lead to an unbalanced supply of amino acids, causing the impairment of pig performance (Wu 2010a, b).
Dietary supplementation with certain amino acids has been reported to increase the efficiency of utilization of dietary protein (Wang et al. 2009; Wu et al. 2011a, b), regulate tissue protein synthesis through the mTOR signaling pathway (Escobar et al. 2006; Yin et al. 2010), enhance the synthesis and secretion of hormones in metabolism, participate in oxidative stress (Li et al. 2011a), modulate the immunity system, and decrease the susceptibility of animals to disease (Li et al. 2007). Dietary amino acids also play critical roles in maintaining gut health and preventing gut diseases through serving as the primary fuels for intestinal mucosa and as substrates for synthesis of intestinal protein and other biological products such as nitric oxide, polyamines and glutathione (Bergen and Wu 2009; Wang et al. 2009). For instance, glutamine could stimulate cell proliferation, inhibit cell apoptosis and is necessary for the integrity of tight junction in enterocytes (Rhoads and Wu 2009; Wu et al. 2011a). Arginine could regulate intestinal cell migration and antioxidative responses through activating the mTOR signaling pathway as well as synthesis of nitric oxide and polyamines (Tan et al. 2010; Wang et al. 2012b; Wu et al. 2013a). Threonine is of great importance for the maintenance of the intestinal mucosal barrier functions through modulating intestinal protein synthesis as well as production and secretion of mucins (Bertrand et al. 2013; Wang et al. 2010). However, the function of the branched-chain amino acids (BCAA) in the intestine is largely unknown.
Dietary amino acids will exert their effects on extra-intestinal tissues after being absorbed by the small intestine (Baker 2009; Rezaei et al. 2013a). Therefore, increasing attention has been paid to effects of different diets on intestinal metabolism and function (Dai et al. 2010; Gilbert et al. 2008). Amino acids are transported into the cell in the free form by specific transporters (Palacín and Kanai 2004) or in a dipeptide or tripeptide form by peptide transporters (Leibach and Ganapathy 1996). Different amino acid transporters have unique substrate specificities (Wu 2013a). For example, transporters have been characterized that are specific for basic amino acids (CAT1, y+LAT/4F2hc), neutral amino acids (B0AT1, ASCT2 and rBAT/b0,+AT) and acidic amino acids (EAAT1, EAAT2) (Kanai and Hediger 2003). Recent work has shown that changes in nutrient content can affect the levels of amino acid transporters in the small intestine, skeletal muscle, and mammary tissue (Gilbert et al. 2008; Laspiur et al. 2009). However, at present little is known about effects of BCAA on the absorption of amino acids in the small intestine of weaning piglets. We hypothesized that dietary supplementation with BCAA to a low-protein diet can regulate intestinal expression of amino acid and peptide transporters in animals.
Dietary composition is one of the major factors that can influence the microbial population in the gastrointestinal tract (Blachier et al. 2010; Yin et al. 2009). It seems likely that reducing dietary protein levels and supplementing amino acids to low-protein diets may affect available amounts of substrates for bacterial proliferation in the gastrointestinal tract (Dai et al. 2011). However, whether BCAA can modulate the microbial population is still unknown. Identifying amino acids that can modulate nutrient transporters and the intestinal microflora in weaning piglets fed an LP diet is of enormous nutritional importance. The primary objective of the current study was to determine the effects of feeding an LP diet supplemented with BCAA on piglet growth performance, expression of amino acid and peptide transporters in the small intestine, and changes in intestinal microflora populations and metabolism in the large intestine of weaning piglets.
Materials and methods
Animals, housing, and experimental design
This study was approved by the Animal Care and Use Committee of China Agricultural University (Beijing, China). A total of 108 piglets (Large White × Landrace × Duroc) weaned at 28 days of age, with an average initial body weight of 7.97 ± 0.11 kg, were assigned randomly to one of 18 pens on the basis of sex and initial body weight. Each treatment had 6 pens (1.8 × 2.1 m2), with three for castrated males and three for females. Pigs were fed three times a day (8:00 a.m., 2:00 p.m. and 6:00 p.m.) using a single-sided 3-hole feeder. Pigs had free access to water via a nipple drinker.
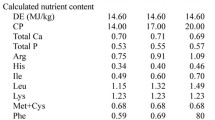
Three diets based on maize, soybean meal and whey powder were prepared for this study (Table 1). The diets comprised a normal protein (NP) diet (containing 20.9 % crude protein) supplemented with lysine, methionine, threonine and tryptophan. A low-protein (LP) diet (17.1 % crude protein) was formulated by decreasing the soybean-meal content, increasing the maize content, and by supplementing the four limiting amino acids mentioned above. In addition, an LP + BCAA (17.9 % crude protein) diet was formulated by supplementing leucine, isoleucine and valine to the LP diet to meet the recommendations of the National Swine Nutritional Guide for BCAA requirements (NSNG 2010). The three diets were formulated to meet or exceed National Research Council (NRC 1998) recommendations for all other nutrients and were supplied in the mash form. Pigs were weighed at the beginning and end of the experiment. The amount of feed in the feeder was monitored and an attempt was made to ensure that feed was always available in the feeder. Feed was weighed before being added to the feeder. Any wasted feed or feed refusals were also weighed and this amount was subtracted from the amount of feed added to the feeder to determine feed disappearance. These values were used to calculate average daily gain of body weight (ADG), average daily feed intake (ADFI), and feed:gain ratio. The nursery-room temperature was initially set at 30 °C and was progressively decreased by 1 °C each week.
Slaughter, tissue and digesta sample collection
Blood samples were collected randomly from six pigs per treatment at the end of the experiment. Pigs were bled via puncture of the jugular vein before the morning feeding. Blood samples were collected into heparin-free vacutainer tubes (Becton–Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA). After blood collection, all samples were transferred to centrifuge tubes and centrifuged for 15 min at 8,000×g at 25 °C. The serum was carefully removed and subdivided into two plastic vials, then stored at −80 °C.
At the end of the 14-day experiment, the 18 pigs used for blood sampling were killed and samples were collected to evaluate intestinal morphology, bacteria in digesta and expression of the intestinal nutrient transporters. The small intestine was dissected from the mesentery and immediately placed on ice. The duodenum was considered as the part of the intestine from the pylorus to the ligament of Treitz, a segment 10 cm proximal to the ileocecal junction was considered as the ileum, and the remainder of the small intestine was considered the jejunum (Wang et al. 2008). Segments (3 cm in length) of mid-duodenum, mid-jejunum, and mid-ileum (the entire alimentary canal without digesta) were fixed in 10 % neutral buffered formalin for subsequent histological measurement. In addition, 3-cm mid-jejunum segments (the entire alimentary canal without digesta) were also immediately flushed with PBS and the digesta collected into a sterile Eppendorf tubes for further RT-PCR and Western blot analysis. Each tube was immediately frozen in liquid nitrogen and stored in a freezer at −80 °C. The proximal colon contents were gently squeezed into the sterile Eppendorf tubes and snap-frozen in liquid nitrogen, then stored at −80 °C for both genomic DNA isolation and analysis of volatile fatty acids.
Intestinal morphology
Samples from each intestinal segment were embedded in paraffin and cut into 5-μm serial sections, and five non-successive sections from each tissue sample were selected and stained with hematoxylin–eosin for identification (Wang et al. 2008). Five well-oriented villi (determined as the distance between the crypt openings and the end of the villi) and their associated crypt (measured from the crypt-villous junction to the base of the crypt) per section were selected and measured under a light microscope (CK-40, Olympus, Tokyo, Japan) at 40× magnification and analyzed with an Image Analyzer (Lucia Software. Lucia, Za Drahou, Czechoslovakia). The means of these measurements were calculated to yield a single value per piglet. These procedures were processed by an observer unaware of the dietary treatments.
RNA isolation and RT-PCR analysis
Total RNA was isolated from a 300 mg of jejunum segment (the entire alimentary canal without digesta) using a RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. RNA (1 μg) was reverse-transcribed to complementary DNA (cDNA) using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Ostu, Japan) according to the manufacturer’s protocol. Primers for the selected genes (Table 2) were designed using Oligo 7.0 Software. Real-time PCR was performed using an Applied Biosystems 7500 Real-Time PCR System (Foster City, CA, USA) with SYBR Green PCR Master Mix (Takara, Ostu, Japan) containing MgCl2, dNTP, and Hotstar Taq polymerase. Quantification of target mRNA was conducted using a relative standard curve generated by a serial dilution (1:107 to 1:1) of amplification products. The PCR system consisted of 5.0 μL of SYBR Green qPCR mix, 1.0 μL of cDNA, 3.6 μL of double distilled water, and 0.4 μL of primer pairs (25 μM forward and 25 μM reverse) in a total volume of 10 μL. The protocols for all genes included a denaturation program (1 min at 95 °C), amplification and quantification program repeated for 35 cycles (5 s at 95 °C, 30 s at 58 °C), followed by the melting curve program at 60–95 °C with a heating rate of 0.1 °C per second and continuous fluorescence measurement. Six samples were used for each treatment and each sample was measured in triplicate.
Detection of volatile fatty acids in colonic digesta
The concentrations of short chain fatty acids were determined by a gas chromatographic method following the procedures of Franklin et al. (2002) with modifications. Approximately, 1.5 g of thawed colonic digesta was suspended in 1.5 mL of distilled water in a screw-capped tube. The entire sample was centrifuged at 15,000×g for 10 min at 4 °C. After that, 1 ml of supernatant was transferred into an ampoule and mixed with 200 μL of metaphosphoric acid (HPO3). The ampoules were placed in an ice bath for 30 min then centrifuged again for 10 min. The sample was injected into a HP 6890 Series Gas Chromatograph (Hewlett Packard, PA, California) equipped with a HP 19091N-213 column with 30.0 m × 0.32 mm i.d. (Agilent, PA, California). The injector and detector temperatures were 185 and 210 °C, respectively.
Western blot analysis
Relative protein levels for CAT-1, rBAT, 4F2hc and PepT-1 obtained from a whole homogenized section of a jejunum segment were determined by the western blotting technique. The frozen jejunum samples were powdered under liquid nitrogen, and lysed in RIPA buffer (150 mM NaCl, 1 % Triton X-100, 0.5 % sodium deoxycholate, 0.1 % SDS, 50 mM Tris–HCl at pH 7.4, plus a protease inhibitor cocktail purchased from Apply Gene (Beijing, China). Protein concentrations were determined using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Equal amounts of proteins (30 mg) were electrophoresed on SDS polyacrylamide gels. Prestained protein markers (Fermentas, Waltham, MA, USA) were analyzed in each gel. Proteins were electrotransferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA) and blocked with 5 % nonfat dry milk overnight at 4 °C. The transfer efficiency was assessed by gel staining with Coomassie Blue. Samples were incubated with corresponding primary antibodies (1:500 dilution for 2 h at 25 °C or overnight at 4 °C) against CAT-1, rBAT, 4F2hc and PepT-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The primary antibodies have been validated for use in swine by the manufacturer. After being washed with Tris-Tween-20 buffer (pH 7.4), membranes were incubated with a secondary antibody (rBAT: horseradish peroxidase-conjugated goat anti-rabbit IgG; CAT-1, PepT-1 and 4F2hc: horseradish peroxidase-conjugated rabbit anti-goat IgG) (Zhongsan Gold Bridge, Beijing, China) at a 1:7,000 dilution for 1 h at room temperature. The membrane was exposed to X-ray film for 1 min. Band densities were detected with the western blot luminance reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and quantified using AlphaImager 2200 (Alpha Innotech, San Leandro, CA, USA).
DNA isolation and PCR-DGGE analysis
DNA extraction of the colonic digesta for DGGE analysis was performed according to the instructions of a DNA Stool Mini Kit (Qiagen, Hilden, Germany). The bacterial universal V3 region of the 16S RNA gene was amplified according to primers published by Muyzer et al. (1993). In brief, the PCR mixture contained 125 ng of genomic DNA, 25 pM of each primer and 25 mL SYBR Green PCR Master Mix 2× (Promega, Madison, WI, USA). The final volume was adjusted to 50 μL with sterile deionized water. The PCR analysis was conducted in a 50 μL volume as described in the routine PCR procedure. The procedure included 5 min at 95 °C; 29 cycles of 30 s at 95 °C, 30 s at 58 °C, and 30 s at 72 °C. The PCR products were analyzed by electrophoresis on 1.0 % (w/v) agarose gel in SYBR green I (Sigma Chemicals, St. Louis, MO, USA) to verify a single product of the expected size. Nucleic acid concentrations were measured using a Gel DocTM XR+ camera with Image LabTM Software (Bio-Rad, Hercules, CA, USA).
DGGE was performed for separation of PCR amplicons on 8 % polyacrylamide gels with a vertical gradient of denaturants of 45–60 %. Electrophoresis began with pre-running for 5 min at 200 V and then for 16 h at 85 V in 0.5 × Tris–acetate–EDTA buffer at a constant temperature of 60 °C using a DCodeTM Universal Mutation Detection System (Bio-Rad). DGGE gels were stained by SYBR Green I (Sigma Chemicals) for 10 min at 25 °C, and photographed with a Gel DocTM XR+ with Image LabTM Software (Bio-Rad) and analyzed by Quantity One Basic 4.6.6 Software for Windows (Bio-Rad). Interesting bands in the denaturing gradient gel electrophoresis profiles were excised with a sterile needle, re-amplified and purified with a Takara Mini Best DNA Fragment Purification Kit (Takara, Dalian, China) according to the manufacturer’s protocol.
The PCR products were cloned into the vector pMD-19T according to the manufacturer’s instructions (Takara) and transformed into Escherichia coli DH5α cells (Tiangen, Beijing, China) via chemical transformation. The transformants were grown on Luria–Bertani agar in the presence of 100 μg/mL ampicillin. The positive recombinants were screened and identified by PCR and sent for sequencing. Homology searches were performed using the BLAST program in the NCBI database (http://blast.ncbi.nlm.nih.gov).
Chemical analyses
The experimental diets were analyzed for crude protein, calcium, and phosphorus according to AOAC (2003) procedures. Dietary amino acids except methionine, cystine, and tryptophan were determined by ion-exchange chromatography using a Hitachi L-8800 AA Analyzer (Tokyo, Japan) after acid hydrolysis with 6 N HCl (reflux for 24 h at 110 °C). Methionine and cystine were determined after oxidation with performic acid and subsequent hydrolysis with 6 M HCl, and the tryptophan content was determined after alkaline hydrolysis at 120 °C for 16 h (AOAC 2003) and separated by reversed-phase HPLC (Agilent 1200, Santa Clara, CA, USA).
Serum AA concentrations were determined by ion-exchange chromatography with physiological fluid analysis conditions (S-433D AA Analyzer, Sykam, Germany). Frozen serum samples were first thawed at 4 °C and then deproteinized with 120 mg of salicylic acid/mL of serum. After samples were placed an ice bath for 20 min, the reaction system was adjusted for pH by adding lithium hydroxide solution (2 mol/L) and then centrifuged at 12,000g (L-80 XP, Beckman, USA) for 30 min. The supernatant fluid was collected and then passed through a filter (0.1 μm) before use for amino acid analysis.
Statistical analysis
Statistical analyses were performed using the statistical software SAS Version 9.2. Data were analyzed by ANOVA according to the GLM procedure of SAS. Log transformation of variables was performed when variance of data was not homogenous among treatment groups, as assessed using the Levene’s test (Wei et al. 2012). Differences among treatment means were determined using Student–Newman–Keuls multiple range test (Fu et al. 2010). Differences at P < 0.05 were considered significant.
Results
Growth performance of weanling piglets
The growth performance of weanling pigs fed the different diets is presented in Table 3. ADG and ADFI of pigs fed the NP treatment were higher than those fed the LP treatment (P < 0.05). The ADG and ADFI of pigs in the LP + BCAA group were similar to those in the NP group (P > 0.05). The feed:gain ratio was greater for pigs fed the LP diet, as compared with pigs in the NP and LP + BCAA groups (P < 0.05).
Serum-free AA concentrations
Serum-free AA concentrations are shown in Table 4. There were significant decreases in the serum concentrations of arginine, histidine, isoleucine, leucine, phenylalanine and valine in pigs fed the LP diet, as compared with those fed the NP diet (P < 0.05). Serum concentrations of arginine, histidine and phenylalanine were also lower in pigs fed the LP + BCAA diet, compared with the NP diet (P < 0.05), while serum concentrations of isoleucine, leucine and valine were higher in pigs fed the LP + BCAA diet, compared with the LP diet (P < 0.05), and did not differ from those in the NP group (P > 0.05).
Intestinal morphology
Pigs fed the LP diet had significantly (P < 0.05) lower villus height in the duodenum, jejunum and ileum than pigs fed the NP diet (Table 5). Supplementation with BCAA to the LP diet restored villous height to that for the NP diet in the jejunum and ileum and partially restored villous height in the duodenum (P < 0.05). Crypt depth and villous height to crypt depth ratio in the jejunum and ileum were not affected by reduction of dietary protein (P > 0.05). However, in the duodenum, the crypt depth of pigs fed the LP diet was lower than for pigs fed the NP diet (P < 0.05). The crypt depth for pigs fed the LP + BCAA was intermediate to that for the other two treatments.
mRNA levels for amino acid and peptide transporters in the jejunum
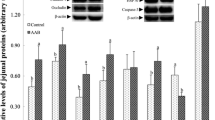
The abundance of ASCT2, B0AT1, CAT-1, rBAT, 4F2hc, b0,+AT, y+LAT and PepT-1 mRNAs in the small intestine of piglets is shown in Fig. 1. There was a significant (P < 0.05) decrease in intestinal ASCT2 mRNA levels in pigs fed the LP diet, compared with the NP diet; however, no difference was detected between the LP and the LP + BCAA groups. The intestinal CAT-1, rBAT and 4F2hc mRNA levels in pigs fed the LP diet were lower compared with the LP + BCAA group (P < 0.05). The abundance of these mRNAs in the NP group was intermediate to the other two treatments. In contrast, intestinal PepT-1 mRNA level in the LP group was higher than that in the LP + BCAA group (P < 0.05), while PepT-1 mRNA level in the NP group was again intermediate to the other two treatments. Intestinal mRNA levels for B0AT1, b0,+AT and y+LAT mRNA did not differ among the three treatment groups (P > 0.05).
Effect of normal protein (20.9 % crude protein, NP), low protein (17.1 % crude protein, LP) or low protein + BCAA (17.9 % crude protein, LP + BCAA) diets on a ASCT2, b B0AT, c CAT-1, d rBAT, e y+LAT, f 4F2hc, g b0,+AT and h PepT-1 mRNA expression in a whole homogenized jejunum segment obtained from piglets 14 days after weaning (n = 6). β-Actin was used as an internal standard to normalize the signal. Means without a common letter differ significantly (P < 0.05)
Protein expression of amino acid and peptide transporters in the small intestine
Results of the western blot analysis indicated the presence of CAT-1, rBAT, 4F2hc and PepT1 in the small intestine (Fig. 2). These four transporters were chosen for western blot analysis because their mRNA levels were significantly affected by dietary CP content and BCAA supplementation. Compared to the NP group, the protein abundance of intestinal PepT-1 was increased in pigs fed the LP diet (P < 0.05). No difference in the PepT-1 protein was detected between the NP and the LP + BCAA groups (P > 0.05). The protein abundance of rBAT-1 was decreased in pigs fed the LP diet (P < 0.05) while there was no difference between the NP and the LP + BCAA groups. The protein abundance of the CAT-1 and the 4F2hc did not differ among pigs fed the different diets (P > 0.05).
Western blot analysis of the proteins CAT-1 (a), PepT-1 (b), 4F2hc (c), and rBAT (d) obtained from a whole homogenized section of a jejunum segment obtained from piglets fed a normal protein (20.9 % crude protein), low protein (17.1 % crude protein) or low protein + BCAA (17.9 % crude protein) diet for 14 days after weaning (n = 6). β-Actin was used as an internal standard to normalize the signal. Means without a common letter differ significantly (P < 0.05)
Volatile fatty acid concentrations in the colon
Concentrations of volatile fatty acid were all significantly influenced by dietary protein levels (Table 6). Specifically, amounts of acetic acid, propionic acid, butyric acid, pentanoic acid, isobutyric acid and isovaleric acid were decreased in pigs fed the LP diet, compared with the NP group (P < 0.05). However, there was no difference between the LP and the LP + BCAA groups (P > 0.05).
DGGE fingerprints
Representative results of the DGGE fingerprints are shown in Fig. 3. Most bands among the three groups of pigs were similar. There was no difference in the numbers of bands, Simpson’s Index of Diversity and Dice’s Coefficient of Similarity (P > 0.05) (Table 7). The bands which differed between the NP and the LP groups were excised from the gels and their species identified by sequence analysis. Compared to the LP group, an increase in the Clostridium-like phenotype was observed in the NP group (Bands 1 and 4, Fig. 3).
Example of DGGE fingerprints of PCR products of V3 regions of 16S rDNA from colonic digesta samples taken from piglets 14 days after weaning. Piglets received normal protein (20.9 % crude protein), low protein (17.1 % crude protein) or low protein + BCAA (17.9 % crude protein) diets. Four representative samples from each group are shown. Arrows (1–4) indicate excised bands that were re-amplified (see Table 8)
Discussion
Low-protein diets are often fed to weanling piglets, which have a compromised ability to digest solid diets (largely of plant origin), to alleviate adverse effects on the intestine (Wang et al. 2009). Specifically, by reducing dietary protein levels, amounts of amino acids available for bacterial proliferation in the gastrointestinal tract are reduced, thereby decreasing the incidence of diarrhea (Rezaei et al. 2013b). In the present study, making the LP and the NP diets isonitrogenous would negate the very reasons for conducting the experiment. The LP and the LP + BCAA diets were essentially isonitrogenous as only small amounts of BCAA were added to the LP diet and the protein content of the two diets was similar (Table 1). Therefore, we believe that any differences observed were due to the presence of BCAA and not due to differences in the dietary protein levels between the LP and LP + BCAA groups.
Both reduced feed intake and metabolic disturbances in piglets fed an LP diet may result from an imbalance in amino acid composition (Wu 2009). While the content of BCAA is relatively high in swine feedstuffs (Hou et al. 2012; Li et al. 2011b; Tan et al. 2012), dietary requirements of these amino acids for protein synthesis in young pigs are also high (Wu et al. 2013b). In mammals, BCAA are degraded via interorgan cooperation, with skeletal muscle as the major site for their transamination and with the liver as the primary organ for the oxidation of their carbon skeletons (Le Plenier et al. 2012; Lei et al. 2012a, b, 2013; Murakami et al. 2012). In the piglet small intestine, BCAA are also degraded by both enterocytes (Chen et al. 2007, 2009) and luminal microorganisms (Dai et al. 2012a, b, 2013). In our study, both the LP (17.1 % crude protein) and the LP + BCAA diets were deficient in protein (NRC 1998). In the LP diet, only lysine, methionine, threonine and tryptophan were supplemented, while the LP + BCAA diet provided a better balance of amino acids by simultaneously supplementing leucine, valine and isoleucine (Dillon 2013). Therefore, these two diets represent a reasonable model for evaluating the effects of BCAA on nutrient transporters and intestinal microbiota in the gut. Among the three BCAA, only leucine is known to activate the mTOR signaling pathway and stimulate tissue protein synthesis (Li et al. 2011a; Suryawan and Davis 2011), isoleucine and valine were added to the LP diet along with leucine to maintain an adequate balance among these amino acids (Suryawan et al. 2013).
Results of the present study showed that reducing the dietary protein level impaired growth performance of weanling pigs, as previously reported by Deng et al. (2009). The lower feed intake observed in piglets that consumed the LP diet may be related to a deficiency in some essential amino acids, whose metabolites regulate the production of neurotransmitters and thus food consumption (Wu 2013b). The ADG of piglets was also decreased likely due to the reduced feed intake. Supplementation with BCAA to the LP diet improved the growth performance of piglets to a level similar to that for the NP diet which is consistent with previous studies showing that BCAA improved the growth performance and feed efficiency in pigs fed an LP diet (Brudevold and Southern 1994; Figueroa et al. 2003; Lordelo et al. 2008; Yin et al. 2010). These improvements likely result from an increase in tissue protein synthesis (Yin et al. 2010). Note that deposition of 1 g protein in the body is associated with the retention of 3 g water (Wu 2013a). Thus, protein growth in skeletal muscle is a major determinant of feed efficiency in young animals.
In the current study, diets with a reduced protein level but supplemented with BCAA cannot sustain adequate concentrations of nutritionally essential amino acids, such as arginine, histidine and phenylalanine in the serum. In young pigs, serum concentrations of some amino acid levels may reflect their dietary intake, but this is not true for certain amino acids due to the complex compartmentalization of their metabolism in vivo (Wu 2013a). BCAA are not synthesized by animal cells and rates of BCAA catabolism are not augmented by an LP diet (Rezaei et al. 2013a). Therefore, serum concentrations of leucine, isoleucine and valine were lower in the LP diet than those in the NP diet. Supplementing BCAA to the LP diet increased the concentrations of leucine, isoleucine and valine in the serum of weanling piglets.
A deficiency of BCAA in diets impairs intestinal protein synthesis in young pigs (Yin et al. 2010). Thus, the LP diet resulted in reduced intestinal mucosal mass in weanling piglets. The folds in the intestine called villi are an important exchange area for digestion and absorption (Pluske et al. 1996). Villous height and crypt depth are critical factors affecting the piglet’s ability to absorb nutrients (Kelly et al. 1991). In the current study, reducing the dietary protein level resulted in a significant reduction of villous height in the duodenum, jejunum and ileum as well as a decline of crypt depth in the duodenum in the LP group. Similar findings were reported for weanling pigs fed a lysine-deficient diet (He et al. 2013). The reduction in villus height would reduce the surface area available for absorption of nutrients and this may partially explain the reduced growth performance and reduced feed efficiency in pigs fed the LP diet. In contrast, the villus height was similar for pigs fed the NP diet and the LP + BCAA diet, indicating an increased surface area for nutrient absorption enhanced growth and the efficiency of nutrient utilization in pigs fed a BCAA-supplemented LP diet.
To provide a molecular mechanism for BCAA to improve intestinal function, additional measurements were made to evaluate the abundance of transporters of peptides and free amino acids in response to dietary amino acid availability. It is well known that small peptides or amino acids are mainly absorbed in the small intestine (Bröer 2008). Intestinal absorption of amino acids and small peptides is mediated, respectively, by amino acid and peptide transporters, which vary in type and specificity (Closs et al. 2006; Prasad et al. 1999). Based on the notion that different nutrient levels in the diet can regulate nutrient transporters in the small intestine (Gilbert et al. 2008; Suryawan and Davis 2011), we hypothesized that the small intestine expresses different levels of amino acid and peptide transporters in response to different intakes of dietary protein and amino acids.
Available evidence shows that, in the small intestine, rates of small-peptide transport by PepT1 are higher than rates of free amino acid transport by amino acid transporters (Daniel 2004). Among the gene encoding for peptide transporters evaluated in this study, PepT1 was the only gene that exhibited a greater expression level in the small intestine of pigs fed the LP diet than piglets which consumed the NP or the LP + BCAA diet. In the piglet small intestine, PepT1 mainly transports dipeptides and tripeptides from the digestion of dietary proteins (Daniel 2004). The lower feed intake influenced by the LP diet may explain the change in intestinal PepT-1 abundance. Ihara et al. (2000) reported that when rats were starved for 4 days, semi-starved (50 % amount of control) for 10 days, or given total parenteral nutrition for 10 days, PepT1 mRNA levels were increased by 179, 161 and 164 % in the starved, parenterally fed, and semi-starved rats, respectively. Furthermore, Chen et al. (2005) observed that PepT-1 mRNA abundance was higher in feed-restricted chickens than ad libitum fed chickens. Therefore, compared with the NP diet, the reduction in feed intake caused by the LP diet may have led to the increase in PepT-1 expression, so as to efficiently transport small peptides from the lumen of the small intestine into enterocytes. This may aid in explaining why bioavailability of dietary amino acids is higher in animals fed an LP diet than an NP diet (Rezaei et al. 2013a). Key regulators of intestinal PepT-1 expression may be BCAA. In support of this view, the abundance of the intestinal PepT-1 protein in pigs fed the LP + BCAA diet returned to the level for the NP group. Therefore, the increased expression of PepT1 in the LP diet may provide a mechanism for conserving dietary protein and compensating for decreased feed intake.
Amino acid transporters that are related to the absorption of BCAA by the small intestine were measured in this study. It would be ideal to analyze the expression of all genes responsible for intestinal amino acid transport in pigs using the currently available technology. However, the DNA sequences of many porcine amino acid transporters are still unknown and were, therefore, unavailable for use in the present experiment. B0AT1, belonging to the B0 system, is the major apical neutral amino acid transporter in the kidney and the small intestine (Bröer 2008). ASCT2 mediates high-affinity transport of neutral amino acids except for aromatic AA. In this research, some amino acid transporters related to the transportation of cationic amino acids (lysine, arginine and histidine) were also quantified at both mRNA and protein levels. For example, rBAT/b0,+AT is a transporter for cationic amino acids and cystine, which is coupled to the efflux of neutral amino acids. In addition, CAT-1 transports cationic amino acids in the kidney and the small intestine, whereas y+LAT1/4F2hc transporter is a mediator of cationic amino acid efflux from epithelial cells (Bröer 2008). All of these amino acid transporters play a vital role in intestinal absorption of amino acids. Our results indicate that dietary intake of protein or BCAA regulates intestinal expression of amino acid transporters to efficiently absorb amino acids from the lumen of the small intestine into enterocytes.
Some researchers have demonstrated the special function of specific amino acid levels in modulating the expression of the amino acid transporters. For example, Torras-Llort et al. (1998) reported that b0,+-like and y+-like transport systems were upregulated in the chicken intestine by increasing dietary lysine levels. Similarly, Wang et al. (2012a) found that different lysine levels could regulate CAT-1 expression in the pig small intestine. The function of the BCAA in the regulation of the amino acid transporters has also been reported in recent years (Laspiur et al. 2009). In particular, Drummond et al. (2010) demonstrated that an increase in dietary leucine intake upregulated the expression of amino acid transporters in human skeletal muscle by stimulating the mTOR pathway. Thus, like other functional amino acids, such as glycine (Wang et al. 2013), glutamate (Brosnan and Brosnan 2013; San Gabriel and Uneyama 2013), glutamine (Ren et al. 2013), tryptophan (Fernstrom 2013), cysteine (Hou et al. 2013), and arginine (Satterfield et al. 2013), BCAA have both nutritional and regulatory roles in animal metabolism. We surmise that supplementing BCAA to the LP diet may increase the expression of intestinal amino acid transporters in weanling piglets through activating the mTOR signaling pathway. Further studies are required to test this hypothesis.
Short chain fatty acids are the major end products from the fermentation processes of gut microflora in the large intestine (Bergen and Wu 2009), and are significantly affected by the type of diet and microbial composition in the gut (van Beers-Schreurs et al. 1998). In our study, we observed that the VFA levels in the colon of pigs fed the NP diet were much higher compared with pigs fed the LP and the LP + BCAA diets, and a relative increase in numbers of the Clostridium-like phenotype was also observed in the NP group. Galfi and Bokori (1990) obtained similar results in which the concentrations of VFA and Clostridium populations were increased when high-protein diets were fed. Also, Zentek et al. (2004) reported that increasing dietary protein levels can increase Clostridium content in the gut. Clostridium and other bacteria are also known to produce VFA (Dai et al. 2011). We speculate that undigested protein in the NP diet that reached the lower intestine of pigs may increase the numbers of Clostridium, leading to increased concentrations of VFA in the colonic digesta.
In conclusion, our data demonstrate that BCAA may be necessary to maintain normal intestinal development and physiological absorption of amino acids via regulating expression of intestinal amino acid and peptide transporters (especially rBAT and PepT-1) in the small intestine. As a functional amino acid, leucine may play an important role in modulating intestinal expression of genes responsible for efficient utilization of dietary protein and maintaining whole-body nitrogen homeostasis. Further studies are needed to reveal the influence of dietary LP or BCAA levels on the microflora in the colon.
Abbreviations
- ADFI:
-
Average daily feed intake
- ADG:
-
Average daily gain of body weight
- ASCT2:
-
Na+-neutral AA exchanger 2
- BCAA:
-
Branched-chain amino acids
- B0AT1:
-
System B0 neutral AA transporter
- CAT1:
-
Cationic amino acid transporter 1
- CP:
-
Crude protein
- DGGE:
-
Denaturing gradient gel electrophoresis
- 4F2hc:
-
4F2 heavy chain
- LP:
-
Low protein
- mTOR:
-
Mammalian target of rapamycin
- NP:
-
Normal protein
- PepT-1:
-
Peptide transporter 1
- rBAT:
-
Related to b0,+ amino acid transporter
- RT-PCR:
-
Real-time polymerase chain reaction
- VFA:
-
Volatile fatty acids
References
AOAC (2003) Official methods of analysis. Association of Official Analytical Chemists, Arlington
Armstrong WD, Cline T (1976) Effects of various dietary nutrient levels on the incidence of colibacillary diarrhea in pigs: intestinal ligation studies. J Anim Sci 42:592–598
Baker DH (2009) Advances in protein-amino acid nutrition of poultry. Amino Acids 37:29–41
Bergen WG, Wu G (2009) Intestinal nitrogen recycling and utilization in health and disease. J Nutr 139(5):821–825
Bertrand J, Goichon A, Déchelotte P et al (2013) Regulation of intestinal protein metabolism by amino acids. Amino Acids 45:443–450
Blachier F, Lancha AH Jr, Boutry C et al (2010) Alimentary proteins, amino acids and cholesterolemia. Amino Acids 38:15–22
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286
Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acids 45:413–418
Brudevold A, Southern L (1994) Low-protein, crystalline amino acid-supplemented, sorghum-soybean meal diets for the 10-to 20-kilogram pig. J Anim Sci 72:638–647
Chen H, Pan YX, Wong EA et al (2005) Dietary protein level and stage of development affect expression of an intestinal peptide transporter (cPepT1) in chickens. J Nutr 135:193–198
Chen LX, Yin YL, Jobgen WS et al (2007) In vitro oxidation of essential amino acids by intestinal mucosal cells of growing pigs. Livest Sci 109:19–23
Chen LX, Li P, Wang JJ et al (2009) Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids 37:143–152
Closs E, Boissel JP, Habermeier A et al (2006) Structure and function of cationic amino acid transporters (CATs). J Membr Biol 213:67–77
Dai ZL, Zhang J, Wu G et al (2010) Utilization of amino acids by bacteria from the pig small intestine. Amino Acids 39:1201–1215
Dai ZL, Wu G, Zhu WY (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16:1768–1786
Dai ZL, Li XL, Xi PB et al (2012a) Regulatory role for l-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 43:233–244
Dai ZL, Li XL, Xi PB et al (2012b) Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 42:1597–1608
Dai ZL, Li XL, Xi PB et al (2013) l-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 45:501–512
Daniel H (2004) Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66:361–384
Deng D, Yin YL, Chu WY et al (2009) Impaired translation initiation activation and reduced protein synthesis in weaned piglets fed a low-protein diet. J Nutr Biochem 20:544–552
Dillon EL (2013) Nutritionally essential amino acids and metabolic signaling in aging. Amino Acids 45:431–441
Drummond MJ, Glynn EL, Fry CS et al (2010) An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298:E1011–E1018
Escobar J, Frank JW, Suryawan A (2006) Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290:E612–E621
Fernstrom JD (2013) Large neutral amino acids: dietary effects on brain neurochemistry and function. Amino Acids 45:419–430
Figueroa J, Lewis A, Miller P et al (2003) Growth, carcass traits, and plasma amino acid concentrations of gilts fed low-protein diets supplemented with amino acids including histidine, isoleucine, and valine. J Anim Sci 81:1529–1537
Franklin M, Mathew A, Vickers J et al (2002) Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J Anim Sci 80:2904–2910
Fu WJ, Stromberg AJ, Viele K et al (2010) Statistics and bioinformatics in nutritional sciences: analysis of complex data in the era of systems biology. J Nutr Biochem 21:561–572
Galfi P, Bokori J (1990) Feeding trial in pigs with a diet containing sodium n-butyrate. Acta Vet Hung 38:3–17
Gilbert ER, Li H, Emmerson DA et al (2008) Dietary protein quality and feed restriction influence abundance of nutrient transporter mRNA in the small intestine of broiler chicks. J Nutr 138:262–271
He LQ, Yang HS, Li TJ et al (2013) Effects of dietary l-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45:383–391
Hou YQ, Wang L, Zhang W et al (2012) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids 43:1233–1242
Hou YQ, Wang L, Yi D et al (2013) N-Acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids 45:513–522
Ihara T, Tsujikawa T, Fujiyama Y et al (2000) Regulation of PepT1 peptide transporter expression in the rat small intestine under malnourished conditions. Digestion 61:59–67
Kanai Y, Hediger MA (2003) The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol 479:237–247
Kelly D, Smyth J, McCracken K (1991) Digestive development of the early-weaned pig. Brit J Nutr 65:181–188
Laspiur JP, Burton JL, Weber PSD et al (2009) Dietary protein intake and stage of lactation differentially modulate amino acid transporter mRNA abundance in porcine mammary tissue. J Nutr 139:1677–1684
Le Plenier S, Walrand S, Noirt R et al (2012) Effects of leucine and citrulline versus non-essential amino acids on muscle protein synthesis in fasted rat: a common activation pathway? Amino Acids 43:1171–1178
Lei J, Feng DY, Zhang YL et al (2012a) Regulation of leucine catabolism by metabolic fuels in mammary epithelial cells. Amino Acids 43:2179–2189
Lei J, Feng DY, Zhang YL et al (2012b) Nutritional and regulatory role of branched-chain amino acids in lactation. Front Biosci 17:2725–2739
Lei J, Feng DY, Zhang YL et al (2013) Hormonal regulation of leucine catabolism in mammary epithelial cells. Amino Acids 45:531–541
Leibach FH, Ganapathy V (1996) Peptide transporters in the intestine and the kidney. Annu Rev Nutr 16:99–119
Li P, Yin YL, Li DF et al (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li FN, Yin YL, Tan BE et al (2011a) Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 41:1185–1193
Li XL, Rezaei R, Li P et al (2011b) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Lordelo M, Gaspar A, Le Bellego L et al (2008) Isoleucine and valine supplementation of a low-protein corn–wheat–soybean meal-based diet for piglets: growth performance and nitrogen balance. J Anim Sci 86:2936–2941
Murakami H, Ito M, Furukawa Y et al (2012) Leucine accelerates blood ethanol oxidation by enhancing the activity of ethanol metabolic enzymes in the livers of SHRSP rats. Amino Acids 43:2545–2551
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microb 59:695–700
NRC (1998) Nutrient requirements of swine. National Academy Press, Washington, DC
NSNG (2010) National Swine Nutrition Guide. Tables on nutrient recommendations, ingredient composition, and use rates. US Pork Center of Excellence, Ames
Opapeju F, Krause D, Payne R et al (2009) Effect of dietary protein level on growth performance, indicators of enteric health, and gastrointestinal microbial ecology of weaned pigs induced with postweaning colibacillosis. J Anim Sci 87:2635–2643
Palacín M, Kanai Y (2004) The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch 447:490–494
Pluske JR, Thompson MJ, Atwood CS et al (1996) Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br J Nutr 76:409–422
Prasad PD, Wang H, Huang W et al (1999) Human LAT1, a subunit of system L amino acid transporter: molecular cloning and transport function. Biochem Biophys Res Commun 255:283–288
Ren WK, Luo W, Wu MM et al (2013) Dietary l-glutamine supplementation improves pregnancy outcome in mice infected with type-2 porcine circovirus. Amino Acids 45:479–488
Rezaei R, Wang WW, Wu ZL et al (2013a) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotechnol 4:7
Rezaei R, Knabe DA, Tekwe CD et al (2013b) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rhoads JM, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
San Gabriel A, Uneyama H (2013) Amino acid sensing in the gastrointestinal tract. Amino Acids 45:451–461
Satterfield MC, Dunlap KA, Keisler DH et al (2013) Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino Acids 45:489–499
Suryawan A, Davis TA (2011) Regulation of protein synthesis by amino acids in muscle of neonates. Front Biosci 16:1445–1460
Suryawan A, Nguyen HV, Almonaci RD et al (2013) Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated. Amino Acids 45:523–530
Tan B, Yin Y, Kong X et al (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
Tan BE, Li XG, Wu G et al (2012) Dynamic changes in blood flow and oxygen consumption in the portal-drained viscera of growing pigs receiving acute administration of l-arginine. Amino Acids 43:2481–2489
Torras-Llort M, Soriano-Garcia J, Ferrer R et al (1998) Effect of a lysine-enriched diet on l-lysine transport by the brush-border membrane of the chicken jejunum. Am J Physiol 274:R69–R75
van Beers-Schreurs HMG, Nabuurs MJA, Vellenga L et al (1998) Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J Nutr 128:947–953
Wang JJ, Chen LX, Li P et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Wang W, Qiao S, Li D (2009) Amino acids and gut function. Amino Acids 37:105–110
Wang W, Zeng X, Mao X et al (2010) Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J Nutr 140:981–986
Wang X, Zeng P, Feng Y et al (2012a) Effects of dietary lysine levels on apparent nutrient digestibility and cationic amino acid transporter mRNA abundance in the small intestine of finishing pigs, Sus scrofa. Anim Sci J 83:148–155
Wang Y, Zhang L, Zhou G et al (2012b) Dietary l-arginine supplementation improves the intestinal development through increasing mucosal Akt and mammalian target of rapamycin signals in intra-uterine growth retarded piglets. Br J Nutr 108:1371–1381
Wang WW, Wu ZL, Dai ZL et al (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010a) Recent advances in swine amino acid nutrition. J Anim Sci Biotech 1:49–61
Wu G (2010b) Functional amino acids in growth, reproduction and health. Adv Nutr 1:31–37
Wu G (2013a) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu X, Ruan Z, Gao Y et al (2010) Dietary supplementation with l-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn-and soybean meal-based diet. Amino Acids 39:831–839
Wu G, Bazer FW, Johnson GA et al (2011a) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Bazer FW, Burghardt RC et al (2011b) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu G, Bazer FW, Satterfield MC et al (2013a) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Wu G, Wu ZL, Dai ZL et al (2013b) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Yin FG, Liu YL, Yin YL et al (2009) Dietary supplementation with Astragalus polysaccharide enhances ileal digestibilities and serum concentrations of amino acids in early weaned piglets. Amino Acids 37:263–270
Yin Y, Yao K, Liu Z et al (2010) Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486
Zentek J, Fricke S, Hewicker-Trautwein M, Ehinger B, Amtsberg G, Baums C (2004) Dietary protein source and manufacturing processes affect macronutrient digestibility, fecal consistency, and presence of fecal Clostridium perfringens in adult dogs. J Nutr 134(8 Suppl):2158S–2161S
Acknowledgments
This research was supported by National Key Basic Research Program (2012CB124704 and 2013CB127302), NSFC (31272217 and 31272450), Chinese Universities Scientific Funds (2012RC024), and the Thousand-People-Talent program at China Agricultural University, and Texas AgriLife Research (No. 8200).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, S., Qiao, S., Ren, M. et al. Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 45, 1191–1205 (2013). https://doi.org/10.1007/s00726-013-1577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1577-y