Abstract
Embryonic loss and intrauterine growth restriction (IUGR) are significant problems in humans and other animals. Results from studies involving pigs and sheep have indicated that limited uterine capacity and placental insufficiency are major factors contributing to suboptimal reproduction in mammals. Our discovery of the unusual abundance of the arginine family of amino acids in porcine and ovine allantoic fluids during early gestation led to the novel hypothesis that arginine plays an important role in conceptus (embryo and extra-embryonic membranes) development. Arginine is metabolized to ornithine, proline, and nitric oxide, with each having important physiological functions. Nitric oxide is a vasodilator and angiogenic factor, whereas ornithine and proline are substrates for uterine and placental synthesis of polyamines that are key regulators of gene expression, protein synthesis, and angiogenesis. Additionally, arginine activates the mechanistic (mammalian) target of rapamycin cell signaling pathway to stimulate protein synthesis in the placenta, uterus, and fetus. Thus, dietary supplementation with 0.83 % l-arginine to gilts consuming 2 kg of a typical gestation diet between either days 14 and 28 or between days 30 and 114 of pregnancy increases the number of live-born piglets and litter birth weight. Similar results have been reported for gestating rats and ewes. In sheep, arginine also stimulates development of fetal brown adipose tissue. Furthermore, oral administration of arginine to women with IUGR has been reported to enhance fetal growth. Collectively, enhancement of uterine as well as placental growth and function through dietary arginine supplementation provides an effective solution to improving embryonic and fetal survival and growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Embryonic death losses in humans and livestock species are estimated to range from 20 to 50 %, with two-thirds of the losses occurring during the peri-implantation period of pregnancy (Bazer et al. 2010, 2011). Additionally, impaired implantation, which occurs between days 20 and 24 of the menstrual cycle, is a major factor limiting the success of assisted reproductive technologies in humans that accounts for 50–75 % of failures to establish pregnancy (Fazleabas 2007). Furthermore, intrauterine growth restriction (IUGR), which increases the risk for neonatal mortality and morbidity, as well as compromising postnatal growth and health, is a significant problem in humans and other mammals (Wu et al. 2012a). New knowledge about nutritional support of embryonic and fetal development is essential for clinicians and producers of food animals to enhance female fertility by reducing early pregnancy losses and improving fetal growth. Because of ethical concerns with studies involving human embryos and fetuses, animal models (e.g., mice, rats, pigs, and sheep) are essential to research that addresses important questions related to uterine biology and pregnancy (Bazer et al. 2012a, b).
Work with farm animals (e.g., pigs and sheep) also has important implications for increasing animal production to provide high-quality animal proteins for human consumption (Wu et al. 2006). For example, profitability of the swine industry critically depends on reproductive efficiency of sows. However, prenatal mortality (up to 50 %) is a significant challenge that must be overcome to improve the reproductive performance of modern high-prolificacy sows. As a result, gilts produce an average of 9.62 piglets born per litter in the USA, which is much lower than the potential of 14 or more piglets per litter based on the total number of oocytes ovulated (USDA 2009). More than 75 % of prenatal losses occur during the first 25 days of gestation (term = 114 days), and there is another period of fetal death between days 40 and 60 of gestation, especially in sows with large litters (Pope 1994). Many factors contribute to embryonic and fetal losses, including ovulation rate, fertilization rate, disease (e.g., virus infection), chromosomal abnormalities, non-uniform development of conceptuses, and intrauterine crowding or uterine capacity (Wu et al. 2006). Of those causes, failure of development of conceptuses (embryo and extra-embryonic membranes) during the peri-implantation period is primarily responsible for embryonic losses before day 30 of gestation and inadequate uterine capacity is the major reason for fetal deaths and suboptimal growth after day 30 (Bazer et al. 2009a, b).
Much effort has been made to improve embryonic/fetal survival in pigs and other mammalian species. Animal breeding can improve litter size, but the efficiency is very low. Litter size in the US swine industry increased at a rate of only 0.052 pigs/year between 1980 and 2000 (Johnson 2000). The reason for low efficiency of genetic selection is low heritability for litter size. Heritability estimates for litter size born is 0.1 and even less for live-born pigs in a litter (~0.07) (Rothschild 1996). An alternative is to increase ovulation rate through superovulation. However, an increase in litter size is seldom realized because high ovulation rates in sows have the potential to cause excessive intrauterine crowding of conceptuses which increases fetal mortality during mid- and late-gestation (Town et al. 2005).
Arginine is a nutritionally essential amino acid for gestating mammals (Wu et al. 2009). In addition to being a building block for proteins, arginine is the precursor for synthesis of many biologically active molecules, including nitric oxide (NO), ornithine, polyamines (putrescine, spermidine, and spermine), creatine, and agmatine (Wu and Morris 1998). Of those, NO and polyamines stimulate cell proliferation and migration, cellular remodeling, angiogenesis, and dilation of blood vessels to increase blood flow, whereas creatine is essential to neurological and skeletal muscle development (Wu et al. 2009). Because much work has been done regarding impacts of arginine nutrition on porcine embryonic and fetal development, findings from these studies are highlighted in the present review. In particular, dietary supplementation with 0.8 % arginine (16 g/sow per day) between days 14 and 25 of gestation (Li et al. 2011) or with 0.83 % arginine (16.6 g/sow per day) between days 30 and 114 of gestation (Mateo et al. 2007) enhances litter size in gilts. A number of studies conducted in different countries, including Australia (De Blasio et al. 2009), China (Gao et al. 2012; Wu et al. 2012b), the Netherlands (Ramaekers et al. 2006), New Zealand (Campbell 2009), and Sweden (Bérard and Bee 2010), have provided additional evidence to support a beneficial role for arginine supplementation in reducing embryonic deaths in gestating sows. Similar results have been demonstrated for pregnant rats (Zeng et al. 2008, 2012). In contrast, two research groups recently reported that dietary supplementation with 1.23 % arginine or 28 g/sow per day during either early (days 18–34) or late (days 75–115) pregnancy had no effect on litter size and could even impair reproductive performance in gilts and sows (Greiner et al. 2012; Zier-Rush et al. 2012). It should be kept in mind that more arginine in the diet is not necessarily better for embryonic or fetal survival in swine. Rather, proper ratios and amounts of amino acids (particularly arginine and lysine) as well as dietary intake of total nitrogen should be taken into consideration in dietary formulations to prevent antagonism among amino acids and the toxicity of ammonia to dams and their embryos/fetuses (Wu 2010a, b). These results underscore the importance of understanding basic knowledge about reproductive biology, as well as amino acid biochemistry and nutrition to improve mammalian reproduction.
Embryogenesis, implantation of blastocysts, and placentation
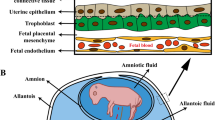
Uterine receptivity to implantation varies among species and involves (1) attachment of trophectoderm to uterine lumenal (LE) and superficial glandular (sGE) epithelia; (2) modification of phenotype of uterine stromal cells; (3) silencing of receptors for progesterone and estrogen in uterine epithelia; (4) suppression of genes for immune recognition in uterine LE/sGE; (5) alterations in membrane permeability to enhance conceptus–maternal exchange of physiological and nutritional factors; (6) angiogenesis and vasculogenesis, increased vascularity of the endometrium; (7) activation of genes for transport of nutrients into the uterine lumen; and (8) enhanced signaling for pregnancy recognition (Bazer et al. 2010, 2012a, b; Johnson et al. 2009). The conceptus (embryo/fetus and associated membranes) requires amino acids and other nutrients for growth and development (Gao et al. 2009a).
In humans, the blastocyst stage of development is reached on about day 5 after fertilization of the oocyte and then enters the uterus where the embryo differentiates into the inner cell mass (which will develop into the fetus) and the outer layer of cells or trophoblast which will develop into the placenta and associated membranes (Bazer et al. 2010). During this period, the conceptus receives all nutrients from uterine secretions and oxygen from its surrounding environment. Embryogenesis is followed by implantation (days 7–9 post-fertilization in humans), which is the first stage in a sequence of events leading to placentation (the formation and growth of the placenta within the uterus). By week 4 after fertilization, the basic structure of the mature placenta will have been established.
In swine, following fertilization, zygotes develop and cleave into 2- and 4-cell stage embryos in the oviduct (Bazer et al. 2010). After entering the uterus around day 3 of gestation, embryos continue to cleave, develop to blastocysts by days 7–8 of gestation and then hatch from the zona pellucida. Thereafter, blastocysts migrate within the uterine lumen to achieve equal spacing among themselves and then undergo dramatic changes in morphology from an expanded spherical shape to tubular and filamentous forms between days 10 and 15 of pregnancy. The diameter of spherical blastocysts is only 5–10 mm by day 10 of gestation. However, when reaching a spherical diameter of 10 mm on about day 11 of gestation, it takes only 3 or 4 h for blastocysts to elongate to tubular and then filamentous conceptuses that are 150–200 mm in length; by day 15, they approach approximately 1,000 mm in length (Geisert and Yelich 1997). This dramatic morphological change occurs initially through cellular remodeling rather than cellular hyperplasia, but the final phase of elongation between days 12 and 15 involves both cellular hyperplasia and cellular remodeling (Geisert et al. 1982).
Maternal recognition of pregnancy starts at day 11 of gestation when the blastocyst begins its dramatic morphological changes and also initiates secretion of estrogen as the pregnancy recognition signal in pigs (Bazer et al. 2009a, b). Porcine conceptuses initiate attachment of trophectoderm to uterine LE on day 13 of pregnancy and implantation is accomplished by about day 18 of gestation in advance of placentation and formation of a true epitheliochorial placenta. Unique characteristics of pigs and other domestic animals (including sheep) are the prolonged pre-implantation periods for elongation of conceptus trophectoderm, followed by orientation of the blastocyst, apposition between trophectoderm and uterine LE, and then adhesion of trophectoderm to uterine LE (Bazer et al. 2008). This prolonged pre-implantation period for blastocyst/conceptus elongation allows for establishment of a maximum surface area of contact between trophectoderm and uterine LE for absorption of molecules secreted or transported by maternal uterine epithelia into the uterine lumen (histotroph). These substances are essential for survival and growth of the conceptus which has superficial and noninvasive attachment between trophectoderm and uterine LE in pigs (Kim et al. 2013).
Elongation of the porcine trophectoderm depends mainly on histotrophic nutrition from uterine LE, sGE, and mid- to deep uterine glandular epithelium (GE) (Spencer and Bazer 2004). However, rapid growth of blood vessels in the yolk sac (day 16–21) and allantois (day 21 to term) of the placenta prepares the conceptus for hematotropic exchange of nutrients and gases between maternal and fetal placental blood (Bazer et al. 2012a, b). Additionally, nutrients are supplied by histotroph via areolae of the chorioallantoic membranes from the post-implantation period of pregnancy on about day 25 to the end of gestation to support growth and development of the conceptus (Johnson et al. 2009). To sustain these dramatic events in conceptus development, many genes for nutrient transport, cellular remodeling, angiogenesis, relaxation of vascular tissues, as well as cell proliferation and migration are expressed (Bazer et al. 2012a, b). Early embryonic losses result from a failure of conceptus development and implantation during the peri-implantation period of pregnancy (Bazer et al. 1988). This essential knowledge about implantation and placentation provides a physiological basis for nutritional management of gestating sows.
In sheep, embryos enter the uterus on day 3 after breeding, develop to spherical blastocysts and then transform to spherical (day 10, 0.4 mm), tubular and filamentous forms between days 12 (1 × 33 mm), 14 (1 × 68 mm) and 15 (1 × 150–190 mm) (Bazer et al. 2012a, b). Meanwhile, extra-embryonic membranes extend into the contralateral uterine horn between days 16 and 20 of pregnancy. Elongation of ovine conceptuses is a prerequisite for central implantation involving apposition and adhesion between trophectoderm of the conceptus (oTr) and uterine LE/sGE before loss of LE occurs to allow intimate contact between oTr and uterine basal lamina proximal to uterine stromal cells between days 18 and 50–60 of gestation (Bazer et al. 2008).
In all species, a functional placenta transports nutrients, respiratory gases, and the products of their metabolism between the maternal and fetal circulations, and this is crucial for fetal survival, growth, and development (Wang et al. 2012). Rates of utero-placental blood flows depend on placental vascular growth (a result of angiogenesis) and placental vascularization that are greatly influenced by the availability of NO and polyamines (Reynolds et al. 2006). To support increased uterine and placental blood flows, placental angiogenesis increases markedly from the first to the second and third trimesters of gestation (term = 147 days) and continues to increase during the last days of gestation. Uptake of nutrients by the uterus or the fetus is determined by both rate of blood flow and concentrations of nutrients in the arterial and venous blood. Thus, uptake of both macro- and micro-nutrients by the uterus is greater in pregnant women than in nonpregnant women (Reynolds et al. 2006). Conversely, impaired placental blood flow contributes to IUGR in mammalian pregnancies (Wu et al. 2004a, b, 2006).
Arginine metabolism in mammals
Arginine synthesis
In most mammals (including humans and pigs), arginine in circulating blood is derived from diets and endogenous sources (including both de novo synthesis and protein degradation) (Wu et al. 2013). While women can choose what they would like to eat, a restricted feeding program (e.g., 2 kg of a typical corn- and soybean meal-based diet) is normally adapted for gestating gilts to prevent both excessive maternal weight gain during pregnancy and low appetite during lactation (Kim et al. 2009). Thus, dietary provision of arginine in the gestation diet is insufficient to meet arginine requirements by pregnant swine. This necessitates endogenous synthesis of arginine to support embryonic, placental, and fetal growth. Because gestating pigs maintain a positive nitrogen balance (meaning no net protein degradation), de novo synthesis makes the sole contribution to endogenous provision of arginine for both the mothers and their fetuses. When gestating pigs are fed a restricted amount of the diet daily, de novo synthesis of arginine from glutamine, glutamate and proline by the mother plus her fetus(es) can be estimated on the basis of arginine composition in the fetus and placenta (Table 1) as well as creatine synthesis. All of these metabolic needs for arginine, except for placental protein accretion after the first half of gestation, increase substantially with advancing stages of gestation (Table 2). In support of this view, the fetal small intestine can synthesize citrulline (the immediate precursor of arginine) from glutamine, glutamate and proline beginning at day 30 of gestation and the capacity of this synthetic pathway increases progressively between days 30 and 114 (term) of pregnancy (Dekaney et al. 2003).
Studies with porcine enterocytes established the enzymological basis for arginine synthesis from glutamine, glutamate and proline (Wu et al. 1994, 2004a, b). Glutamine is converted into glutamate by phosphate-activated glutaminase. Pyrroline-5-carboxylate (P5C) is formed from glutamate by P5C synthase. P5C can also be synthesized from proline by proline oxidase (Wu 1997). P5C is amidated by ornithine aminotransferase to form ornithine, which reacts with carbamoylphosphate to yield citrulline by carbamoylphosphate synthase I. All of the enzymes involved in citrulline and arginine synthesis exist in the small intestine of fetal pigs (Dekaney et al. 2003). Arginase activity is nearly absent in fetal enterocytes, thereby maximizing the release of arginine from the gut (Wu and Morris 1998).
Arginine catabolism
Overall picture
Due to extensive catabolism of arginine by arginase in the small intestine (Dai et al. 2011; Wu 2010a, b), only 60 % of dietary arginine enters the portal circulation of pregnant gilts (Wu et al. 2007a, b). The quantitatively major products of intestinal arginine degradation include ornithine and proline, which are released into the portal circulation. Approximately 8 % of arginine in the portal vein is extracted by the liver where it is used for synthesis of protein and urea (Wu et al. 2007a, b). Thus, considering the true ileal digestibility (85 %) of arginine in a corn- and soybean meal-based diet, only 47 % of protein-bound arginine in the diet is available for utilization by extra-intestinal and extra-hepatic tissues in gestating swine (Fig. 1).
Digestion of dietary protein-bound arginine (Arg) in the gastrointestinal tract of nonruminant mammals, including humans and swine. Proteases and peptidases in the lumen of the small intestine hydrolyze proteins and large peptides, respectively, to eventually form Arg-containing tripeptides and dipeptides. A proportion of these dipeptides are hydrolyzed by peptidases to yield free Arg. The Arg-containing dipeptides or tripeptides in the lumen of the small intestine can be directly transported into the enterocytes through their apical membrane by H+ gradient-driven peptide transporter 1 (PepT1). This peptide transporter does not transport free Arg or peptides containing four or more AA residues. Arg-containing small peptides are rapidly hydrolyzed by intracellular peptidases to form free Arg in enterocytes and bacteria. A small proportion of Arg peptides may exit the enterocytes via their basolateral membrane into the blood stream, but the identity of basolateral peptide transporters remains unknown. Free Arg in the lumen of the small intestine is taken up by enterocytes primarily via the Na+-independent cationic amino acid transporters (CAT1, CAT2 and CAT3), as well as other transporters (y+, y+L, b0,+, and B0,+). In sheep, dietary protein is fermented in the rumen for synthesis of microbial protein, whose digestion in the abomasum and the small intestine is similar to that in nonruminants (Wu 2013a). Approximately 40 % of luminal Arg is catabolized by the small intestine, with the responsible cell types including enterocytes, other mucosal cells, and bacteria (Wu et al. 2009; Dai et al. 2012a, b). AA amino acids, NM nitrogenous metabolites, PA polyamines, PepT1 H+ gradient-driven peptide transporter 1, SI small intestine
Arginine transport
Transport of arginine is the first step in its utilization by cells since this nutrient cannot be taken up in a significant quantity from extracellular fluid by simple diffusion. Specific transporters are needed for transporting arginine across the cell membrane (Morris 2009). As a cationic amino acid, arginine shares the same transporters with lysine, ornithine, and histidine. System y+, which was the first transport system identified as a cationic amino acid transporter (CAT), is selective for cationic amino acids and is Na+-independent (Devés and Boyd 1998). Three different CAT genes (SLC7A1, SLC7A2, and SLC7A3) have been cloned, which encode for four homologous proteins CAT-1, CAT-2A, CAT-2B, and CAT-3, respectively (Gao et al. 2009b). In addition, systems bo,+ and Bo,+, which are expressed in mouse blastocysts (Van Winkle et al. 1985), can transport neutral amino acids as well as cationic amino acids (Van Winkle et al. 1988). These two systems are distinguished by their dependence on Na+ in that System Bo,+ is Na+-dependent, while system bo,+ is Na+-independent. The fourth transport system named y+L has high affinity for both neutral and cationic amino acids (Devés and Boyd 1998). Transport of cationic amino acids through this system is Na+-independent and its apparent affinity for neutral amino acids decreases dramatically when Na+ in the medium is replaced with K+. To avoid an imbalance among basic amino acids, the level of dietary arginine supplementation for mammals (e.g., pigs) should not be greater than 2.0 % on an as-fed basis (90 % dry matter; Go et al. 2012).
Multiple pathways for arginine utilization
There are multiple pathways in cells for arginine utilization. Arginine serves as the precursor for synthesis of many biological molecules, including ornithine, polyamines (putrescine, spermine and spermidine), proline, glutamine, creatine, agmatine, NO, and protein (Wu and Morris 1998; Wu et al. 2011a, b). The classic pathway of arginine catabolism is initiated by arginase to produce ornithine. Ornithine is subsequently converted to polyamines, proline, glutamate, and glutamine. Arginase exists as two distinct isoenzymes in mammals (Morris 2009). Type I arginase is a cytosolic enzyme mainly expressed in the liver. Type II arginase is expressed in mitochondria of extra-hepatic tissues including kidney, brain, small intestine, mammary gland and macrophages, as well as human and ovine placentae (Wu et al. 2009). All enzymes of the urea cycle are expressed in the small intestine of gestating swine (Wu 2010b). This may serve as a first line of defense against the toxicity of ammonia which is generated from amino acid metabolism in the small intestine (Wu 1995). Arginine-derived ornithine is an important precursor for synthesis of polyamines and proline in maternal and conceptus tissues (Fig. 2). Proline plays an important role in polyamine synthesis in both the porcine placenta and the fetal small intestine, which do not contain arginase I or II activity (Wu et al. 2008).
Synthesis of polyamines from arginine, proline, and methionine mammals, including humans, swine, and sheep. Arginine is hydrolyzed to ornithine plus urea by arginase I and arginase II in many cell types (possibly except for porcine placentae and neonatal small intestine). Synthesis of putrescine from ornithine is catalyzed by ornithine decarboxylase (a cytosolic enzyme) in all cell types. In placental mitochondria, arginine may be decarboxylated to form agmatine by arginine decarboxylase (ADC) and agmatine is then converted into putrescine by agmatinase. DCAM decarboxylated 5-adenosylmethionine, α-KG α-ketoglutarate, MTA methylthioadenosine, SAMD S-adenosyl-methionine decarboxylase, SAM S-adenosylmethionine
Arginine is the precursor of NO, which is catalyzed by NO synthase (NOS) (Bredt and Snyder 1994). There are three isoforms of NOS: neuronal NOS (nNOS; also known as NOS1), inducible NOS (iNOS; also known as NOS2), and endothelial NOS (eNOS; also known as NOS3). NOS1 and NOS3 are expressed constitutively in a cell-specific manner and produce low levels of NO (Gao et al. 2009c). However, NOS2 is induced by certain immunological stimuli (including endotoxin and inflammatory cytokines) to generate large amounts of NO which is a major endothelial cell-dependent relaxing factor. Nicotinamide adenine dinucleotide phosphate hydrogen (NADPH), calmodulin, flavin adenine dinucleotide, flavin mononucleotide, and tetrahydrobiopterin (BH4) are essential cofactors for enzymatic activities of all isoforms of NOS (Wu and Meininger 2009). In addition, NOS1 and NOS3, but not NOS2, require calcium for generation of NO. Nearly all cell types can recycle citrulline into arginine via argininosuccinate synthase and lyase, and this intracellular arginine–citrulline cycle helps sustain sufficient concentrations of arginine to support NO production (Wu et al. 2009). Elevating extracellular concentrations of arginine from 0.0 to 10 mM increases NO synthesis in cells and tissues, including endothelial cells, macrophages, and the placenta, in a dose-dependent manner (Wu and Meininger 2002). Arginine increases NOS activity and NO production in the porcine placenta by increasing BH4 synthesis and bioavailability (Wu and Meininger 2009).
Another pathway for arginine catabolism in animals is agmatine synthesis. In rats, arginine decarboxylase (ADC) decarboxylates arginine to produce agmatine in a cell-specific manner (Wu et al. 2009). Degradation of agmatine by agmatinase (a mitochondrial enzyme) yields putrescine. Catabolism of arginine via ADC has long been recognized in plants and bacteria, and this pathway was previously thought to be absent in animals until ADC was discovered in the rodent brain (Li et al. 1994). Expression of the ADC gene in mammalian cells was confirmed by cloning and characterization of human ADC (Zhu et al. 2004). ADC has 48 % amino acid sequence homology with ODC1, but has no ODC1 activity. ADC is also present in liver, kidney, adrenal gland, and macrophages (Morris 2009) but absent in porcine enterocytes (Wu et al. 1996b). At present, it is unknown whether ADC is expressed in the conceptuses of farm animals.
Arginine is required for creatine synthesis (Wu and Morris 1998). The guanidino group from arginine is transferred to the amino group of glycine by arginine:glycine amidinotransferase (AGAT), yielding guanidinoacetate. Guanidinoacetate is methylated to creatine by guanidinoacetate N-methyltransferase in the presence of S-adenosylmethionine (SAM, a metabolite of methionine; Wu et al. 2009). AGAT is the first rate-limiting enzyme for creatine synthesis. Although hepatocytes can readily convert guanidinoacetate to creatine, creatine cannot be produced directly from its precursors (arginine, glycine, or methionine) in the liver (da Silva et al. 2009). In rats and pigs, AGAT is predominantly expressed in the kidney, whereas high guanidinoacetate N-methyltransferase activity occurs in the liver (Wu and Morris 1998). This suggests inter-organ cooperation for creatine synthesis. Our results indicate that de novo synthesis of creatine is a major pathway for arginine utilization in gestating swine (Table 2).
Physiological functions of arginine and its metabolites in gestating mammals
Multiple catabolic fates and unique chemical features enable arginine to have versatile functions in cardiovascular, neurological, endocrine, and immunological systems (Wu et al. 2009). Moreover, physiological levels of arginine activate the MTOR cell signaling pathway, which plays crucial roles in protein synthesis, cell proliferation, and modulation of the intracellular cytoskeletal structure (Bazer et al. 2010; Kong et al. 2012; Lei et al. 2011). As an endothelium-derived relaxing factor, NO regulates blood flow across tissues (including the uterus and placenta) and, therefore, the transfer of nutrients from gestating sows to their fetuses (Reynolds et al. 2006). NO also stimulates placental angiogenesis and, thus, placental vascular growth. NO acts by binding to the heme group of soluble guanylate cyclase, thereby increasing generation of cyclic guanosine monophosphate (cGMP) from guanosine-5′-triphosphate (GTP). The cGMP activates cGMP-dependent protein kinases and the phosphorylation of target proteins that elicit a series of physiological responses (e.g., relaxation of vascular smooth muscle cells and mitochondrial biogenesis). In addition, NO inhibits the release of endothelin-1 (a vasoconstrictor) by endothelial cells and prevents leukocyte adhesion to the endothelium and platelet aggregation (Wu et al. 2009).
NOS gene knockout mice have proved to be an excellent model system to evaluate the functions of NO on reproductive process in females (Maul et al. 2003; Pallares et al. 2008). Results indicate that NO is essential for ovulation, embryonic development, and implantation (Jablonka-Shariff et al. 1999). There is no significant influence of NOS2 deficiency on length of the estrous cycle or ovulation rate. However, cycle length was increased and ovulation rate was markedly decreased in NOS3 knockout mice (Jablonka-Shariff et al. 1999). The number of implanted blastocysts was also lower in NOS3 knockout than wild-type mice (Pallares et al. 2008). Remodeling of the uterine vascular wall is essential for increasing uterine blood flow which is required for successful pregnancy outcomes (Pallares et al. 2008). Thus, knockout of the NOS3 gene decreased the remodeling capacity of the uterine arteries during pregnancy in mice (van der Heijden et al. 2005). This may be a major cause for the decline in fetal and neonatal survival in NOS3 knockout mice. Viable embryos at mid-gestation and litter size at term were reduced without changes in implantation rates or early development of implantation sites in NOS3 knockout mice (van der Heijden et al. 2005). This impairment was associated with reduced cellularity and abnormally thickened walls of arteries in the uterine decidual tissue in the absence of NOS3 gene expression. Results of all of these studies suggest that physiological levels of NO are essential for a successful outcome of pregnancy (Burnett et al. 2002).
Arginine may regulate the synthesis of carbon monoxide (CO) and hydrogen sulfide (H2S) from glycine and cysteine, respectively (Li et al. 2009). These gases have important biological functions in the cardiovascular system. NO stimulates H2S production in vascular tissues, whereas H2S inhibits the arginine–NO pathway in aorta and endothelial cells (Li et al. 2009). Additionally, endothelial NO has a permissive role in CO- and perhaps H2S-induced vascular dilation. Furthermore, arginine increases brown adipose tissue development in the fetus (Satterfield et al. 2012, 2013) as reported for postnatal rats (Wu et al. 2012c) via multiple cell signaling pathways. Thus, there may be cross-talk between various gaseous signaling pathways, and physiological levels of NO regulate vascular tone and hemodynamics in synergy with other gaseous vasoactive factors (Dai et al. 2013).
Polyamines are essential for a healthy pregnancy. ODC1 activity increases sharply between days 6 and 8 of gestation which is just after implantation (days 4–5 of gestation) in mice (Fozard et al. 1980). Similar changes occur for uterine levels of putrescine and spermidine. Compared with the control group, adding 2 % dl-α-difluoromethylornithine (α-DFMO, an irreversible inhibitor of ODC1) to the drinking water from days 5 to 8 of gestation prevented embryonic development and caused pregnancy loss by day 18. Polyamines are also essential for blastocyst implantation as implantation of blastocysts is inhibited by α-DFMO (Zhao et al. 2008). The benefit of polyamines in pregnancy may relate to its regulation of synthesis of steroid hormones as well as embryonic, placental and fetal growth and development. The activity of ovarian ODC1 increases immediately after ovulation and is required to enhance secretion of progesterone by the corpora lutea (Bastida et al. 2002). Ovarian growth and the formation of Graafian follicles were also inhibited by blocking ODC1 activity in immature female mice (Bastida et al. 2005). The decrease in concentrations of progesterone and estradiol at diestrus caused by α-DFMO treatment was associated with its inhibitory effects on expression of genes for steroidogenic factor 1, cytochrome cholesterol side chain cleavage enzyme, and steroidogenic acute regulatory protein in the ovary (Bastida et al. 2005). Furthermore, depletion of polyamines has been implicated in embryonic diapause, which is a poorly understood phenomenon of reversible arrest of blastocyst development prior to implantation (Lefèvre et al. 2011). These results indicate a crucial role for polyamines in embryogenesis and implantation of blastocysts.
Beneficial effects of dietary arginine supplementation on embryonic/fetal survival and growth in mammals
Studies involving pregnant swine
Studies involving pregnant swine were guided by the notion that the unusual abundance of arginine in porcine allantoic fluid during early pregnancy has important physiological functions in survival and growth of the conceptus (Wu et al. 1995, 1996a, 1998). Mateo et al. (2007) determined that dietary supplementation of arginine for gestating gilts increased litter size at birth. These authors found that dietary supplementation with 0.83 % arginine (as 1.0 % arginine–HCl; 16.6 g arginine/sow per day) between days 30 and 114 of gestation increased the number of live-born piglets by 2 per litter and litter birth weight by 24 % (Mateo et al. 2007). This novel finding indicates that arginine is a nutritionally essential amino acid to maximize reproductive performance in swine.
The rationale for supplementing 0.83 % arginine (as 1 % arginine–HCl) to the basal diet (12 % crude protein, 0.70 % arginine, and 0.57 % lysine; a digestibility value of 85 %) for pregnant gilts is as follows. First, because of a potential nutritional imbalance among basic amino acids, the total content of arginine in the diet should be <2 % (on an as-fed basis; 90 % dry matter) so that the ratio of digestible arginine to digestible lysine in the diet is <3.0. Second, because of the short half-life (1.7 h) of orally administered arginine in pregnant gilts due to (1) extensive catabolism of arginine (40 % of its dietary intake) by the small intestine and (2) rapid utilization of arginine by multiple pathways in tissues (Wu et al. 2007a, b), a sufficient dosage of supplemental arginine is required to sustain elevated levels of arginine (at least 25 % greater than plasma arginine concentrations in non-supplemented gilts) for 4 h. Third, arginine concentrations in the plasma of non-supplemented gilts are 240 μM at 1 h after feeding. On the basis of the pharmacokinetics of arginine in pregnant gilts, achieving a concentration of arginine in the plasma of supplemented gilts that is 25 % greater than the value in non-supplemented gilts at 4 h after feeding would require a peak value of ~500 μM at 1 h after feeding in supplemented gilts (Wu et al. 2007a). This would require a ~130 % increase in dietary content of digestible arginine, namely the supplementation of 0.78 % arginine to the basal diet. For convenience, we decided to supplement 1 % arginine–HCl (0.83 % arginine) to the diet for pregnant gilts.
Several lines of experimental evidence further support the conclusion that arginine supplementation is effective in enhancing embryonic/fetal survival in pigs (Table 3). First, under practical production conditions, there were increases in placental weight (+16 %), the number of live-born piglets per litter (+1.1), and litter birth weights for live-born piglets (+1.7 kg) for gilts and multiparous sows that received dietary supplementation with 0.83 % arginine between days 22 and 114 of gestation (Gao et al. 2012). Second, under practical production conditions, dietary supplementation with 1 % arginine to gilts and sows between days 14 and 28 of gestation increased the number of live-born piglets by approximately 1 at birth (Ramaekers et al. 2006; Campbell 2009). Third, compared with control gilts, dietary supplementation with 0.4 or 0.8 % l-arginine between days 14 and 25 of gestation increased placental growth by 21–34 % and the number of viable fetuses per litter by approximately 2 (Li 2011). Fourth, dietary supplementation with 1 % arginine between days 14 and 28 of gestation increased the number of fetuses per litter by 3.7 on day 70 of gestation in superovulated gilts (Bérard and Bee 2010). Fifth, supplementing 1 % arginine to the diet for gilts beginning on day 17 of gestation for 16 days increased the number of live-born piglets per litter by 1.2 (De Blasio et al. 2009). Finally, dietary supplementation with 0.83 % arginine between days 90 and 114 of gestation increased average birth weight of live-born piglets by 16 % (Wu et al. 2012b). An increase in the number of live-born pigs will markedly increase the profit margin associated with reproduction and lactation in dams. Additionally, a reduction in the number of low birth weight piglets will greatly improve the management of neonatal pigs and maximize pre-weaning survival and growth (Rezaei et al. 2013; Wu et al. 2011a, b).
The timing and dose of arginine supplementation are critical to its beneficial effects in improving pregnancy outcomes in swine (Wu et al. 2010). This is demonstrated by adverse effects of arginine supplementation immediately after mating on porcine embryonic survival and growth. Specifically, as compared with the control group of gilts, dietary supplementation with 0.8 % l-arginine between days 0 and 25 of gestation decreased uterine weight (−20 %), total number of fetuses (−24 %), number of corpora lutea (CL; −17 %), total fetal weight (−34 %), total volume of allantoic and amniotic fluids (−34 to 42 %), concentrations of progesterone in maternal plasma (−33 %), as well as total amounts of progesterone (−35 %), estrone (−40 %), and estrone sulfate (−37 %) in allantoic fluid (Li et al. 2010). In the ovary, follicular development and discharge of mature oocytes with the formation of CL depend on cell signaling via mitogen activated protein kinases 3 and 1 [also known as extracellular-regulated protein kinases 1 and 2 (ERK1/2)] (Duggavathi and Murphy 2009) and liver receptor homolog 1 (Lrh1) (Duggavathi et al. 2008). There is evidence that Lrh1 is essential for ovulation in mice through a mechanism involving expression of NOS3 (Johnson et al. 2009). Based on available data, we suggest that increased production of NO through arginine supplementation between days 0 and 14 of gestation may impair ERK1/2 signaling and Lrh1 function in the porcine ovary, thereby reducing the number of follicles that ovulate and, therefore, the number of CL and concentrations of progesterone in maternal plasma. Regression of CL rarely occurs in pregnant pigs under normal feeding conditions (e.g., 2 kg daily of a typical corn- and soybean meal-based diet) because CL are the main source for progesterone required for establishment and maintenance of pregnancy (Bazer et al. 2009a, b). Prostaglandin F2a (PGF2α) is a luteolytic agent for CL regression when it is secreted from the uterine epithelia into the uterine circulation (endocrine secretion) rather than into the uterine lumen (exocrine secretion) (Bazer and Thatcher 1977; Henderson and McNatty 1975). Thus, PGF2α production is known to be modulated by estrogen secreted by the conceptus (Bazer and Thatcher 1977). NO can also stimulate this biochemical event by up-regulating expression of cyclooxygenase II, a key enzyme for prostaglandin synthesis (Roberto da Costa et al. 2008; Salvemini et al. 1993). Thus, dietary l-arginine supplementation, which promotes systemic NO synthesis in animals (Wu et al. 2010), may lead to CL regression through a PGF2α-dependent pathway. Additionally, because ovulation in swine usually takes place at about 44 h after the onset of estrus (Bazer et al. 2012a, b), initiation of l-arginine supplementation within 24 h after onset of estrus may inhibit or interfere with follicular development and ovulation, thereby decreasing the number of CL in gilts.
Dietary arginine supplementation at dosages >1 % or 25 g arginine/day has been reported by Greiner et al. (2012) and Zier-Rush et al. (2012) to have little beneficial effect on reproductive performance of swine. For example, dietary supplementation with 28 g arginine/sow per day between days 18 and 34 or between days 75 and 115 did not increase litter size (Greiner et al. 2012; Zier-Rush et al. 2012) with either reducing (Greiner et al. 2012) or having no effect on (Zier-Rush et al. 2012) birth weights of piglets. Interpretation of these results is complicated by the lack of data on the content of protein, arginine, and other amino acids in the basal diet. The lack of beneficial effects of arginine on gestating swine in both studies is likely due to (1) an imbalance among amino acids and particularly, antagonism between arginine and lysine, in the supplemented diet; (2) increased intake of dietary nitrogen, resulting in increased production of ammonia that may reduce utero-placental blood flow, impair the Krebs cycle, and cause a metabolic burden to the mother and her conceptuses; and (3) missing of the early period of implantation of conceptuses when short-term arginine supplementation is initiated on day 18 after breeding. These results should not be considered as a lack of a beneficial role for arginine in swine reproduction. Rather, they underscore the importance of understanding basic knowledge about reproductive biology, as well as amino acid biochemistry and nutrition in designing balanced diets to benefit the pork industry worldwide.
Studies involving pregnant sheep
Arginine and its precursor (citrulline) are abundant in ovine uterine fluid (Gao et al. 2009a) and allantoic fluid (Kwon et al. 2003) during early to mid-gestation. Intravenous administration of arginine can improve embryonic survival in pregnant sheep (Luther et al. 2008). To test the hypothesis that increasing utero-placental blood flow enhances fetal growth in ewes, 0, 75, or 150 mg/day sildenafil citrate (Viagra) was administered from days 28 to 115 of gestation to underfed [50 % of National Research Council (NRC) requirements] or adequately fed ewes (100 % of NRC requirements) (Satterfield et al. 2010). On day 115, concentrations of amino acids and polyamines in maternal plasma and conceptuses were lower in underfed ewes than in adequately fed ewes. Viagra treatment increased: (1) amino acids and polyamines in amniotic and allantoic fluids and fetal serum; and (2) fetal weights in underfed ewes without affecting maternal body weights (Satterfield et al. 2010). Likewise, intravenous administration of arginine prevents IUGR in underfed ewes (Lassala et al. 2010). Specifically, beginning on day 28 of gestation, Suffolk ewes were fed a diet providing 100 or 50 % (underfed) of NRC nutrient requirements and, between day 60 of gestation and parturition, underfed ewes received intravenous infusions of saline or 155 μmol l-arginine per kg body weight three times daily, whereas control ewes received saline. The birth weights of lambs from saline-infused underfed ewes were 23 % lower than those of lambs from control-fed dams (Lassala et al. 2010). The administration of arginine to underfed ewes increased concentrations of arginine in maternal plasma (69 %), fetal BAT mass (48 %), and birth weights of lambs by 21 %, as compared to saline-infused underfed ewes (Satterfield et al. 2013). Similar results were observed for diet-induced obese ewes (Satterfield et al. 2012). Furthermore, intravenous administration of arginine (345 μmol arginine–HCl per kg BW three times daily) between days 100 and 121 of gestation reduced the percentage of lambs born dead by 23 %, increased the percentage of lambs born alive by 59 %, and enhanced birth weights of quadruplets by 23 %, without affecting maternal body weight (Lassala et al. 2011).
Studies involving pregnant rats and mice
Compared to the isonitrogenous control (2.2 % l-alanine), dietary supplementation with 1.3 % l-arginine–HCl either throughout pregnancy or during the first 7 days of pregnancy increases the numbers of implantation sites and litter size by approximately 3 per dam (Zeng et al. 2008, 2012). A series of experiments were conducted to elucidate the underlying mechanisms (Zeng et al. 2013). Specifically, rats were fed the basal diets supplemented with 1.3 % l-arginine–HCl or 2.2 % l-alanine (isonitrogenous control) on day 1 of pregnancy. On day 4 of pregnancy, rats received intrauterine administration of l-N G-nitroarginine methyl ester (an NOS inhibitor), wortmannin (a PI3K inhibitor), or rapamycin (a MTOR inhibitor). Arginine supplementation increased, but administration of l-N G-nitroarginine methyl ester decreased, the numbers of implantation sites and embryos. Treatment with rapamycin or wortmannin increased embryonic mortality, and dietary arginine supplementation could not reverse this adverse effect. Western blot analysis revealed that levels of uterine p-PKB and p-S6K1 proteins were greater in pregnant rats fed the arginine-supplemented diet, and that wortmannin or rapamycin decreased expression of uterine iNOS and eNOS. Collectively, these results suggest that the PI3K/PKB/mTOR/NO signaling pathway mediates the beneficial role for dietary arginine supplementation to enhance implantation of blastocysts in rats.
Recently, Ren et al. (2012) determined whether dietary supplementation with arginine could ameliorate reproductive failure in pregnant mice with viral infection. After female adult mice were fed for 14 days a standard rodent diet supplemented with either 0.6 % arginine or 1.22 % alanine (isonitrogenous control), they were mated with fertile male mice. On day 7 of gestation, mice were challenged with a dose of porcine circovirus type 2 that caused embryonic losses. Arginine supplementation decreased the high rate of abortion in porcine circovirus type 2-infected pregnant mice and the high rate of mortality of their Moreover, the postnatal weight gain of offspring were greater in the arginine group as compared to the control group. These exciting findings have important implications for the use of arginine to improve embryonic and fetal survival in virus-infected farm animals (e.g., pigs and sheep).
Studies involving pregnant women
Arginine is an essential nutrient for survival and growth of human embryos. Oral administration of l-arginine (16 g/day for 8 days) can improve uterine blood flow, ovarian response to gonadotrophin, endometrial receptivity, and pregnancy rate in women who responded very poorly to in vitro fertilization (Battaglia et al. 1999). Based on results of animal studies, clinical trials were conducted to evaluate effects of arginine supplementation on improving fertility and preventing IUGR in pregnant women. For example, during late (week 33) gestation, daily intravenous infusion of arginine (20 g/day) for 7 days to women with unknown causes of IUGR increased birth weight at term (week 39) by 6.4 % (Xiao and Li 2005). Moreover, results of a more recent study indicated that l-arginine administration to women with an IUGR fetus was effective in reducing placental apoptosis and improving fetal growth and development (Shen and Hua 2011). Specifically, after the diagnosis of fetal growth restriction, pregnant women received either a conventional treatment or the conventional treatment plus intravenous administration of l-arginine (15 g in 500 ml of 5 % glucose, once daily). (Information on the period or duration of arginine supplementation was not provided by the authors of the published results.) There were 30 subjects in each group. The prescribed conventional treatment included: left-lateral position, oxygen inhalation for 30 min two times daily, intravenous administration of magnesium sulfate, oral salbutamol sulfate (2.4 mg every 8 h), oral multi-vitamin B (3 × 1 tablets; i.e., three tablets/day), intravenous fat emulsion (250 ml/day), and intravenous infusion of 5 % glucose (500 ml). Compared with the control, arginine administration reduced the level of Bax (an apoptotic protein) in the placenta, increased average birth weight by approximately 53 g, and decreased the incidence of small-for-gestational-age newborns (25 vs 50 %) (Table 4). Finally, a recent meta-analysis of data collected from seven registered clinical trials involving 916 patients has shown that l-arginine supplementation can beneficially reduce diastolic pressure and prolong pregnancy in patients with gestational hypertension with or without proteinuria (Gui et al. 2013). Thus, arginine holds great promise to ameliorate preeclampsia in human pregnancy.
Conclusion and perspectives
Embryonic and fetal losses are substantial in both women and livestock species. However, effective solutions to this reproductive problem are limited. Failure of conceptus development and implantation during the peri-implantation period of pregnancy is regarded as the major cause for embryonic losses in mammals. Arginine is a physiologically versatile amino acid. It is a nitrogenous precursor for synthesis of ornithine, polyamines (putrescine, spermidine, and spermine), proline, glutamine, creatine, agmatine, and NO. In addition, arginine activates the MTOR cell signaling pathway, as well as enhances antioxidative defense, placental growth (including vascular growth), and utero-placental blood flow (Fig. 3). These biochemical events contribute to cell proliferation and migration, cellular remodeling, angiogenesis, dilation of blood vessels, and various cell signaling pathways (Wu 2009; Wu et al. 2011a, b), leading to improved embryonic/fetal survival and growth in mammals (Fig. 3).
Roles of arginine in improving embryonic and fetal survival and growth in mammals, including humans, swine, and sheep. Dietary supplementation with arginine enhances placental synthesis of NO and polyamines within physiological ranges and, therefore, placental development and utero-placental blood flow. This results in increased transfer of nutrients and oxygen from mother to conceptuses, thereby supporting their survival, growth and development. The ornithine used for polyamine synthesis is derived from proline catabolism via proline oxidase in porcine placental and other tissues as well as from arginine hydrolysis via arginase in a variety of porcine tissues, including the small intestine, liver and kidneys. BH4 tetrahydrobiopterin, Cit citrulline, MTOR mechanistic target of rapamycin, GTP-CH GTP cyclohydrolase-I, ODC ornithine decarboxylase, NO nitric oxide, NOS nitric oxide synthase
We have acquired a great deal of knowledge about important roles of arginine nutrition in gestating swine and sheep as animal models for human pregnancy. Such work is expected to lead to improved efficiencies in animal production systems. For example, in the past two decades, there has been only slow progress to enhance litter size in pigs using genetic and animal breeding approaches. However, dietary arginine supplementation provides an effective approach to solve this problem. Besides increasing litter size, an additional benefit of dietary arginine supplementation is to improve immune function in gestating sows (Li et al. 2007), thereby preventing infectious diseases as well as associated losses of conceptuses. Thus, the National Research Council recently classified arginine as a conditionally essential amino acid for gestating swine (NRC 2012).
Arginine is truly a functional amino acid for mammalian pregnancy (Wu 2013b; Wu et al. 2013). Thus, numerous well-designed studies have demonstrated beneficial effects of arginine supplementation on reproductive performance in pigs and sheep, as well as rats and mice. However, many issues remain to be addressed. For example, are the effects of arginine mediated directly by this nutrient or by its metabolites, such as NO, polyamines, creatine, or agmatine? Moreover, how does arginine regulate gene expression in the uterus and placenta to enhance nutrient transport and placental growth? What is the optimum window of opportunity for arginine supplementation to improve embryonic survival? What is the ideal pattern of amino acids in diets for gestating (including swine, sheep, and women) during early mid, and late pregnancy? Do different breeds of gestating pigs and sheep have different responses to arginine supplementation? Adequate answers to these questions are required to effectively improve embryonic survival and fetal growth through optimizing dietary arginine supplementation.
Caution should be taken when arginine is supplemented to humans and other animals during gestation. For example, we have reported that dietary supplementation with 0.83 % arginine between days 0 and 14 of pregnancy reduces litter size in gilts (Li et al. 2010). Likewise, there are reports that supplementing 1.2 % arginine to the diet for gilts and multiparous sows on either days 18–34 or days 75–115 of gestation had no effect on litter size and even reduced litter birth weight. These undesirable outcomes should not be considered as a lack of a beneficial role for arginine in survival and growth of the conceptus, particularly the fetus. Rather, the results underscore the importance of understanding basic knowledge about reproductive biology, as well as amino acid biochemistry and nutrition in designing balanced diets to enhance the efficiency of reproduction in mammals. We expect the findings from research with large farm animals (e.g., pigs and sheep) to provide a strong scientific basis for supplementing arginine to pregnant women to improve embryonic and fetal survival and development.
Abbreviations
- ADC:
-
Arginine decarboxylase
- AGAT:
-
Arginine:glycine amidinotransferase
- CAT:
-
Cationic amino acid transporter
- α-DFMO:
-
dl-α-Difluoromethylornithine
- GE:
-
Glandular epithelium
- IUGR:
-
Intrauterine growth restriction
- LE:
-
Luminal epithelium
- MTOR:
-
Mammalian target of rapamycin
- NO:
-
Nitric oxide
- NOS:
-
NO synthase
- ODC:
-
Ornithine decarboxylase
- P5C:
-
Pyrroline-5-carboxylate
- SAM:
-
S-adenosylmethionine
References
Bastida CM, Tejada F, Cremades A et al (2002) The preovulatory rise of ovarian ornithine decarboxylase is required for progesterone secretion by the corpus luteum. Biochem Biophys Res Commun 293:106–111
Bastida CM, Cremades A, Castells MT et al (2005) Influence of ovarian ornithine decarboxylase in folliculogenesis and luteinization. Endocrinology 146:666–674
Battaglia C, Salvatori M, Maxia N et al (1999) Adjuvant l-arginine treatment for in vitro fertilization in poor responder patients. Hum Reprod 14:1690–1697
Bazer FW, Thatcher WW (1977) Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2α by uterine endometrium. Prostaglandins 14:397–401
Bazer FW, Thatcher WW, Martinat-Botte F et al (1988) Conceptus development in Large White and prolific Chinese Meishan pigs. J Reprod Fertil 84:37–42
Bazer FW, Burghardt RC, Johnson GA et al (2008) Interferons and progesterone for establishment and maintenance of pregnancy: interactions among novel cell signaling pathways. Reprod Biol 8:179–211
Bazer FW, Spencer TE, Johnson GA et al (2009a) Comparative aspects of implantation. Reproduction 138:195–209
Bazer FW, Spencer TE, Johnson GA (2009b) Interferons and uterine receptivity. Sem Reprod Med 27:90–102
Bazer FW, Wu G, Spencer TE et al (2010) Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod 16:135–152
Bazer FW, Wu G, Johnson GA et al (2011) Uterine histotroph and conceptus development: select nutrients and secreted phosphoprotein 1 affect MTOR cell signaling in ewes. Biol Reprod 85:1094–1107
Bazer FW, Song GW, Kim JY et al (2012a) Uterine biology in sheep and pigs. J Anim Sci Biotech 3:23
Bazer FW, Song GH, Kim JY et al (2012b) Mechanistic mammalian target of rapamycin (MTOR) cell signaling: effects of select nutrients and secreted phosphoprotein 1 on development of mammalian conceptuses. Mol Cell Endocrinol 354:22–33
Bérard J, Bee G (2010) Effects of dietary l-arginine supplementation to gilts during early gestation on foetal survival, growth and myofiber formation. Animal 4:1680–1687
Bredt DS, Snyder SH (1994) Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem 63:175–195
Burnett TG, Tash JS, Hunt JS (2002) Investigation of the role of nitric oxide synthase 2 in pregnancy using mutant mice. Reproduction 124:49–57
Campbell R (2009) Pork CRC—NZ seminar series: arginine and reproduction. http://www/nzpib.co.nz
da Silva RP, Nissim I, Brosnan ME et al (2009) Creatine synthesis:hepatic metabolism of guanidino-acetate and creatine in the rat in vitro and in vivo. Am J Physiol Endocrinol Metab 296:E256–E261
Dai ZL, Wu G, Zhu WY (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16:1768–1786
Dai ZL, Li XL, Xi PB et al (2012a) Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 42:1597–1608
Dai ZL, Li XL, Xi PB et al (2012b) Regulatory role for l-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino Acids 43:233–244
Dai ZL, Wu ZL, Yang Y et al (2013) Nitric oxide and energy metabolism in mammals. BioFactors. doi:10.1002/biof.1099
De Blasio M, Roberts C, Owens J et al (2009) Effect of dietary arginine supplementation during gestation on litter size of gilts and sows. http://www.australianpork.com.au
Dekaney CM, Wu G, Jaeger LA (2003) Gene expression and activity of enzymes in the arginine biosynthetic pathway in porcine fetal small intestine. Pediatr Res 53:274–280
Devés R, Boyd CA (1998) Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev 78:487–545
Duggavathi R, Murphy BD (2009) Ovulation signals. Science 324:890–891
Duggavathi R, Volle DH, Mataki C et al (2008) Liver receptor homolog 1 is essential for ovulation. Genes Dev 22:1871–1876
Erb RE, Tillson SA, Hodgen GD et al (1970) Urinary creatinine as an index compound for estimating rate of excretion of steroids in the domestic sow. J Anim Sci 30:79–85
Fazleabas AT (2007) Physiology and pathology of implantation in the human and nonhuman primate. Semin Reprod Med 25:405–409
Fozard JR, Part ML, Prakash NJ et al (1980) l-ornithine decarboxylase: an essential role in early mammalian embryogenesis. Science 208:505–508
Gao HJ, Wu G, Spencer TE et al (2009a) Select nutrients in the ovine uterine lumen: I. Amino acids, glucose and ions in uterine lumenal flushings of cyclic and pregnant ewes. Biol Reprod 80:86–93
Gao HJ, Wu G, Spencer TE et al (2009b) Select nutrients in the ovine uterine lumen: III. Expression of cationic amino acid transporters in ovine uterus and peri-implantation conceptuses. Biol Reprod 80:602–609
Gao HJ, Wu G, Spencer TE et al (2009c) Select nutrients in the ovine uterine lumen: V. Nitric oxide synthase, GTP cyclohydrolase and ornithine decarboxylase in ovine uteri and peri-implantation conceptuses. Biol Reprod 81:67–76
Gao KG, Jiang ZY, Lin YC et al (2012) Dietary l-arginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids 42:2207–2214
Geisert RD, Yelich JV (1997) Regulation of conceptus development and attachment in pigs. J Reprod Fertil Suppl 52:133–149
Geisert RD, Brookbank JW, Roberts RM et al (1982) Establishment of pregnancy in the pig: II. Cellular remodeling of the porcine blastocyst during elongation on day 12 of pregnancy. Biol Reprod 27:941–955
Go GW, Wu G, Silvey DT et al (2012) Lipid metabolism in pigs fed supplemental conjugated linoleic acid and/or dietary arginine. Amino Acids 43:1713–1726
Greiner L, Usry JL, Neill C et al (2012) The evaluation of supplemental l-arginine during gestation on sow reproductive performance. J Anim Sci 90(Suppl 2):33–34 (abstract)
Gui S, Jia J, Niu X et al (2013) Arginine supplementation for improving maternal and neonatal outcomes in hypertensive disorder of pregnancy: a systematic review. J Renin Angiotensin Aldosterone Syst (Epub ahead of print)
Henderson KM, McNatty KP (1975) A biochemical hypothesis to explain the mechanism of luteal regression. Prostaglandins 9:779–797
Jablonka-Shariff A, Ravi S, Beltsos AN et al (1999) Abnormal estrous cyclicity after disruption of endothelial and inducible nitric oxide synthase in mice. Biol Reprod 61:171–177
Johnson R (2000) History of litter size selection. www.nsif.com/conferences/2000/johnson.html
Johnson GA, Bazer FW, Burghardt RC et al (2009) Conceptus-uterus interactions in pigs: endometrial gene expression in response to estrogens and interferons from conceptus. Soc Reprod Fertil Suppl 66:321–332
Kim SW, Hurley WL, Wu G et al (2009) Ideal amino acid balance for sows during gestation and lactation. J Anim Sci 87:E123–E132
Kim JY, Song GH, Wu G et al (2013) Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6 K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod. doi:10.1095/biolreprod.112.105080
Kong XF, Tan BE, Yin YL et al (2012) l-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem 23:1178–1183
Kwon H, Spencer TE, Bazer FW et al (2003) Developmental changes of amino acids in ovine fetal fluids. Biol Reprod 68:1813–1820
Lassala A, Bazer FW, Cudd TA et al (2010) Parenteral administration of l-arginine prevents fetal growth restriction in undernourished ewes. J Nutr 140:1242–1248
Lassala A, Bazer FW, Cudd TA et al (2011) Parenteral administration of l-arginine enhances fetal survival and growth in sheep carrying multiple pregnancies. J Nutr 141:849–855
Lefèvre PL, Palin MF, Chen G et al (2011) Polyamines are implicated in the emergence of the embryo from obligate diapause. Endocrinology 152:1627–1639
Lei XQ, Feng CP, Liu C et al (2011) Regulation of protein expression by l-arginine in endothelial cells. Front Biosci S3:655–661
Li XL (2011) Regulation of porcine conceptus survival and growth by l-arginine. PhD dissertation, Texas A&M University, College Station
Li G, Regunathan S, Barrow CJ et al (1994) Agmatine: an endogenous clonidine-displacing substance in the brain. Science 263:966–969
Li P, Yin YL, Li D et al (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li XL, Bazer FW, Gao H et al (2009) Amino acids and gaseous signaling. Amino Acids 37:65–78
Li X, Bazer FW, Johnson GA et al (2010) Dietary supplementation with 0.8% l-arginine between days 0 and 25 of gestation reduces litter size in gilts. J Nutr 140:1111–1116
Li XL, Rezaei R, Li P et al (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Luther JS, Windorski EJ, Schauer CS et al (2008) Impacts of l-arginine on ovarian function and reproductive performance in ewes. J Anim Sci 86(E-Suppl 2):ii
Mateo RD, Wu G, Bazer FW et al (2007) Dietary l-arginine supplementation enhances the reproductive performance of gilts. J Nutr 137:652–656
Maul H, Longo M, Saade GR et al (2003) Nitric oxide and its role during pregnancy: from ovulation to delivery. Curr Pharm Des 9:359–380
Morris SM Jr (2009) Recent advances in arginine metabolism: roles and regulation of the arginases. Br J Pharmacol 157:922–930
National Research Council (NRC) (2012) Nutrient requirements of swine. National Academy Press, Washington, DC
Pallares P, Garcia-Fernandez RA, Criado LM et al (2008) Disruption of the endothelial nitric oxide synthase gene affects ovulation, fertilization and early embryo survival in a knockout mouse model. Reproduction 136:573–579
Pope WF (1994) Embryonic mortality in swine. In: Zavy M, Geisert R (eds) Embryonic mortality in domestic species. CRC Press, Boca Raton, pp 53–77
Ramaekers P, Kemp B, van der Lende T (2006) Progenos in sows increases number of piglets born. J Anim Sci 84(Suppl 1):394 (abstract)
Ren W, Yin YL, Liu G et al (2012) Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42:2089–2094
Reynolds LP, Caton JS, Redmer DA et al (2006) Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol (Lond) 572:51–58
Rezaei R, Wang WW, Wu ZL et al (2013) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotech 4:7
Roberto da Costa RP, Costa AS, Korzekwa AJ et al (2008) Actions of a nitric oxide donor on prostaglandin production and angiogenic activity in the equine endometrium. Reprod Fertil Dev 20:674–683
Rothschild MF (1996) Genetics and reproduction in the pig. Anim Reprod Sci 42:143–151
Salvemini D, Misko TP, Masferrer JL et al (1993) Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA 90:7240–7244
Satterfield MC, Bazer FW, Spencer TE et al (2010) Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J Nutr 140:251–258
Satterfield MC, Dunlap KA, Keisler DH et al (2012) Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids 43:1593–1603
Satterfield MC, Dunlap KA, Keisler DH et al (2013) Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino Acids. doi:10.1007/s00726-011-1168-8
Shen SF, Hua CH (2011) Effect of l-arginine on the expression of Bcl-2 and Bax in the placenta of fetal growth restriction. J Matern Fetal Neonatal Med 24:822–826
Spencer TE, Bazer FW (2004) Conceptus signals for establishment and maintenance of pregnancy. Reprod Biol Endocrinol 2:49
Town SC, Patterson JL, Pereira CZ et al (2005) Embryonic and fetal development in a commercial dam-line genotype. Anim Reprod Sci 85:301–316
USDA (2009) Changes in the U.S. swine industry. National Agricultural Statistics Service home page. http://www.usda.gov/nass
van der Heijden OW, Essers YP, Fazzi G et al (2005) Uterine artery remodeling and reproductive performance are impaired in endothelial nitric oxide synthase-deficient mice. Biol Reprod 72:1161–1168
Van Winkle LJ, Christensen HN, Campione AL (1985) Na+ dependent transport of basic, zwitterionic, and bicyclic amino acids by a broad-scope system in mouse blastocysts. J Biol Chem 260:12118–12123
Van Winkle LJ, Campione AL, Gorman JM (1988) Na+ independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J Biol Chem 263:3150–3163
Wang JJ, Wu ZL, Li DF et al (2012) Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal 17:282–301
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wu G (1995) Urea synthesis in enterocytes of developing pigs. Biochem J 312:717–723
Wu G (1997) Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol Gastrointest Liver Physiol 272:G1382–G1390
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010a) Functional amino acids in growth, reproduction and health. Adv Nutr 1:31–37
Wu G (2010b) Recent advances in swine amino acid nutrition. J Anim Sci Biotech 1:49–61
Wu G (2013a) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton 503
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids. doi:10.1007/s00726-013-1500-6
Wu G, Meininger CJ (2002) Regulation of nitric oxide synthesis by dietary factors. Annu Rev Nutr 22:61–86
Wu G, Meininger CJ (2009) Nitric oxide and vascular insulin resistance. BioFactors 35:21–27
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Knabe DA, Flynn NE (1994) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299:115–121
Wu G, Bazer FW, Tuo W (1995) Developmental changes of free amino acid concentrations in fetal fluids of pigs. J Nutr 125:2859–2868
Wu G, Bazer FW, Tuo W et al (1996a) Unusual abundance of arginine and ornithine in porcine allantoic fluid. Biol Reprod 54:1261–1265
Wu G, Knabe DA, Flynn NE et al (1996b) Arginine degradation in developing porcine enterocytes. Am J Physiol Gastrointest Liver Physiol 271:G913–G919
Wu G, Pond WG, Ott TL et al (1998) Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. J Nutr 128:894–902
Wu G, Ott TL, Knabe DA et al (1999) Amino acid composition of the fetal pig. J Nutr 129:1031–1038
Wu G, Knabe DA, Kim SW (2004a) Arginine nutrition in neonatal pigs. J Nutr 134:2390–2783
Wu G, Bazer FW, Cudd TA et al (2004b) Maternal nutrition and fetal development. J Nutr 134:2169S–2172S
Wu G, Bazer FW, Wallace JM et al (2006) Intrauterine growth retardation: implications for the animal sciences. J Anim Sci 84:2316–2337
Wu G, Bazer FW, Cudd TA et al (2007a) Pharmacokinetics and safety of arginine supplementation in animals. J Nutr 137:1673S–1680S
Wu G, Bazer FW, Davis TA et al (2007b) Important roles for the arginine family of amino acids in swine nutrition and production. Livest Sci 112:8–22
Wu G, Bazer FW, Datta S et al (2008) Proline metabolism in the conceptus: implications for fetal growth and development. Amino Acids 35:691–702
Wu G, Bazer FW, Davis TA et al (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu G, Bazer FW, Burghardt RC et al (2010) Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci 88:E195–E204
Wu G, Bazer FW, Johnson GA et al (2011a) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Bazer FW, Burghardt RC et al (2011b) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu G, Imhoff-Kunsch B, Girard AW (2012a) Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinatal Epidemiol 26(Suppl 1):4–26
Wu X, Yin YL, Liu YQ et al (2012b) Effect of dietary arginine and N-carbamoylglutamate supplementation on reproduction and gene expression of eNOS, VEGFA and PlGF1 in placenta in late pregnancy of sows. Anim Reprod Sci 132:187–192
Wu ZL, Satterfield MC, Bazer FW et al (2012c) Regulation of brown adipose tissue development and white fat reduction by l-arginine. Curr Opin Clin Nutr Metab Care 15:529–538
Wu G, Wu ZL, Dai ZL et al (2013) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Xiao XM, Li LP (2005) l-arginine treatment for asymmetric fetal growth restriction. Int J Gynecol Obstet 88:15–18
Zeng XF, Wang FL, Fan X et al (2008) Dietary arginine supplementation during early pregnancy enhances embryonic survival in rats. J Nutr 138:1421–1425
Zeng XF, Mao XB, Huang ZM et al (2012) Arginine enhances embryo implantation in rats through PI3 K/Akt/mTOR/NO signaling pathway during early pregnancy. PLoS ONE 7(7):e41192
Zeng XF, Mao XB, Huang ZM et al (2013) Arginine enhances embryo implantation in rats through PI3 K/PKB/mTOR/NO signaling pathway during early pregnancy. Reproduction 145:1–7
Zhao YC, Chi YJ, Yu YS et al (2008) Polyamines are essential in embryo implantation: expression and function of polyamine-related genes in mouse uterus during peri-implantation period. Endocrinology 149:2325–2332
Zhu M, Iyo A, Piletz J et al (2004) Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochim Biophys Acta 1670:156–164
Zier-Rush CE, Kuntzman A, Schmidt T et al (2012) Arginine supplement in early and late pregnant sows did not improve litter size or birth weight. J Anim Sci 90(Suppl 2):34 (abstract)
Acknowledgments
Work in our laboratories was supported by National Research Initiative Competitive Grants from the Animal Reproduction Program (2008-35203-19120, 2009-35206-05211 and 2011-67015-20028) and Animal Growth & Nutrient Utilization Program (2008-35206-18764) of the USDA National Institute of Food and Agriculture, AHA (10GRNT4480020), Texas A&M AgriLife Research (H-8200), the National Natural Science Foundation of China (u0731001, 30810103902, 31172217, 31272449, and 31272450), China Postdoctoral Science Foundation (2012T50163), Chinese Universities Scientific Funds (2012RC024), and the Thousand-People Talent program at China Agricultural University. Important contributions of our graduate students and colleagues to the recent development of this field of research are gratefully appreciated.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, G., Bazer, F.W., Satterfield, M.C. et al. Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45, 241–256 (2013). https://doi.org/10.1007/s00726-013-1515-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1515-z