Abstract
Background
Intraoperative imaging is increasingly being used in resection of brain tumors. Navigable three-dimensional (3D)-ultrasound is a novel tool for planning and guiding such resections. We review our experience with this system and analyze our initial results, especially with respect to malignant gliomas.
Methods
A prospective database for all patients undergoing sononavigation-guided surgery at our center since this surgery’s introduction in June 2011 was queried to retrieve clinical data and technical parameters. Imaging was reviewed to categorize tumors based on enhancement and resectability. Extent of resection was also assessed.
Results
Ninety cases were operated and included in this analysis, 75 % being gliomas. The 3D ultrasound mode was used in 87 % cases (alone in 40, and combined in 38 cases). Use of combined mode function [ultrasound (US) with magnetic resonance (MR) images] facilitated orientation of anatomical data. Intraoperative power Doppler angiography was used in one-third of the cases, and was extremely beneficial in delineating the vascular anatomy in real-time. Mean duration of surgery was 4.4 hours. Image resolution was good or moderate in about 88 % cases. The use of the intraoperative imaging prompted further resection in 59 % cases. In the malignant gliomas (51 cases), gross-total resection was achieved in 47 % cases, increasing to 88 % in the “resectable” subgroup.
Conclusions
Navigable 3D US is a versatile, useful and reliable intraoperative imaging tool in resection of brain tumors, especially in resource-constrained settings where Intraoperative MR (IOMR) is not available. It has multiple functionalities that can be tailored to suit the procedure and the experience of the surgeon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aim of surgery in brain tumors is safe maximal resection. Evidence is mounting that increasing the extent of resection positively impacts survivals and oncological outcomes [18]. With improved technical adjuncts and equipped with a varying therapeutic armamentarium, neurosurgeons are pushing for more radical resections. Planning of the surgical procedure is therefore a very crucial step. Central to the plan is an accurate localization of the tumor, its extent, and its relationship to vital neurovascular structures. Navigation permits customized tailoring of small craniotomies. However, because of the phenomenon of brain shift once the dura is opened, navigation based on images acquired preoperatively alone has limited use. A reliable intraoperative image update is increasingly needed in the operating room. Intraoperative magnetic resonance imaging (IOMR), though ideal, is very costly and not readily available. A more practical (and cost efficient) alternative is the intraoperative ultrasound (IOUS). IOUS [11, 25] is a very effective tool for localizing lesions. There are, however, certain limitations of conventional two-dimensional (2D) US [25]. Navigable three-dimensional (3D) US is a new technology that combines features of navigation with a high-resolution 3D US [27]. It surmounts most of the limitations of conventional 2D US by essentially overcoming the orientation problem and providing more user-friendly multiplanar imaging capabilities. We recount our experience with one such 3D navigable US system specifically for resection of brain tumors. Our aim was to assess its practical utility and impact on intraoperative decisions (regarding continuation of resection) with respect to tumor resection at a tertiary care oncology center. One of the major applications of this technology is improving resections of intraparenchymal tumors. To assess this, we also analyzed the extent of resection achieved in malignant gliomas.
Materials and methods
This was a retrospective analysis. We have been using the navigable 3D US system SonoWand [SONOWAND AS, Trondheim, Norway] since June 2011. The system has been described in detail elsewhere [5]. Navigable 3D US essentially combines navigation technology with a high-end dedicated cranial insonation probe capable of generating 2D as well as 3D images. The cranial probe is precalibrated and registered to the navigation system. It can rapidly (30–40 seconds) acquire a series of 2D images (about 200–300) that are computed automatically into a 3D volume that can then be displayed on the navigation system in either the traditional ACS (axial, coronal, sagittal) planes, or a more user-friendly and intuitive “dual-anyplane” mode. The US data can also be superimposed on preoperative MR (when available) to provide a better orientation of the cross-sectional anatomy. Using this US data set, the neurosurgeon is able to navigate. This data set can then be repeatedly updated (as and when necessary) during the course of the surgery. The system can be used as either a stand-alone navigation system using preoperative images; as a stand-alone ultrasound machine providing real-time intraoperative 2D images as well as a navigable 3D ultrasound (which allows navigation based solely on the IOUS without requiring preoperative MR images); or in a combined mode using both preoperative images and intraoperative US.
MRI protocol
this was obtained a day or two prior to the planned surgery in some of the cases. We used fiducial-based as well as anatomical registration. For the former, standard skin-adhering fiducials were placed on the scalp. Preoperative MRI was obtained with a 3-tesla system (3 T HDXE, GE) . Post-contrast axial 3-DSPGR (BRAVO, slice thickness 1.6 mm, 0 spacing) and axial T2 sequences (FSE-XL, slice thickness 2 mm, 0 gap) were routinely use. The images were burnt on a disc and transferred to the navigation system prior to the surgery. Image registration was done on the system, and after positioning, patient-to-image registration was completed.
The operative procedure
Patient positioning was always planned to ensure that the operative cavity would be as vertical as possible, so that it could be filled completely with saline in order to optimize ultrasound image quality. Craniotomy was planned either using the preoperative images for navigation in certain cases, or using standard surface marking principles. Awake surgery with intraoperative clinical monitoring was used whenever the tumor was close to the eloquent cortex. After craniotomy and before opening the dura, a baseline ultrasound acquisition was carried out. Initially, a 2D acquisition was performed and ultrasound parameters adjusted to obtain the best image resolution. Then, anatomical landmarks were identified if possible and the lesion was characterized (delineation, size and extent, echo-characteristics). Whenever preoperative MR was available, the system automatically superimposed the live 2D US images on the corresponding MR, thus allowing excellent orientation. Once the lesion was identified, a rapid 3D US acquisition was performed. This essentially involves scanning a sector of the volume of interest by sweeping the US probe over it in a slow and predetermined pattern, so as to cover the entire region of interest. The phased-array probe was preferred for most deep lesions, with the linear probe being used for superficial, subcortical lesions. The dura was then opened, and surgery proceeded adhering to routine microneurosurgical principles. Occasionally, thickened/calcified dura resulted in poor image resolution. In these cases, the baseline US was performed after opening the dura. Tumor resection proceeded, guided by the 3D US images using a trackable pointer to navigate. Repeat 3D US images were obtained as many times as required during the surgery to update the information as tumor debulking proceeded. The surgeons recorded their impression regarding the completeness of resection at the end of the surgery. An US was acquired after this, and if residual tumor was detected, the surgeon either opted for proceeding with the resection or stopping (if it was close to eloquent areas). This was documented prospectively in a proforma filled immediately after the surgery. A final US was obtained at the end of the procedure and after dural closure (to look for any hematoma collection).
Data analysis
During the procedure at various times, screenshots (Fig. 1) of the navigation display screen were captured and recorded and subsequently compiled into case capsules. These were entered into a database. These case capsules were reviewed and the database queried to extract the information for this analysis (such as indication for use, mode of application, type of registration and error, type of probe used, and other subjective details). Descriptive analysis was performed.
Snapshot of a left frontal glioma operated using Direct 3D US mode. Upper row depicts the “anyplane” view, and the lower row depicts the corresponding images in a plane perpendicular to the “anyplane” view. Left panel shows the initial (pre-resection) US. Middle panel shows thick rim of residual tumor in the middle of the surgery. Right panel shows the post-resection US scan with no residual tumor
Extent of resection (EOR) analysis
This was performed in the subset of malignant gliomas (n = 51). These gliomas were classified based on their resectability, as decided by the operating surgeons (AM, PS). Lesions were considered resectable when they were well circumscribed and localized with a possibility of obtaining a complete radiological resection. Lesions were further classified as “enhancing” (when there was complete or predominant contrast enhancement on the preoperative MRI) and “non-enhancing” (when there was minimal or no contrast enhancement). Postoperative contrast MR was obtained within 72 hours in all cases. When this was not possible (logistical reasons), a postoperative computed tomography (CT) scan was obtained and a delayed MR was performed subsequently. The MR scans were analysed for extent of resection. Absence of all contrast enhancement was considered as gross total resection (GTR) for the enhancing tumors. For the non-enhancing tumors, absence of T2 and FLAIR changes on the postoperative MR scan were considered as GTR. If there was any doubt, the delayed scans (at 3 months) were also reviewed to unequivocally confirm the absence of T2/FLAIR changes. In seven cases, immediate postoperative MR could not be obtained (MR not working in four instances, and patient uncooperative in three cases). In each of these, a CT scan was obtained and a delayed MR performed as soon as possible. Both CT and MR findings were reviewed to ascertain the extent of resection in these cases.
Results
Between June 2011 and February 2013, the SonoWand system was used in 90 cases. The distribution of cases is shown in Table 1. A large majority of the cases (75 %) were gliomas. Malignant gliomas were the single most common group of tumors operated. Seven of these were recurrent tumors. The mean duration of surgery was 4.4 hours (range 2–9 hours).
The modes of use of the system and details about the technical parameters are depicted in Table 2. The navigable US function was used in 87 % of the cases (stand-alone US in 44 % of case, and combined with preoperative MR-based navigation in 42 % of cases). Fiducial-based registration was used in most cases when preoperative images were required for navigation. We also used anatomical landmark-based registration in a few cases. The mean accuracy for fiducial-based registration was better than for anatomical landmarks (2.8 mm v/s 7.8 mm).
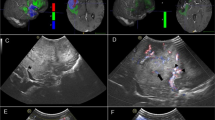
The mini-craniotomy (MC) probe was used for the catheter placement/ cyst drainage procedures employing a single 2.5 centimeter burr hole. For resective surgeries, both linear and phased array probes were used (n = 75). The image resolution was good in 44 (59 %), moderate in 22 (29 %), and poor in 9(12 %) cases where US was used. When we reviewed the latter, we realized that all were because of poor acoustic coupling (calcified or thick dura) or artifacts due to adjacent bone in the skull-base cases. Power Doppler angiography is a very useful function of this system and (Fig. 2) was used in about one-third of the cases, especially in the skull base cases where it often was the predominant intention of using the system.
The impact of the use of the intraoperative imaging system on the course of the surgery is shown in Table 3. In 59 % cases where the system was used with the intention of resection control, further resection was prompted by the newly acquired intraoperative images. In another 21 %, the IOUS showed residual tumor that was not resected because of proximity to various eloquent areas. Extent of resection of malignant gliomas: a separate analysis was conducted in the 51 malignant gliomas. These accounted for 56 % of all the cases. The location of these tumors and histological spectrum is shown in Table 4. Glioblastoma was the single most common tumor (52 %). Half of malignant gliomas were considered resectable (n = 26). Based on the enhancement pattern, 36 of the 51 (70 %) were classified as enhancing tumors (Figs. 3 and 4). GTR was achieved in 29 of the cases overall (47 %). The GTR rate was 88 % for the resectable group (23 of 26) and much lower at 24 % for the unresectable group (6 of 25). Resectability was similar in the enhancing and non-enhancing groups (53 % and 47 % respectively). GTR rates were higher (61.1 %) for the enhancing group than for the non-enhancing ones (47 %) (Table 5).
Enhancing resectable tumor: Preoperative and postoperative contrast enhanced T1 weighted MR images [top and middle rows respectively, axial images (left panel) and coronal images (right panel)]. Bottom row depicts screenshot from the intraoperative ultrasound imaging (left panel showing pre-resection image and center panel showing post resection cavity)
Non-enhancing resectable tumor. Preoperative images (top row): T2 weighted axial (left), post contrast T1 image (center) and pre-resection ultrasound image (right—note the hyperechoic texture and focal increased echogenicity—white arrow). Postoperative images (bottom row): T2 weighted images—axial (right) and coronal (center) and post-resection US image showing no residual tumor
Perioperative outcomes: Neurological deterioration was experienced in seven cases (8 %). Seventeen patients improved (19 %) and the remaining 66 cases maintained their neurological conditions postoperatively. There was no operative mortality.
Discussion
History of 2D IOUS in Neurosurgery
Intraoperative US has been used in neurosurgery since the early 1970s [3]. Our own previous experience with 2D US has been extremely encouraging [11]. However, many limitations with 2D US exist, and these have been highlighted by Unsgård et al. [25]. Advances in image processing and computational capabilities, coupled with refined stereotactic principles, gave birth to navigation in the late 1980s that has transformed the way neurosurgical procedures are performed [9, 30, 31]. There is no doubt that navigation based on preoperatively acquired images (CT or MR) enables the neurosurgeon to confidently plan accurate and small (“tailored”) craniotomies. However, soon after the introduction of navigation, it was evident that once the dura is opened, there is a change in the intracranial anatomy obviating the usefulness of the preoperatively acquired images (the phenomenon called “brainshift”). Moreover, inaccuracies related to image and patient registration add to the problem. Thus, purely navigation-based systems are not enough and intraoperative imaging update is essential. In this setting, intraoperative MR has emerged as the modality of choice. There is unequivocal evidence to support the benefit of IOMR in improving resections as well as in improving overall outcomes [8, 10, 20]. However, his tool still remains beyond reach for a majority of neurosurgical centers, and thus its usefulness cannot be widely applied. This is especially true for resource-constrained settings. The navigable 3D US fills this void, allowing navigation to proceed directly without the need for preoperative images [27]. Admittedly, getting oriented to the US images takes a while. However, as is true of any new technology, after a steep learning curve, the utility and confidence in using it is enormous. In our experience, using the combined mode (preoperative MR plus intraoperative US) initially during the learning phase facilitates the orientation and makes the user more confident. Over a period of time, one can graduate to using direct IOUS images. One big advantage of using US images directly is that it obviates the need for acquiring preoperative MR images. Often (as we see at our referral center), patients come with complete MRI done elsewhere. Repeating an MR for navigation specifically is not always possible and certainly not in the emergency setting. Further, patients with large tumors often have raised intracranial pressure or cognitive deficits and may not always be cooperative for a MR imaging. In such cases, the “3D Direct” mode is very useful. Besides, using the direct mode eliminates the inaccuracies due to image and patient registration. Indeed, in our hands while using the navigation mode of the same system, we had a minimum registration error of 2.8 mm. It may be theoretically possible to minimize this further by meticulous registration techniques; however, it can never be completely eliminated.
Proof of principle
The 3D US has been extensively evaluated by a few groups, the largest experience being from the group at Trondheim that was actively involved in the development of the system, as well as many other centers [13, 14, 23, 27, 28]. The Trondheim group showed (using meticulous histological correlation of biopsies with the US as well as MR images) that navigable 3D US was as good and reliable as navigated MR for delineating high-grade and low-grade gliomas as well as metastases [28]. They reported high specificity and positive predictive values (PPV), indicating the safety of using this system for guiding resections. But they also found a low negative predictive value, implying that when the IOUS was negative, there was a possibility of tumor still being left behind. Future improvements in image resolution capabilities are expected to resolve these issues. Interestingly, the same study also found a higher PPV for low-grade gliomas. In a follow-up study, the same group from Trondheim evaluated the accuracy of the system during the resection (in the subsequent phase of the surgery) [16]. They found that due to imaging artifacts imparted by blood and other changes in the adjacent tissue due to handling, the specificity and PPV dropped. Careful attention during hemostasis and tissue handling, as well as employing strategies to overcome these artifacts, can improve image quality [19]. Considering its potential of use at a fraction of the cost of IOMR, this could be a cost-effective alternative, especially in resource-constrained settings. We were keen to evaluate the practical usefulness of the adjunct in guiding tumor-resections (primarily with respect to the change it necessitated in our resection extent intraoperatively). Not only was it a very reliable guide during resection, but we found that in about 59 % of the cases where it was used with the intention of controlling the resection, it made a positive impact, detecting residual tumors and prompting the surgeon to go ahead with surgery.
Navigable Ultrasound for resection of high-grade gliomas
Solheim et al. have shown that the SonoWand system was effectively used in an unselected consecutive cohort of high-grade gliomas [21]. In this study, they were able to achieve acceptable results (37 % GTR with 13 % morbidity). They also showed that if the intent of surgery was to achieve a radical resection, a GTR was achieved in 55 % (versus only 2 % GTR when the intent was only debulking). Our overall GTR rate of 47 % compares favorably to this. In the same study by Solheim et al., the GTR rate in the “resectable” group was 63 %. Interestingly, in that study, many neurosurgeons (including residents) were part of the operating team, and despite the heterogeneity in surgeon experience and expertise, the rate of GTR of 63 % was fairly high. We were able to achieve higher GTR rates in our subgroup of “resectable” tumors (88 %). This could be primarily because the surgeries were always performed by the two faculty neurosurgeons (AM, PS) only. This rate is comparable to GTR rates achieved using other intraoperative adjuncts such as aminolevulinc acid (ALA)-guided FGR and IOMR [2, 20]. FGR is, however, useful for only enhancing tumors, whereas IOMR and IOUS have no such limitation. Though IOMR may be better, IOUS has the benefit of a significantly lower expense as well as ease of use. Further, Solheim et al. also noted that the system was routinely used in a majority of their surgeries and by a wide range of surgeons (including residents), attesting to the ease-of-use and wide applicability of the technology. In a subsequent study, the same group showed that survival in Glioblastoma multiformes (GBMs) improved in the years after the routine introduction of the SonoWand system [17]. This is encouraging because it means that the learning curve is not as steep as perceived, and more importantly, it can be routinely used without significant logistical and infrastructure constraints. In at least 45 % of all cases (and 59 % of cases where the US mode was used), we were intraoperatively prompted to continue resection thereby reducing residual disease (which would otherwise have been left).
Scope of use
Besides intra-axial brain tumors, the system has been used in spinal surgeries [1, 7], hemangioblastomas [4], meningiomas [6], vascular malformations [26] and many other cases. A special intrasellar probe has also been devised to facilitate trans-sphenoidal use during pituitary surgery [22]. In short, the scope of use is vast, limited only by the ability to insonate and delineate the lesion of interest. It can also be used to guide endoscopic procedures similar to standard navigation systems, with the added benefit of having updated intraoperative US images [29]. Another very unique, powerful and useful application is the Power Doppler function, which permits excellent intraoperative angiograms to be obtained. It has been described to be useful in resection of hemangioblastomas [4], arteriovenous malformations [26] and even other tumors [15]. We have found this extremely useful in visualizing major vessels around tumors especially skull-base cases, (Fig. 2). Advanced image processing (functional MR data as well as diffusion images) is also possible [12]. In the future, improvements in image resolution and enhancement of combined modality image processing are likely to widen the scope of its use.
Limitations
Intraoperative ultrasound, though useful, has its limitations. Lack of a full-head view results in difficulties in orientation of the image and can potentially limit its usefulness. Further, the usefulness is reliant on a good quality image, acquiring of which requires experience and attention to details. Artifacts during the resection may compromise the image accuracy. Other intraoperative visualization techniques like fluorescence guided-resections may be complementary to it and need to be considered [24]. Lastly, the ultrasound images lack functional information and intraoperative clinical electrophysiological functional monitoring cannot be replaced, especially in close proximity to eloquent structures.
Conclusions
The navigable 3D US system is a very useful intraoperative image guidance tool in neuro-oncology, often facilitating better and radical resections. Despite a learning curve, it is easy to use and has a varied scope of applications without significant increase in costs. It facilitates safe radical resections in malignant gliomas and could serve as a cost-efficient alternative to intraoperative MRI in resource-constrained settings.
Abbreviations
- IOUS:
-
Intraoperative Ultrasound
- 2D:
-
Two dimensional
- 3D:
-
Three dimensional
- IOMR:
-
Intraoperative Magnetic resonance Imaging
- CT:
-
Computed Tomography
- ALA:
-
Aminolevulinc acid
- PD:
-
Power doppler
References
Bonsanto MM, Metzner R, Aschoff A, Tronnier V, Kunze S, Wirtz CR (2005) 3D ultrasound navigation in syrinx surgery—a feasibility study. Acta Neurochir 147:533–540, discussion 540–531
Diez Valle R, Tejada Solis S, Idoate Gastearena MA, Garcia de Eulate R, Dominguez Echavarri P, Aristu Mendiroz J (2011) Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neuro-Oncol 102:105–113
Dohrmann GJRJ (2001) History of intraoperative ultrasound in neurosurgery. Neurosurg Clin N Am 12:155–166
Glasker S, Shah MJ, Hippchen B, Neumann HP, van Velthoven V (2011) Doppler-sonographically guided resection of central nervous system hemangioblastomas. Neurosurgery 68:267–275, discussion 274–265
Gronningsaeter A, Kleven A, Ommedal S, Aarseth TE, Lie T, Lindseth F, Lango T, Unsgard G (2000) SonoWand, an ultrasound-based neuronavigation system. Neurosurgery 47:1373–1379, discussion 1379–1380
Jakola AS, Gulati M, Gulati S, Solheim O (2012) The influence of surgery on quality of life in patients with intracranial meningiomas: a prospective study. J Neuro-Oncol 110:137–144
Kolstad F, Rygh OM, Selbekk T, Unsgaard G, Nygaard OP (2006) Three-dimensional ultrasonography navigation in spinal cord tumor surgery. Technical note. J Neurosurg Spine 5:264–270
Kubben PLTMKJ, Schijns OEMG, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H (2011) Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12:1062–1070
Kurimoto M, Hayashi N, Kamiyama H, Nagai S, Shibata T, Asahi T, Matsumura N, Hirashima Y, Endo S (2004) Impact of neuronavigation and image-guided extensive resection for adult patients with supratentorial malignant astrocytomas: a single-institution retrospective study. Minim Invasive Neurosurg : MIN 47:278–283
Mehdorn HMSF, Dawirs S, Hedderich J, Dörner L, Nabavi A (2011) High-field iMRI in glioblastoma surgery: improvement of resection radicality and survival for the patient? Acta Neurochir Suppl 109:103–106
Moiyadi A, Shetty P (2011) Objective assessment of utility of intraoperative ultrasound in resection of central nervous system tumors: a cost-effective tool for intraoperative navigation in neurosurgery. J Neurosci Rural Pract 2:4–11
Rasmussen IA Jr, Lindseth F, Rygh OM, Berntsen EM, Selbekk T, Xu J, Nagelhus Hernes TA, Harg E, Haberg A, Unsgaard G (2007) Functional neuronavigation combined with intra-operative 3D ultrasound: initial experiences during surgical resections close to eloquent brain areas and future directions in automatic brain shift compensation of preoperative data. Acta Neurochir 149:365–378
Rohde VCVA (2011) Intraoperative 3-dimensional ultrasound for resection control during brain tumour removal: preliminary results of a prospective randomized study. Acta Neurochir Suppl 109:187–190
Roth J, Biyani N, Beni-Adani L, Constantini S (2007) Real-time neuronavigation with high-quality 3D ultrasound SonoWand in pediatric neurosurgery. Pediatr Neurosurg 43:185–191
Rygh OM, Nagelhus Hernes TA, Lindseth F, Selbekk T, Brostrup Muller T, Unsgaard G (2006) Intraoperative navigated 3-dimensional ultrasound angiography in tumor surgery. Surg Neurol 66:581–592, discussion 592
Rygh OM, Selbekk T, Torp SH, Lydersen S, Hernes TA, Unsgaard G (2008) Comparison of navigated 3D ultrasound findings with histopathology in subsequent phases of glioblastoma resection. Acta Neurochir 150:1033–1041, discussion 1042
Saether CA, Torsteinsen M, Torp SH, Sundstrom S, Unsgard G, Solheim O (2012) Did survival improve after the implementation of intraoperative neuronavigation and 3D ultrasound in glioblastoma surgery? A retrospective analysis of 192 primary operations. J Neurol Surg Part A Cent Eur Neurosurg 73:73–78
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8
Selbekk T, Jakola AS, Solheim O, Johansen TF, Lindseth F, Reinertsen I, Unsgard G (2013) Ultrasound imaging in neurosurgery: approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir 155:973–980
Senft CBA, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003
Solheim O, Selbekk T, Jakola AS, Unsgard G (2010) Ultrasound-guided operations in unselected high-grade gliomas–overall results, impact of image quality and patient selection. Acta Neurochir 152:1873–1886
Solheim O, Selbekk T, Lovstakken L, Tangen GA, Solberg OV, Johansen TF, Cappelen J, Unsgard G (2010) Intrasellar ultrasound in transsphenoidal surgery: a novel technique. Neurosurgery 66:173–185, discussion 185–176
Steno A, Karlik M, Mendel P, Cik M, Steno J (2012) Navigated three-dimensional intraoperative ultrasound-guided awake resection of low-grade glioma partially infiltrating optic radiation. Acta Neurochir 154:1255–1262
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicenter phase III trial. Lancet Oncol 7:392–401
Unsgaard G, Gronningsaeter A, Ommedal S, Nagelhus Hernes TA (2002) Brain operations guided by real-time two-dimensional ultrasound: new possibilities as a result of improved image quality. Neurosurgery 51:402–411, discussion 411–402
Unsgaard G, Ommedal S, Rygh OM, Lindseth F (2005) Operation of arteriovenous malformations assisted by stereoscopic navigation-controlled display of preoperative magnetic resonance angiography and intraoperative ultrasound angiography. Neurosurgery 56:281–290
Unsgaard G, Rygh OM, Selbekk T, Muller TB, Kolstad F, Lindseth F, Hernes TA (2006) Intra-operative 3D ultrasound in neurosurgery. Acta Neurochir 148:235–253, discussion 253
Unsgaard G, Selbekk T, Brostrup Muller T, Ommedal S, Torp SH, Myhr G, Bang J, Nagelhus Hernes TA (2005) Ability of navigated 3D ultrasound to delineate gliomas and metastases–comparison of image interpretations with histopathology. Acta Neurochir 147:1259–1269, discussion 1269
Unsgard G, Solheim O, Lindseth F, Selbekk T (2011) Intra-operative imaging with 3D ultrasound in neurosurgery. Acta Neurochir Suppl 109:181–186
Willems PW, Taphoorn MJ, Burger H, Berkelbach van der Sprenkel JW, Tulleken CA (2006) Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg 104:360–368
Wirtz CR, Albert FK, Schwaderer M, Heuer C, Staubert A, Tronnier VM, Knauth M, Kunze S (2000) The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res 22:354–360
Acknowledgments
We would like to thank all the members of our neuro-oncology working group and Mr. Thomson Verghese (clinical nurse coordinator) for assistance in the clinical work and data collection.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Medial frontal glioblastoma. Snapshot from the navigation screen showing the post contrast MR image (left) and corresponding Us image (center). Note the US image shows hyperechoic tumor beyond the contrast enhancing MR tumor component. Also, the ventricular system is very well delineated on the US image, facilitating orientation. (JPEG 195 kb)
Supplementary Fig. 2
Posterior frontal glioma. Snapshot from the navigation screen. Left panel shown the MR image with a white outlined rectangle denoting the sector scanned by the US. The corresponding US image is shown in the center panel. It depicts the structural heterogeneity of the tumor. Also noteworthy is the brainshift that is apparent as compared to the preoperative MRI. (JPEG 174 kb)
Supplementary Fig. 3
Medial frontal glioma. Snapshot from the navigation screen. Left panel shows the preoperative contrast enhanced MR images in “dual anyplane” view. The center panel shows the corresponding US images. Note that the tumor is hyperechoic (and therefore not cystic but containing solid necrotic areas), though the MR shows a hypointense non-enhancing central core. (JPEG 239 kb)
Supplementary Fig. 4
Parietal Glioma. Snapshot from the navigation screen. Left panel shows the preoperative contrast enhanced MR images in “dual anyplane” view. The center panel shows the corresponding US images. Note that the tumor is hypoechoic (and therefore cystic) as reflected in the MR, which shows a hypointense non-enhancing central core. (JPEG 193 kb)
Rights and permissions
About this article
Cite this article
Moiyadi, A.V., Shetty, P.M., Mahajan, A. et al. Usefulness of three-dimensional navigable intraoperative ultrasound in resection of brain tumors with a special emphasis on malignant gliomas. Acta Neurochir 155, 2217–2225 (2013). https://doi.org/10.1007/s00701-013-1881-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1881-z