Abstract
Background
Intraoperative ultrasound for intracranial neurosurgery was largely abandoned in the 1980s due to poor image resolution. Despite many technological advances in ultrasound since then, the use of this imaging modality in contemporary practice remains limited. Our aim was to evaluate the utility of modern intraoperative ultrasound in the resection of a wide variety of intracranial pathologies.
Methods
A total of 105 patients who underwent intracranial lesion resection in a contiguous fashion were prospectively included in the study. Ultrasound images acquired intraoperatively were used to stratify lesions into one of four grades (grades 0–3) on the basis of their ultrasonic echogenicity and border visibility.
Results
Forty-two out of 105 lesions (40 %) were clearly identifiable and had a clear border with normal tissue (grade 3). Fifty-five of 105 lesions (52 %) were clearly identifiable but had no clear border with normal tissue (grade 2). Eight of 105 lesions (8 %) were difficult to identify and had no clear border with normal tissue (grade 1). None (0 %) of the lesions could not be identified (grade 0). High-grade gliomas, cerebral metastases, meningiomas, ependymomas, and haemangioblastomas all demonstrated a median ultrasonic visibility grade of 2 or greater. Low-grade astrocytomas and oligodendrogliomas demonstrated a median ultrasonic visibility grade of 2 or less.
Conclusion
Intraoperative ultrasound can be of tremendous benefit in allowing the surgeon to appraise the location, extent, and local environment of their target lesion, as well as to reduce the risk of preventable complications. We believe that our grading system will provide a useful adjunct to the neurosurgeon when deciding for which lesions intraoperative ultrasound would be useful.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the important goals for a neurosurgeon is to remove the target lesion without causing neurological deficit. There are many ways in which a surgeon is able to maximise their capacity to achieve this outcome. The ability to localise the tumour with a good degree of accuracy, to estimate the volume of tumour resected, and the proximity of any remnants to normal brain anatomy are all important factors in achieving the primary goal. With this in mind, several methods of intraoperative imaging have been developed to facilitate safe and efficacious tumour removal. Intraoperative ultrasound, neuronavigation, intraoperative MRI and, in high-grade gliomas, fluorescene-guided techniques (using 5-Aminolevulinic acid (5-ALA)) have all been shown to support safe, more extensive tumour resection [8, 10, 12, 16].
Ultrasound was developed for medical purposes by the American Navy in the 1940s but was first used cranially in the 1950s [1]. It utilises sound waves at frequencies above the human auditory threshold to image planes of tissue in two dimensions. Its use intraoperatively in cranial cases was largely abandoned during the 1980s due to the poor resolution obtained at that time. The technology has advanced significantly since then and users are now able to integrate preoperative MRI/CT images with intraoperative, ‘real-time’ ultrasound pictures if required. Even without 3D integration the operator can precisely localise the lesion in ’real time’ and view the relationships to important anatomical structures as well as being able to re-scan during the operation to assess the degree of resection.
Intraoperative ultrasound has been shown in several studies to provide benefit in the resection of various lesions including (1) glioma, where good intraoperative ultrasound image quality was associated with the achievement of gross total resection with subsequent good functional outcomes [11], (2) meningioma, where its use facilitated the identification of the surrounding vasculature permitting safer resection [13], and (3) haematoma evacuation, where a recent review article concluded that improvements in image quality enhanced navigation and evacuation assessment [4].
Despite progress within this field, the re-adoption of intraoperative cranial ultrasound has not been widespread. When examining why this might be, we found that neurosurgeons were reticent due to several factors. The most important of these factors was the perception that the views obtained intraoperatively would be poor and of little surgical use. As a consequence a general consensus formed that either the lesion would not be visualised or that no useful information would be gleaned regarding the extent of resection or the proximity of adjacent structures. With this in mind we felt that a grading system of ultrasonic visibility would help to demonstrate the expected value of ultrasound in certain situations and would be a useful adjunct to the surgeon’s armamentarium in intraoperative decision making. We believed that it would encourage the neurosurgeon as to the use of ultrasound in a wide variety of intracranial pathologies. It would also apprise as to when the use of ultrasound would be of limited benefit.
Here we present our experience of 105 consecutive cases of intracranial lesion resection performed by the lead author (MI) in a prospective fashion using intraoperative ultrasound navigation alongside our resultant grading analysis.
Methods
We used Philips 3500, Philips 4000 (Amsterdam, Netherlands), Hitachi Aloka ProSound SSD 5000 (Tokyo, Japan) and SonoWand (Trondheim, Norway) ultrasound consoles with probes of sector 5 and 7.5 MHz probes and linear 12 MHz to acquire intraoperative images in a standard fashion [5, 14]. The probe selected was dependent upon tumour depth and size, with the best possible image selected as the reference. For superficial lesions, located less than 2 cm from the cortex and with a maximum diameter of less than 3 cm, a 12-MHz linear probe was used. For deeper and/or larger lesions we used a 7.5-MHz sector probe and occasionally a 5-MHz probe. For AVM assessment, B-Mode was used for the grading system with Colour mode and Power Angio mode used as adjuncts. Intraoperative ultrasound images were assessed and correlated to preoperative MRI/CT, intraoperative neuronavigation as well as tumour histology. The following two factors were considered: 1) overall tumour visibility by the operating neurosurgeon, 2) distinction between the lesion margins and the surrounding brain (Table 1). The assessment of the images was initially performed intraoperatively by the operating neurosurgeon (MI). The best image was saved and assessed postoperatively by another independent, experienced neurosurgeon. This second assessment was performed blindly, without review of the preoperative images.
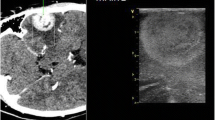
We developed this grading system after careful review of the known limitations of the current imaging modalities and of how ultrasound could benefit lesion identification. We are aware that certainty as to the classification of a lesion cannot be expressed preoperatively. However, from a pragmatic perspective we believe that a radiological differential diagnosis would provide the information required to allow our grading system to be of use. We propose a four-tiered system that takes into account the echogenicity of the lesion and its border visibility. This four-tiered system, reflecting four degrees of visibility, was used in preference to a two-tiered system (visible and invisible) due to the heterogeneity of the visibility amongst neurosurgical lesions. We reason that this will subsequently allow the practicing neurosurgeon improved accuracy when determining the value of intraoperative ultrasound. This is a practical grading system designed by surgeons for surgeons (Fig. 1).
Ultrasonographic visibility of brain lesions. a) grade III: the lesion is clearly identifiable and has a clear border with normal tissue; artistic representation, preoperative CT with contrast and intraoperative ultrasound; coronal image. T, tumour; F, falx; LV, lateral ventricles; b) the lesion is clearly identifiable but has no clear border with normal tissue; CT with contrast—Mesial temporal tumour, Power Angio mode. Tumour and its relationship to neighbouring vessels is visible. T, tumour; WC, Willis Circle; PCA, Posterior Cerebral Artery; c) the lesion is difficult to visualise and has no clear border with normal tissue but remains identifiable on MRI (T2). T, tumour; F, falx; CP,choroid plexus in the lateral ventricles
Data was collected in a contiguous fashion with the patient data being made anonymous. Informed patient consent was taken prior to surgery to allow for participation in the study. The collection of images was not done at the expense of prolongation of the surgery itself.
Results
Using our grading system we were able to show that we could both visualise and clearly demarcate the lesion boundary in B-mode in 42 out of 105 intracranial lesions (40 %). Fifty-five patients had lesions that were clearly visible on ultrasound but in which we were unable to identify a definite border with normal tissue (52 %). In no cases were we unable to visualise the lesion whatsoever and in only eight cases (8 %) did we have a poorly visible lesion in B-mode (four low-grade gliomas, two arteriovenous malformations [AVMs], one granuloma, one oligodendroglioma) (Table 2). However, changing the standard B-mode to Colour-Angio or Power-Angio mode placed AVM in the group of clearly visible lesions. These two modes, which allow assessment of blood flow, have additional value for the operating surgeon by providing useful information regarding the relationship between a tumour and adjacent blood vessels (Fig. 1 b). The use of Doppler Mode with the area of interest placed on the identified vessel can also be helpful in differentiating between arterial and venous flow patterns.
Discussion
Using our grading system we were able to grade 42 out of 105 intracranial lesions (40 %) as grade 3. The lead author found that the information gained in these cases provided benefit with regards to the localisation and completeness of resection. Fifty-five patients (52 %) scored a 2 on our grading system. In these patients ultrasound was found to be useful from a localisation perspective and this helped to minimise dissection through normal cerebral tissue. No cases scored 0 (not visible) and only eight cases scored 1. In these cases the use of ultrasound was helpful in order to estimate the lesion location but was not useful for appreciation of the degree of resection.
Maximal tumour resection is associated with increased survival, better long-term tumour control, and a reduced incidence of the malignant transformation of lower grade lesions [2, 9]. A high Karnofsky performance score (i.e. low postoperative neurological deficits) is also a predictor for long-term survival in tumour patients [7]. The application of intraoperative imaging techniques to intracranial tumours with the aim of improving these parameters is therefore laudable. We have identified a group of five tumours (high-grade glioma, cerebral metastasis, meningioma, ependymoma, haemangioblastoma) in which we found intraoperative ultrasound a valuable tool. All of these tumours scored a median grade of 2 or greater in our grading system, which meant that the tumours were easily identifiable using intraoperative ultrasound. We also applied our grading system to several rarer tumours and found that in ganglioglioma and gangliocytoma it was also useful. The tumours that we found ultrasound to be less useful for were low-grade astrocytomas and oligodendrogliomas, both of which scored a median grade of 2 or less. The low-grade astrocytomas were particularly difficult to image, scoring 1 in 57 % of cases. However, the ability to recognise these tumours has improved with experience and adjustment of the console settings (Fig. 2).
Neuronavigation is the stalwart of current neurosurgical resective practice but has several limitations in terms of an inability to compensate for brain shift as well as potential inaccuracies due to registration anomalies. The current vogue for intraoperative MRI provides an alternative, although it too has its drawbacks, including the high cost and level of disruption required for installation of the device and the equipment required, as well as the time taken to image the intraoperative patient to any useful degree of accuracy. 5-ALA-guided resection of high-grade glioma is accurate in showing the tumour but is limited to a specific type of tumour and does not provide anatomical information. It also requires the purchase of photodynamic equipment for tumour visualisation, making it more expensive. Intraoperative ultrasound is a cheap and ubiquitous alternative or complementary method to those outlined above.
The current literature examining the uses of intraoperative ultrasound is contradictory as to its usefulness amongst various tumour types. A recent study using intraoperative ultrasound in an unselected group of 142 patients with high-grade glioma showed that medium or good views were independently associated with achieving gross-total resection. They also noted a correlation between ultrasound image quality and clinical as well as radiological results [10]. A further study that compared a cohort of high-grade with low-grade gliomas found difficulty in defining a tumour boundary in the high-grade lesions using intraoperative ultrasound. This difficulty was attributed to parenchymal oedema and as such they found identification of the tumour boundary easier in their low-grade glioma subset [15]. Good correlation between initial ultrasound appearances and those of intraoperative MRI has also been shown in low-grade glioma surgery [3]. When comparing the intraoperative findings on ultrasound with the histopathological diagnosis, a recent study initially showed good correlation, however, reliability became worse as the resection progressed, eventually declining to 26 % [6]. A contemporary study examining the use of intraoperative ultrasound in meningioma found it of value in explicating the location of adjacent vasculature, thus facilitating safe tumour resection [11].
Our dataset included six patients with vascular lesions, including three patients with cavernomas. Rebleed following complete surgical resection of these lesions is rare and so accurate localisation and demarcation of the boundaries to facilitate total removal is advantageous. In our series, cavernomas were easy to identify with a median grade of 3. We also included data from three AVM resections and found that ultrasound was good at localising the AVM in one patient but was not useful in the other two if B-mode was used. However, Power Angio/Colour modes helped us to visualise and characterise abnormal vessels with much higher accuracy, placing this type of pathology in the group of clearly visible lesions with clear borders. Ultrasound is currently used in AVM surgery whereby Doppler is employed to identify arterial feeders entering the nidus. Doppler mode was helpful to differentiate between arterial and venous flow patterns with reasonable accuracy. We found no particular benefit with regards to its use in B-mode to identify AVM location or extent. This is in contrast to using Colour/Power Angio modes as well as in contrast to its usefulness in cavernomas, where it was used to successfully localise small subcortical lesions.
We also applied our grading scale to intracerebral haematomas (ICH). They scored a median grade of 3 and were thus easily identifiable using ultrasound. The lead author found this useful in the emergency setting, whereby safe localisation was made without the delays associated with neuronavigational setup whilst still avoiding unnecessary parenchymal injury. It was also useful in assessing the degree to which the haematoma had been evacuated. In one case, the fresh haematoma in the tumour bed was identified after dural closure, facilitating timely wound re-exploration and adequate haemostasis. A recent review article also discusses the reticence of the neurosurgical community to employ ultrasound despite considerable improvements within the field. In the authors experience they find ultrasound useful both in reducing the transcortical trajectory required to access ICH as well as in identifying the relationship of various lesions to the surrounding brain in real time [4].
Finally, we applied our grading scale to abscesses of which we identified six. They scored a median grade of 2 and were easy to localise in all cases. The gold standard treatment for abscess management is currently under debate, with both burr hole aspiration and formal craniotomy for resection yielding similar results [17]. Ultrasound can be used and, in conjunction with our findings, would be useful for either of these options. Another advantage of ultrasound in this context was to localise the tip of the draining needle within the abscess cavity. This is of relevance in very viscous abscesses where aspiration can be difficult. It also allowed visualisation of the collapse of the abscess wall and, as such, assessment for complete drainage of the collection.
We found that the linear probes with higher frequency are helpful in localization of small superficial lesions, offering a higher accuracy with less noise. However, for larger and deeper lesions this probe was less helpful due to an attenuation effect and a lower frequency probe was found to be more efficient. An additional benefit of the lower frequency probe was the possibility to assess the entire cranial cavity, assisting with anatomical orientation.
We are aware that our study is limited by its moderate sample size and by the low instance, or absence, of certain lesions within its cohort. In mitigation we are not attempting to present a categorical review of the indications for intraoperative ultrasound. Instead, we reason that for certain intracranial lesions, intraoperative ultrasound can be of tremendous benefit in allowing the surgeon to appraise the location, extent and local environment of their target as well as reduce preventable complications. We also believe that this study might inform a new generation of surgeons who may be unfamiliar with the technique, as well as try to reanimate a generation indifferent to its use. Our scoring system aims to provide surgeons with practical information as to the utility of intraoperative ultrasound for the lesion upon which they are about to operate, and as such the advantages of intraoperative ultrasound can work as either a standalone tool or in symbiosis with other intraoperative localization techniques.
Ideally, we would have liked to include more examples of rare tumour types as well as to increase the number of those encountered more frequently, but for the sake of continuity we felt it appropriate to present the experience of a single surgeon and thus reduce the risk of interobserver bias. Other sources of error would be the learning curve that said surgeon will have pursued and the experience with the technique that was present at the start of the study. We feel that every new technique encounters these issues and that routine use of ultrasound, even in cases where there is no stringent need for it and provided that no significant delay in the surgery occurs, should be considered for gaining the valuable experience necessary in more complex cases.
Conclusion
We present the first attempt to classify intracerebral lesions on their intraoperative ultrasound appearances. We believe that our grading system provides a useful adjunct to the neurosurgeon when deciding in which lesions intraoperative ultrasound would be efficacious. We found that this relatively inexpensive, real-time and easily available adjunct to the neurosurgical armamentarium was helpful in the localization of the majority of intracranial lesions. This included situations whereby neuronavigation had failed to localize the pathology. We are optimistic that this will lead to resurgence in use for this simple but effective clinical tool in units where its usefulness was underestimated, and will encourage neurosurgeons who do not have much experience with ultrasound to use it with more confidence and more often.
References
Ballantine HT Jr, Bolt RH, Hueter TF, Ludwig GD (1950) On the detection of intracranial pathology by ultrasound. Science 112(2914):525–528
Claus EB, Horlacher A, Hsu L, Schwartz RB, Dello-Iacono D, Talos F, Jolesz FA, Black PM (2005) Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer 103:1227–1233
Gerganov VM, Samii A, Giordano M, Samii M, Fahlbusch R (2011) Two dimensional high-end ultrasound imaging compared to intraoperative MRI during resection of low grade gliomas. J Clin Neurosci 18:669–673
Goren O, Monteith SJ, Hadani M, Bakon M, Harnof S (2013) Modern intraoperative imaging modalities for the vascular neurosurgeon treating intracerebral hemorrhage. Neurosurg Focus 34(5):E2
Ivanov M, Wilkins S, Poeata I, Brodbelt A (2010) Intraoperative ultrasound in neurosurgery - a practical guide. Br J Neurosurg 24(5):510–517
Rygh OM, Selbekk T, Torp SH, Lydersen S, Hernes TA, Unsgaard G (2008) Comparison of navigated 3D ultrasound findings with histopathology in subsequent phases of glioblastoma resection. Acta Neurochir (Wien) 150(10):1033–1041
Scoccianti S, Magrini SM, Ricardi U, Detti B, Buglione M, Sotti G, Krengli M, Maluta S, Parisi S, Bertoni F, Mantovani C, Tombolini V, De Renzis C, Lioce M, Fatigante L, Fusco V, Muto P, Berti F, Rubino G, Cipressi S, Fariselli L, Lupattelli M, Santoni R, Pirtoli L, Biti G (2010) Patterns of care and survival in a retrospective analysis of 1059 patients with glioblastoma multiforme treated between 2002 and 2007: a multicentre study by the Central Nervous System Study Group of Airo. Neurosurgery 67(2):446–458
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12(11):997–1003
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345
Solheim O, Selbekk T, Jakola AS, Unsgård G (2010) Ultrasound-guided operations in unselected high grade gliomas—overall results, impact of image quality and patient selection. Acta Neurochir 152:1873–1886
Solheim O, Selbekk T, Lindseth F, Unsgard G (2009) Navigated resection of giant intracranial meningiomas based on intraoperative 3D ultrasound. Acta Neurochir (Wien) 151:1143–1151
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ; ALA-Glioma Study Group (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Tang H, Sun H, Xie L, Tang Q, Gong Y, Mao Y, Xie Q, Zheng M, Wang D, Zhu H, Zhu J, Feng X, Yao Z, Chen X, Zhou L (2013) Intraoperative ultrasound assistance in resection of intracranial meningiomas. Chin J Cancer Res 25:339–345
Unsgaard G, Rygh OM, Selbekk T, Müller TB, Kolstad F, Lindseth F, Hernes TA (2006) Intra-operative 3D ultrasound in neurosurgery. Acta Neurochir (Wien) 148(3):235–253
Wang J, Liu X, Hou WH, Dong G, Wei Z, Zhou H, Duan YY (2008) The relationship between intra-operative ultrasonography and pathological grade in cerebral glioma. J Int Med Res 36:1426–1434
Wirtz CR, Albert FK, Schwaderer M, Heuer C, Staubert A, Tronnier VM, Knauth M, Kunze S (2000) The benefit of neuronavigation for neurosurgery analysed by its impact on glioblastoma surgery. Neurol Res 22(4):354–360
Xiao F, Tseng MY, Teng LJ, Tseng HM, Tsai JC (2005) Brain abscess: clinical experience and analysis of prognostic factors. Surg Neurol 63(5):442–450
Acknowledgements
The lead author would like to express his great appreciation to Mr. Andrew Brodbelt (Consultant Neurosurgeon at the Walton Centre for Neurology and Neurosurgery, Liverpool, UK) for his valuable support with the data collection and for his enthusiastic encouragement of this research.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
*This study has not been presented at a conference
Rights and permissions
About this article
Cite this article
Mair, R., Heald, J., Poeata, I. et al. A practical grading system of ultrasonographic visibility for intracerebral lesions. Acta Neurochir 155, 2293–2298 (2013). https://doi.org/10.1007/s00701-013-1868-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1868-9