Abstract
Background
The use of intraoperative ultrasound (iUS) has increased in the last 15 years becoming a standard tool in many neurosurgical centers. Our aim was to assess the utility of routine use of iUS during various types of intracranial surgery. We reviewed our series to assess ultrasound visibility of different pathologies and iUS applications during the course of surgery.
Materials and methods
This is a retrospective review of 162 patients who underwent intracranial surgery with assistance of the iUS guidance system (SonoWand). Pathologic categories were neoplastic (135), vascular (20), infectious (2), and CSF related (5). Ultrasound visibility was assessed using the Mair classification, a four-tiered grading system that considers the echogenicity of the lesion and its border visibility (from 0 to 3; grade 0, pathology not visible; grade 3, visible with clear border with normal tissue). iUS applications included lesion localization, approach planning to deep-seated lesions, and lesion removal.
Results
All pathologies were visible on iUS except one aneurysm. On average, extra-axial tumors were identified more easily and had clearer limits compared to intra-axial tumors (extra-axial 17% grade 2, 83% grade 3; intra-axial 5.5% grade 1, 46.5% grade 2, 48% grade 3). iUS provided precise and safe transcortical trajectories to deep-seated lesions (71 patients; tumors, hemangiomas, ICHs); iUS was judged to be less useful to approach skull base tumors and aneurysms. iUS was used to judge extent of resection in 152 cases; surgical artifacts reduced sonographic visibility in 25 cases: extent of resection was correctly checked in 127 patients (53 gliomas, 15 metastases, 39 meningiomas, 4 schwannomas, 4 sellar region tumors, 6 hemangiomas, 3 AVMs, 2 abscesses).
Conclusions
iUS was highly sensitive in detecting all types of pathology, was safe and precise in planning trajectories to intraparenchymal lesions (including minimally mini-invasive approaches), and was accurate in checking extent of resection in more than 80% of cases. iUS is a versatile and feasible tool; it could improve safety and its use may be considered in routine intracranial surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few decades, there have been a number of technological innovations during intracranial surgery. In the 1970s, the surgical microscope revolutionized intracranial surgery. At first, it was reserved for a few selected centers and for the most complex cases: at present, the operating microscope is a neurosurgical standard. In the 1990s, neuronavigation systems were introduced and progressively accepted and now have become one of the standard tools for surgical planning and intraoperative guidance in both intracranial and spinal surgery. There are, however, some limitations including deliquoration, spatulas, and progressive tumor debulking that make navigation based on preoperative images inaccurate. Therefore, the need for intraoperative imaging has become more and more important and includes modalities such as intraoperative magnetic resonance (iMRI), computed tomography (iCT), and ultrasound (iUS). The major limitation of iMRI and iCT is that the images are not in real time. In addition, they require long acquisition times, special surgical equipment, and special workspaces, and the technology requires huge economic investments. iUS imaging initially had little acceptance among neurosurgeons, likely due to inexperience with images interpretation. Nevertheless, its use has increased in the last decade likely due to the introduction of three-dimensional (3D) US and integration with navigation systems. Traditionally, the main use of iUS is evaluation of extent of tumor resection, especially during resection of intra-axial tumors. With progressive experience, however, iUS has been used during the treatment of other pathologies (abscesses, hematomas, hemangiomas…) and other purposes, such as positioning of external ventricular devices (EVD), evaluation of adjacent vessels using the power Doppler function, and US-guided biopsies. Herein, we present our experience using navigated iUS imaging (SonoWand) during 162 intracranial surgeries. Our aim was to assess the utility of routine use of iUS during various types of intracranial surgery. We reviewed our series to assess ultrasound visibility of different pathologies and iUS applications during the course of surgery.
Materials and methods
Between June 2014 and December 2016, 162 patients underwent intracranial surgery in our department with intraoperative 3D ultrasound (3D-iUS)-based image guidance system (SonoWand). We have retrospectively reviewed patients and operative data to evaluate the potential benefits of navigated 3D-iUS. All procedures were performed by two experienced neurosurgeons (DP and RB) both well-trained in intraoperative ultrasound. We included neoplastic, vascular, and other (hydrocephalus) pathologies in the study (Table 1). We grouped with the term intraparenchymal pathologies all lesions (101 patients) that required a transcortical/transcerebral approach; 31 lesions were cortical and 70 lesions were subcortical. Ventricular catheter placement procedures were considered separately.
The SonoWand system can be used as either a stand-alone neuronavigation system using preoperative images (CT or MRI) or as a stand-alone ultrasound machine providing real-time intraoperative 2D images as well as a navigable 3D ultrasound (which allows navigation based solely on the iUS with no need of preoperative images). The system is also equipped with a combined mode which uses both preoperative images and iUS. The system has been previously described [6]. The navigable iUS function was used in a combined manner with preoperative images in 139 cases and as a stand-alone US (either 2D or navigable 3D US) in the remaining 23 cases. For navigation cases, a volumetric CT head with contrast or MRI with gadolinium with standard skin-adhering fiducials placed on the scalp was obtained on the day prior to surgery. Images were transferred to the system using a USB device. After anesthesia and patient positioning, the patient-to-image registration was performed. Patient positioning was optimized to ensure that the operative cavity could be filled completely with saline in order to optimize US image quality. The skin and bone flaps were planned with the neuronavigator (when preoperative images were available). The first US scan was performed after the craniotomy with the dura mater still intact, when brain-shift is virtually absent compared to preoperative images, in order to localize the lesion and to define its margins, dimensions, morphology, and echogenic features. Furthermore, anatomical landmarks such as falx, tentorium, ventricles, skull base, and major vessels are identified. The system automatically superimposes the live iUS images on the corresponding preoperative CT/MRI (when available) thus allowing anatomical orientation and comparison of US details with the corresponding CT or MRI [10]. Once the baseline acquisition is obtained and the surgical procedure advances, it is possible to repeat other US scans (either 2D or 3D navigable) whenever it is necessary to update the information as brain-shift occurs and neuronavigation accuracy is lost. Two kinds of US probes were used: a phased-array probe and a linear-array probe. The phased-array (4–8 MHz) produces a conic beam of ultrasound and is suitable for deep-seated lesions. For lesions close to the surface (cortical and subcortical pathologies), a flat linear-array probe (12 MHz) is more suitable. The probe generates a rectangular image. The 3D-US system allows simultaneous acquisition of both tissue signals and power Doppler angiography. US angiography was assessed to visualize vessels engulfed by the tumor or in the neighboring tissue. Major branches from feeding vessels were also visualized in tumors. Intraoperative neurophysiological monitoring was used in 5 cerebello-pontine angle lesions, 2 fourth ventricle tumors, and 14 supratentorial lesions adjacent to motor eloquent structures (primary motor cortex and corticospinal tract). All patients underwent an immediate postoperative CT scan (in order to exclude the presence of a surgical complication).
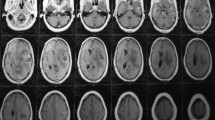
US data was reviewed for visibility of the pathology and additional applications. In order to describe the ultrasound visibility of different kinds of pathologies, we used the classification proposed by Mair et al. in 2013 [8], a grading system for US visibility of typical intracranial lesions and its discrimination at the border. Grade 0 describes lesions that are not visible. Grade 1 describes tumors difficult to visualize without an exact border with normal brain tissue. Grade 2 is a clearly identifiable lesion lacking a clear border with normal brain tissue and grade 3 is a lesion clearly identifiable with a clear border with normal tissue (Fig. 1).
US applications during surgical procedure were the following:
-
Lesion localization (visualization of the pathology and understanding position, limits, and adjacent structures)
-
Planning the approach to deep-seated lesions (subcortical and skull base)
-
Lesion removal guidance
-
Final US evaluation of the surgical field
Initial visibility and utility assessment were performed at the time of surgery by the operating neurosurgeon; all intraoperative US images were stored and independently reviewed postoperatively by the other experienced surgeon. The two evaluations were then matched and the final visibility grade and utility assessment for each lesion and procedure were established.
Results
Echographic visibility
Table 1 summarizes the Mair grade of the treated pathologies. In cases of an EVD placement procedure, we assessed the ability to visualize the ventricles, which resulted grade 3 in all cases. iUS was used in 135 cases of intracranial neoplasms. All tumors were identifiable with US imaging. In 130 cases (97%), the lesion was easily and clearly visualized (Mair grades 2–3), and in just 5 cases (3%), it was difficult to identify the lesion or differentiate it from the surrounding cerebral tissue (Mair grade 1). On average, extra-axial tumors such as meningiomas, schwannomas, and craniopharyngiomas were identified more easily and had clearer limits compared to intra-axial tumors. Comparing gliomas and metastatic lesions, the latter was more discernable. Regarding gliomas, high-grade lesions (WHO III-IV) appeared to be more neatly visible than low-grade lesions: respectively 51 and 33% Mair grade 3. In high-grade gliomas, the cystic elements and the enhancing nodules are easily distinguished from the cerebral tissue. The only cases in which Mair grade was 1 were a cerebral lymphoma, a pleomorphic xanthoastrocytoma, and two multicentric glioblastomas. All four patients showed a diffuse signal alteration on preoperative MRI in both T2-weighed and FLAIR images, with a large diffusion in the respective cerebral lobes interpreted as wide disease infiltration. Fourteen cases of vascular pathologies were treated; echographic visibility was assessed: four out of five aneurysms were clearly identified with Doppler angiography (considered Mair grade 3) and one ICAOpht aneurysm was not visible probably due to proximity to the anterior clinoid process (Mair grade 0). The AVMs were visualized with Doppler angiography (Mair grade 3). Of the six deep-seated cavernous hemangiomas (4 supratentorial and 2 cerebellar) visualized with iUS, all were classified Mair grade 3; in one case with an associated hematoma, the hemangioma remained discernible even after evacuation of the hematoma despite the brain-shift. Intraparenchymal hemorrhages (6 cases) demonstrated an average low US visibility (one grade 1, four grade 2, and one grade 3) even though sufficient for localization and approach planning. Brain abscesses (2 cases) had high US visibility (Mair grade 3). Overall, regardless of histology, perilesional edema was associated with worse ability to distinguish the lesions’ limits (Fig. 2).
CECT head scan of a frontal metastasis and corresponding iUS. Posterior margin of the lesion is clearly visible on CT and iUS with a clear border with surrounding normal brain tissue (white arrow). At the medial margin, the lesion is clear on CT scan but is lacking a clear border with normal brain tissue on iUS (black arrow) due to surrounding edema (Mair grade 2)
Ultrasound applications
Table 2 summarizes ultrasound applications for each kind of pathology.
Lesion localization
The system’s ability to localize the lesion was directly proportional to the US visibility grade. All pathologies were localized except for one aneurysm.
Planning the approach to deep-seated pathologies
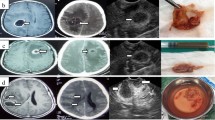
The iUS utility in planning the trajectory to deep-seated lesions did not always correlate with the assigned Mair grade. Deep-seated extra-axial pathologies, mainly skull base tumors, although clearly visualized with iUS, were nonetheless approached through the classical surgical cisternal pathways, without the need of iUS guidance. In our experience, only one such case, a subtemporal approach to a middle temporal base meningioma, was the iUS useful during the approach. Similarly, the use of US did not impact dissection and clipping strategies during aneurysm clipping. In these patients, iUS was helpful for the placement of ventricular catheters in one case and hematoma evacuation in another one. All deep-seated intraparenchymal pathologies were approached with iUS guidance (70 patients; brain tumors, cavernous hemangiomas, hematomas, brain abscesses) (see Table 2). In these cases, iUS was used to plan a correct and safe trajectory to the lesion, avoiding major vessels (Fig. 3) and eloquent cortex (integration of iUS and neurophysiological monitoring). The transcortical approach was planned using 3D-navigated iUS in 65 patients and in five cases, a 2DiUS real-time control was used. Eight patients underwent biopsy of an intra-axial neoplasm (due to multiplicity). The target was localized with iUS and a minimally invasive approach was performed with minimal brain transgression. All the specimens were histologically positive (absence of false positive on iUS). iUS-guided EVD placement was performed in eight patients, five during shunt procedures (4 slit-ventricles and 1 malformative hydrocephalus) and three during surgery for aneurysm, hematoma, and brain abscess. Catheter placement with 2DUS real-time guidance was performed in six patients and 3DUS navigation in the remaining two patients. Correct placement was achieved in all patients.
Left panel shows axial, coronal, and sagittal view on CECT head scan of an insular glioma (Mair grade 2). Right panel shows the corresponding 3DiUS images superimposed to axial, coronal, and sagittal plans on CECT scan. The green line represents the trajectory to the target planned to avoid the vessels. Note the considerable brain-shift
Lesion removal guidance
Extent of lesion removal was assessed with iUS in 152 procedures of neoplastic and non-neoplastic pathologies (except aneurysms and EVD placement) by acquiring repetitive iUS scan to compensate brain-shift as surgery proceeded (Fig. 4). As surgery proceeds, we have frequently observed a worsening of the iUS image quality due to surgical artifacts. Lesion removal was accurately checked in 127 patients out of 152. The phenomenon of echographic visibility reduction was not observed in cavernous hemangiomas, brain abscesses, AVMs, and extra-axial tumors (meningiomas, sellar region tumors, schwannomas); these pathologies remained easily visible throughout the operation with minimal or no decrease in iUS visibility. In these patients (48 tumors, 6 hemangiomas, 2 brain abscesses, 3 AVMs), quantification of extent of resection was possible as well as quantification of the potential residual (10 subtotal resection over 48 tumors).
Frontal glioblastoma (Mair grade 3). a 3DiUS acquisition prior to resection: axial, coronal, and sagittal plan superimposed to the corresponding preoperative CT head scan. b–d Progressive tumor debulking with contemporary display of tissue and power Doppler signal (note the position of the anterior cerebral artery with respect to the cavity wall)
Image quality was worse in the cases of intra-axial tumors (including metastasis); iUS tumor removal guidance was still considered useful, however, because iUS precision is better in excision quantification than the microscopic vision alone. Among 87 cases of intra-axial tumors, US removal control was possible in 68 patients. Forty underwent incomplete resection: 32 subtotal resection (STR) and 8 biopsies. Among 31 patients (25 STR, 6 biopsy), the residual lesion was identified and assessed, and the decision to not proceed further with the procedure was based either on anatomical criteria or the neurophysiological monitoring. In the remaining nine patients, iUS was not clear because of surgical artifacts (5 glioblastoma, 3 low-grade astrocytoma, 1 lymphoma). Forty-seven patients underwent gross total removal (GTR): HGG (29), LGG (5), metastasis (13). In 37 cases (21 HGG, 3 LGG, 13 metastasis), iUS visibility remained clear during the procedure and the system was considered helpful in tumor resection. In the remaining 10 cases, the images were not good enough and the excision was completed relying on microscopic vision and anatomical landmarks.
In our experience, a decrease in echographic visibility was encountered during intraparenchymal hematomas evacuation. The iUS was used to plan the transcortical trajectory, but in all six patients, we could not evaluate the progression of evacuation, with an average of one grade-lowering on the Mair classification. Power Doppler angiography was used to localize major vessels encased or adjacent to the lesion and major feeding arteries (tumors, AVMs). Displaying simultaneously iUS tissue signal and power Doppler allowed us to localize and spare vascular structures during both the approach and the resection (Fig. 5). Repeated intraoperative Doppler acquisitions were also used to check progressive deafferentation of AVMs and hypervascularized tumors. On the opposite of the tissue signal, we did not observe any decrease of the Doppler images’ quality throughout the procedure.
Final iUS evaluation of the surgical field
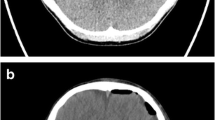
The final iUS, performed at the end of the procedure in all patients, demonstrated two cases of hemorrhage in the surgical field, one of which was recognized and evacuated. The other case was misinterpreted for a residual of an insular glioblastoma and left in situ only to be identified later on the postoperative CT scan.
Discussion
Echographic visibility
Echographic visibility of brain lesions is difficult to be objectively quantified. There is a well-known learning curve of interpreting and recording iUS images and there can be various artifacts in surgical cavities that contribute to a deterioration of ultrasound image quality and lesion delineation during resection [12]. We used an echographic visibility grading system proposed by Mair et al. in 2013 [8]. It is a four-tiered system that takes into account the echogenicity of the lesion and its border visibility (Fig. 1). Although it is an unvalidated scale, it is almost identical to others [14, 16]. Similar to what other authors have described [8, 11, 17, 21, 22], all lesions, except one aneurysm, were identifiable by iUS. Most of the lesions (primary and metastatic tumors, cavernous hemangiomas, abscesses) appear hyperechoic compared to the surrounding normal brain parenchyma. Edema also appears slightly hyperechoic. Hemorrhage has different echogenic aspects, depending on the phase of the bleeding: in the acute phase, for example, it appears hyperechoic. Extra-axial and metastatic tumors were on average identified more easily and had clearer limits compared to intra-axial tumors. Among the latter, diffusely infiltrating lesions showed the lowest grade of visibility with poor limits of definition (Table 1). Depiction of tumor borders was dependent on intrinsic factors (nature of the lesion) and extrinsic factors (surrounding parenchyma). Considering intrinsic aspects, diffuse infiltrative lesions (such as infiltrative gliomas and lymphomas) do not have a histologically defined border and echographic differentiation from normal brain is difficult; extra-axial lesions (i.e., meningiomas and hemangiomas) on the other hand have a well-defined anatomical (hence echographic) demarcation with normal parenchyma [2]. Brain metastases are also usually well circumscribed and have a defined border on MRI and in the corresponding ultrasound images [2, 8, 22]. Extrinsic features refer to perilesional edema or hemorrhage that, being slightly hyperechogenic, reduce the contrast between the hyperechogenic signal of the lesion and the signal of the brain (Fig. 2). For this reason, diffusely infiltrating lesions with poor edema might be better visualized than high-grade lesions with perilesional edema. For the same reasons, a meningioma or hematoma with surrounding edema could appear less definable [2, 22, 23]. In high-grade gliomas, although infiltrative in nature, iUS is capable of identifying different multiform portions of the solid lesion such as necrosis, cysts, bleedings, and irregular dense tumor. Again, visibility improves as perilesional edema decreases.
US applications
Lesion localization and planning the approach
The ability to localize the lesion is directly proportional to its grade of US visibility. Utility of the US system for approach planning seems to be directly proportional to the lesion’s grade of US visibility, as well as the kind of lesion and the ability to understand the anatomical orientation. In deep extra-axial pathologies (skull base tumors, aneurysms), an anatomical pathway is generally used during the approach (subfrontal, subtemporal, transsilvian), and therefore iUS is less useful. Nevertheless, out of this group of pathologies, there are some selected cases in which the ability to visualize the surgical target and the adjacent structures is useful during the approach. In our experience, iUS has proven itself helpful for planning the approach to deep-seated intraparenchymal pathologies. Every time a transcortical route is used, iUS guidance is used to plan a safe and precise trajectory, avoiding unnecessary parenchymal injury. Our findings are confirmed by previous literature examining the use of iUS in the surgical treatment of brain tumors, cavernous hemangiomas, cerebral abscesses, and ICHs [8, 9, 20,21,22]. The ability to precisely localize the lesion reduces invasiveness and therefore potential surgical morbidity. It allows minimally invasive procedures as well. The transcortical trajectory can be planned with both the 2D real-time iUS and the 3D iUS [21]. In our experience, we did not observe difference between 2D and 3D iUS in terms of safety and precision to reach the target. 2D real-time iUS (conventional iUS and navigated US) allows ongoing visualization of the surgical progression and can identify the relationships between the lesion and artifact from the surgical instruments. Moreover, brain-shift is less of an issue as the image updates are in real time. One disadvantage is that the US probe must constantly be on the surgical field, thus occupying part of the already narrow space. Another disadvantage is that the images are limited to the 2D insonance plan; navigating the probe may facilitate anatomical understanding [10]. 3DUS allows the ability of visualizing the images on the three orthogonal planes and making a direct comparison of iUS and preoperative MRI in the same plane of view [9, 20]. The problem of brain-shift is minimal as it is possible to acquire a new echographic volume in a few seconds at anytime [21]. 3DUS could be used as a navigational tool even without preoperative imaging (Fig. 6). In these events, we can navigate directly based on the reconstructed 3D US data [9, 21]. In both our and other authors’ opinions, this iUS application is useful in the treatment of deep-seated cavernous hemangiomas [9, 25], cerebral abscesses (with minimally invasive approach) [8], ICH [5], or US-guided biopsies and US-guided placement of ventricular catheters. We biopsied eight deep-seated lesions using simultaneous visualization of the tissue US signal and power Doppler. The target was localized and all histological exams were positive. The EVD positioning in normal conditions is a simple procedure and is based on anatomical landmarks; in some cases, however, such as ventricular anomalies (slit-ventricle, malformative hydrocephalus), atypical catheter placements, or atypical patient position, it can be problematic. In these events, iUS allows direct visualization of the ventricles, increasing the precision and safety of the procedures. We used the trajectory based on 3D-navigated iUS in two cases (using the technique described by Jakola et al. [7]) and on 2D real-time iUS guidance in the two remaining cases. We obtained correct catheter placement with both methods.
Resection control
The use of iUS for resection control during surgery of intracerebral tumors is documented in numerous reports; however, our aim was to assess iUS utility in all neurosurgical procedures and we therefore analyzed this application of iUS for non-neoplastic pathologies as well (cavernous hemangiomas, cerebral abscesses, hematomas). The ability to check the resection with ultrasound depends on the pathology’s echographic visibility both at the beginning and during surgery; it is common to observe a reduction of image quality during the procedure. Surgical artifacts can decrease the lesion’s US visibility and particularly the definition of its edges. We were able to accurately check resection in 127 cases out of 152 procedures (Table 2). In our experience, extra-axial pathologies (extra-axial tumors, cavernous hemangiomas, and AVMs) showed high visibility throughout the procedure with minimal decrease in image quality and high capacity of distinguishing borders from the surrounding cerebral tissue. Nevertheless, these are lesions in which the resection can be usually checked with microscopic view; therefore, iUS utility is limited to some specific cases. Echographic control during evacuation of cerebral abscesses, hematomas, or cystic lesions through a minimally invasive approach has been reported [8, 19, 21]. We used iUS with good results during evacuation of two cerebral abscesses and two cases of intraparenchymal hematomas (one associated with an aneurysm, the other with a cavernous hemangioma). In the remaining six hematomas, iUS resulted accurate to plan transcortical approach, but we could not check the evacuation due to low quality of US images. Solheim et al. in 2009 [15] proposed a method of ultrasound-guided resection of giant meningiomas (also effective with schwannomas and craniopharyngiomas): the method enables image-guided resection through narrow approaches that minimize traction on brain parenchyma. US tissue signal could be used to assess the amount of residual tumor (minimizing the risk of over- or underestimation of residual tumor), whereas power Doppler angiography allows the identification of normal arteries adjacent or encased in the tumor as well as feeding vessels [15]. We performed this kind of approach with satisfactory results in meningiomas, schwannomas, craniopharyngiomas, and AVMs (with control of progressive deafferentation with power Doppler).
One of the most used iUS applications is resection control of intra-axial tumors. The aim is to sonographically identify residual lesion which is not visible to the bare eye, in order to refine the resection. Several authors assessed the usefulness of this technique; however, randomized trials are still lacking. Woydt et al. [24] and Chacko et al. [1] compared 2D US imaging results after resection to histopathology by taking probes from various points at the resection margin. They concluded that iUS could detect residual tumor tissue with high specificity and thus improve gross total resection. The role of 3D ultrasound was widely studied by Trondheim’s group [12, 13, 16, 22]. Their studies show that ultrasound is highly accurate in delineating glioblastomas (and metastasis) before resection, but it appears less accurate during and after resection. During resection, there seem to be some overestimation of the tumor and on the contrary, small tumor remnants and infiltrated tissue in the cavity wall are underestimated after resection [12, 22]. In our study, a retrospective unselected population, we evaluated the utility of iUS (for resection control) based on the echographic visibility of the tumor throughout the procedure. On a total of 87 intraparenchymal tumors (HGG, LGG, metastasis, lymphoma), we could perform iUS-based resection control in 68 cases (37 GTR, 26 STR, 5 biopsies). We deemed useful in all cases, except from biopsies (in which quantification of residual tumor was not considered relevant). iUS allowed us to perform a direct control of resection. In the remaining 19 cases (10 GTR, 7 STR, 2 biopsy), image quality was inadequate to perform an accurate assessment of the resection. In the subgroup of giant intra-axial tumors, resection control could be difficult because of system’s inability to visualize the whole lesion together with its margins. This drawback could be overcome by alternating internal tumor debulking and US acquisitions until the borders become clear. Saeter et al. performed a retrospective analysis on the use of 3D ultrasound in 192 glioblastomas surgery. Their study demonstrated that survival rates improved within the same period that intraoperative ultrasound and neuronavigation were introduced and established in their department [13]; however, these findings are not confirmed by larger randomized controlled trial and the authors concluded that one must be cautious to claim causality between iUS and prolonged glioblastoma survival. In two comparative studies, 2DiUS resulted in slightly inferior to iMRI in resection control [3, 4]. Tronnier et al., however, showed that detection of HGG and metastasis and intraoperative delineation of tumor remnants through low-field iMRI (0.2 T) or 3DiUS was comparable in both imaging modalities whereas in the case of a low-grade glioma, iUS was more helpful [18]. Comparison between iUS and other intraoperative imaging (iCT or iMRI) is beyond the scope of this article; our aim was to assess the utility of routine use of iUS in intracranial surgery. Our study as well as literature data [1, 11,12,13, 16, 20, 22, 24] suggests that iUS has high sensitivity in detecting tumor remnants (higher than “bare eyes”) and hence could help improving gross total resection.
Final US evaluation of the surgical field
The final iUS scan showed in two cases the presence of hematoma in the surgical field. In one of these cases, the hematoma went unnoticed before using iUS. We firmly believe that our ability in interpreting the final iUS scans will improve by gaining experience; however, at the moment, we are not ready to replace postoperative CT scan with iUS scan because it does not provide a global intracranial view.
Conclusions
In this study, we presented our experience using navigated iUS imaging (SonoWand) during 162 intracranial surgeries. Our aim was to assess the utility of routine use of iUS during various types of intracranial surgery. We reviewed our series to assess ultrasound visibility of different pathologies and iUS application during the course of surgery. We found that intraoperative ultrasound was highly sensitive in detecting all types of pathology. The technology resulted applicable to all kind of intracranial surgical procedure, even in urgency regime, has no need for any particular operating room setting and fast in image-acquiring timing. Navigated probes facilitate anatomical orientation and comparison between the US signal and preoperative MR and/or CT imaging. It can be quickly equipped, even in cases in which its utilization was not planned preoperatively.
Regarding the applications, resulted in useful and safe in planning trajectories to subcortical lesions (even with minimally invasive approaches). iUS was accurate in checking extent of resection in more than 80% of cases; resection control appeared easy and accurate in extra-axial pathologies. In intra-axial tumors, resection control might become difficult during surgery due to drop of US visibility. Impact of iUS on long-term oncological outcome has still to be assessed, but iUS showed high sensitivity in detecting tumor remnants and hence could help improving gross total resection. Simultaneous visualization of US tissue and power Doppler signals allows both to reduce the risk of vessel injury and to check deafferentation of tumors and AVMs. We think that intraoperative ultrasound is a versatile and feasible tool that many other colleagues probably will find useful with some practice.
Abbreviations
- HGG:
-
High-grade glioma
- LGG:
-
Low-grade glioma
- ICH:
-
Intracerebral hematoma
- AVM:
-
Artero-venous malformation
- US:
-
Ultrasound
- iUS:
-
Intraoperative US
- CECT:
-
Contrast-enhanced CT scan
References
Chacko AG, Kumar NK, Chacko G, Athyal R, Rajshekhar V (2003) Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours—a comparative study with computed tomography and histopathology. Acta Neurochir 145(9):743–748
Coburger J, König RW (2016). Intraoperative findings in brain tumor surgery. In: Prada F, Solbiati L, Martegani A, Di Meco F, editors. Intraoperative ultrasound (IOUS) in neurosurgery. From standard-B-mode to elastosonography. Switzerland Springer, pp 41–59
Gerganov VM, Samii A, Akbarian A, Stieglitz L, Samii M, Fahlbusch R (2009) Reliability of intraoperative high-resolution 2D ultrasound as an alternative to high-field strength MR imaging for tumor resection control: a prospective comparative study. J Neurosurg 2009 111(3):512–519
Gerganov VM, Samii A, Giordano M, Samii M, Fahlbusch R (2011) Two-dimensional high-end ultrasound imaging compared to intraoperative MRI during resection of low-grade gliomas. J Clin Neurosci 18(5):669–673
Goren O, Monteith SJ, Hadani M, Bakon M, Harnof S (2013) Modern intraoperative imaging modalities for the vascular neurosurgeon treating intracerebral hemorrhage. Neurosurg Focus 34(5):E2
Gronningsaeter A, Kleven A, Ommedal S, Aarseth TE, Lie T, Lindseth F, Langø T, Unsgård G (2000) SonoWand, an ultrasound-based neuronavigation system. Neurosurgery 47(6):1373–1379
Jakola AS, Reinertsen I, Selbekk T, Solheim O, Lindseth F, Gulati S, Unsgård G (2014) Three-dimensional ultrasound-guided placement of ventricular catheters. World Neurosurg 82(3–4):536.e5–536.e9
Mair R, Heald J, Poeata I, Ivanov M (2013) A practical grading system of ultrasonographic visibility for intracerebral lesions. Acta Neurochir 155(12):2293–2298
Miller D, Benes L, Sure U (2011) Stand-alone 3D-ultrasound navigation after failure of conventional image guidance for deep-seated lesions. Neurosurg Rev 34(3):381–387
Miller D, Heinze S, Tirakotai W, Bozinov O, Sürücü O, Benes L, Bertalanffy H, Sure U (2007) Is the image guidance of ultrasonography beneficial for neurosurgical routine? Surg Neurol 67(6):579–587
Moiyadi AV, Shetty PM, Mahajan A, Udare A, Sridhar E (2013) Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Acta Neurochir 155(12):2217–2225
Rygh OM, Selbekk T, Torp SH, Lydersen S, Hernes TA, Unsgaard G (2008) Comparison of navigated 3D ultrasound findings with histopathology in subsequent phases of glioblastoma resection. Acta Neurochir 150(10):1033–1041
Sæther CA, Torsteinsen M, Torp SH, Sundstrøm S, Unsgård G, Solheim O (2012) Did survival improve after the implementation of intraoperative neuronavigation and3D ultrasound in glioblastoma surgery? A retrospective analysis of 192 primary operations. J Neurol Surg A Cent Eur Neurosurg 73(2):73–78
Shinoura N, Takahashi M, Yamada R (2006) Delineation of brain tumor margins using intraoperative sononavigation: implications for tumor resection. J Clin Ultrasound 34(4):177–183
Solheim O, Selbekk T, Lindseth F, Unsgård G (2009) Navigated resection of giant intracranial meningiomas based on intraoperative 3D ultrasound. Acta Neurochir 151(9):1143–1151
Solheim O, Selbekk T, Jakola AS, Unsgård G (2010) Ultrasound-guided operations in unselected high-grade gliomas—overall results, impact of image quality and patient selection. Acta Neurochir 152(11):1873–1886
Sosna J, Barth MM, Kruskal JB, Kane RA (2005) Intraoperative sonography for neurosurgery. J Ultrasound Med 24(12):1671–1682
Tronnier VM, Bonsanto MM, Staubert A, Knauth M, Kunze S, Wirtz CR (2001) Comparison of intraoperative MR imaging and 3D-navigated ultrasonography in the detection and resection control of lesions. Neurosurg Focus 10(2):E3
Unsgaard G, Gronningsaeter A, Ommedal S, Nagelhus Hernes TA (2002) Brain operations guided by real-time two-dimensional ultrasound: new possibilities as a result of improved image quality. Neurosurgery 51(2):402–411
Unsgaard G, Ommedal S, Muller T, Gronningsaeter A, Nagelhus Hernes TA (2002) Neuronavigation by intraoperative three-dimensional ultrasound: initial experience during brain tumor resection. Neurosurgery 50(4):804–812
Unsgaard G, Rygh OM, Selbekk T, Müller TB, Kolstad F, Lindseth F, Hernes TA (2006) Intra-operative 3D ultrasound in neurosurgery. Acta Neurochir 148(3):235–253
Unsgaard G, Selbekk T, Brostrup Müller T, Ommedal S, Torp SH, Myhr G, Bang J, Nagelhus Hernes TA (2005) Ability of navigated 3D ultrasound to delineate gliomas and metastases—comparison of image interpretations with histopathology. Acta Neurochir 147(12):1259–1269
Wang J, Liu X, Hou WH, Dong G, Wei Z, Zhou H, Duan YY (2008) The relationship between intra-operative ultrasonography and pathological grade in cerebral glioma. J Int Med Res 36(6):1426–1434
Woydt M, Krone A, Becker G, Schmidt K, Roggendorf W, Roosen K (1996) Correlation of intra-operative ultrasound with histopathologic findings after tumour resection in supratentorial gliomas. A method to improve gross total tumour resection. Acta Neurochir 138(12):1391–1398
Woydt M, Krone A, Soerensen N, Roosen K (2001) Ultrasound-guided neuronavigation of deep-seated cavernous haemangiomas: clinical results and navigation techniques. Br J Neurosurg 15(6):485–495
Acknowledgements
We would like to thank Dr. Justin Mascitelli (Neurosurgeon at Barrow Neurological Institute) for the English revision and for his precious intellectual support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Policicchio, D., Doda, A., Sgaramella, E. et al. Ultrasound-guided brain surgery: echographic visibility of different pathologies and surgical applications in neurosurgical routine. Acta Neurochir 160, 1175–1185 (2018). https://doi.org/10.1007/s00701-018-3532-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3532-x