Abstract
Background
The use of IOUS is increasingly common in recent neurosurgical practice. IOUS has become very valuable in neurosurgery. It plays a key role in the localization of space-occupying lesion location and decreasing operation time and hence improves both surgical efficiency and safety. Ultrasound is very useful in the determination of the lesion location, its most superficial portion, and in differentiation between solid tumors and cystic components.
Results
Intraoperative ultrasonography has a significant edge over the other intraoperative aids for image guidance in brain surgery, especially in terms of independence, cost, and adaptability to multiple different clinical scenarios. Ultrasound-based neuro-navigation is an easy-to-use, fast, and safe technique of real-time imaging for various neurosurgical procedures.
Conclusion
We conclude that ultrasound-based neuro-navigation is an easy-to-use, fast, and safe technique of real-time imaging for various neurosurgical procedures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
The use of ultrasound in brain space-occupying lesions excision is an alternative tool to intraoperative navigation or magnetic resonance imaging (MRI) for delineating tumor borders and improving the chances of radical excision [1].
The purpose of brain lesions excision is maximal resection while preserving healthy tissues. The extent of resection is a prognostic factor in survival time, functional recovery, and tumor recurrence rates [2].
The residual lesional volume may persist postoperatively, which may result in disease recurrence, as a result of the erroneous association between preoperative imaging and intraoperative anatomy as well as the inadequate delineation of certain lesions from normal tissue. To avoid these, better delineation of normal from pathological tissue intraoperatively may improve outcomes such as the increased chance of radical excision and prevention of neural damage [3].
Intraoperative ultrasound imaging can detect significant lesions residual. MRI systems are time-consuming and of high cost and not available in our center [1, 4].
Patients and methods
Study design
This is a prospective randomized study conducted on 20 patients admitted to the neurosurgery department at Fayoum university hospital with intracranial space-occupying lesions in the period from June 2018 till April 2020 operated upon by using intraoperative ultrasound; the efficacy it is using in achieving radical excision or aspiration of the lesions was assessed according to lesion size and amount of residual in the postoperative CT or MRI in comparison with the preoperative radiological studies.
Ultrasound equipment
Intraoperative ultrasound was performed with Philips ClearVue 350 ultrasonography system by using L12-4 linear and C5-2 convex active probes
Inclusion criteria
All patients with intracranial space-occupying lesions are indicated for surgery, with no sex or age predilection.
Exclusion criteria
Recurrent brain space-occupying lesions, the presence of pneumocephalus, and patients who received chemotherapy or radiotherapy preoperative.
Imaging studies
CT brain and/or MRI brain with or without IV contrast was done on patients
The lesion volume was measured from the CT and MRI brain using the modified McDonald’s criteria.
Counseling and consent
Patients were informed about the underlying neurological problem, the role of surgery, surgical technique, postoperative care, and expected mortality and morbidity and their percentages. The consent was in detail and written.
Preoperative medications
Phenytoin was used with a loading dose of 15 mg/kg followed by a maintenance dose of 5–8 mg/kg in supratentorial lesions, antibiotics (third-generation cephalosporin) at the induction of the anesthesia (2 gm), and continued for at least 3 days after surgery 1 gm every 12 h.
Operative technique
Intraoperative ultrasound was performed by the operator for assessment of lesion dimensions, relations, and echogenicity differentiation according to the location and depth of the lesion to the probe applied. The transducer and cord were placed in a transparent plastic surgical sterile sheath, and irrigation of the operative field with saline was done for proper transmission of the acoustic beam between the sheathed scan head and the dura.
The sheathed scan head was placed on the dura for scanning before opening the dura mater where we identified the site and size of the lesion before any brain shift, which could result from CSF drainage after dural opening. The distance from the pathology to the dura, the location, and the size of the pathology was measured and compared with preoperative available MRI and CT images.
Dural opening then follows, and the scan head was placed directly to the surface of the brain tissue for further scanning during which continuous irrigation with saline was performed.
Localization of the pathology, its consistency, and borders with the normal brain were studied, followed by colored mode scanning to identify any close vascular structures the returning B-mode scan to look for any relation of the lesion with nearby anatomical landmarks like the ventricles or the tent.
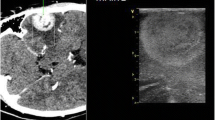
After collecting these data, we started tumor excision from the nearest safe part as the tumor debulking proceeded, and repeated scanning was performed to evaluate any residual and its new relation to surrounding structures. After assuming total excision, another scan was performed after filling the cavity with sterile saline to get an idea about the size of the created cavity and compare it to the original size and any residue was looked for and excised (Figs. 1, 2, 3, 4).
Postoperative care
Drains were removed within 48 h postoperatively. Intravenous antibiotic continued for 3 days, and a maintenance dose of antiepileptic drugs and fluids. Routine postoperative dressings were changed every 48 h, and the sutures were removed 10–14 days after the operation.
Follow-up and evaluation after operation
Follow-up CT scan and MRI were done within 48 h postoperatively. The pathology size preoperative was compared to postoperative size. The accuracy of excision and presence of residual were documented for each patient.
Statistical analysis
-
Data were collected and coded to facilitate data manipulation and double entered into Microsoft Access, and data analysis was performed using Statistical Package of Social Science (SPSS) software version 18 in windows 7.
-
Simple descriptive analysis in the form of numbers and percentages for qualitative data, and arithmetic means as central tendency measurement, standard deviations as a measure of dispersion for quantitative parametric data.
-
For qualitative data
-
Chi-square test to compare two of more than two qualitative groups.
-
-
Sensitivity and specificity test for testing a new test with ROC curve “Receiver Operating Characteristic.”
-
The P value ≤ 0.05 was considered the cutoff value for significance.
Results
The mean age of the study group was (44.86 ± 19.5) years old ranging between 13 and 75 years old, as regards sex distribution 55% were males, and 45% were females.
Our study illustrates that 25% of the group diagnosed with ICH and high-grade glioma, followed by 15% diagnosed with low-grade glioma and 10% with meningioma, epidermoid cyst and abscess followed by 5% for metastasis.
Classification of our lesions consistency: 40% of the study group had a solid lesion, and 25% had a hemorrhage, then 25% showed cystic lesions, and 10% were heterogeneous (solid and cystic)
Anatomical site of lesions: 40% of the study group showing lesions in the parietal area, 30% in the frontal area, 20% in the posterior fossa, and finally 10% in the temporal area.
Size of lesions
The mean lesion size by CT/MRI was (34.5 ± 15.4) cm ranging between 13 and 80 cm, but using the US the mean lesion size was (34.62 ± 15.5) cm ranging between 12 and 81 cm (Fig. 5).
There is a statistically significant difference with a p value < 0.05 between two radiological diagnoses of brain lesion residual with a high percentage of false-negative results noted among ultrasound results (31.3%) (Fig. 6).
Sensitivity and specificity test ultrasound in comparison with CT/MRI in the detection of residual brain lesion during surgery with sensitivity (100%) and specificity (68.8%), with total accuracy of (84.4%) (Fig. 7).
The postoperative assessment revealed that 5 cases of positive intraoperative US residues and also 11 cases with negative residues were confirmed through postoperative CT/MRI and 4 cases (31%) revealed positive postoperative CT/MRI residues; however, negative intraoperative US residue sounds that IOUS is a good sensitive procedure (Figs. 8, 9).
Discussion
As a result of various illnesses such as infections, hematomas, and tumors containing proteinaceous fluid and/or cellular/necrotic components, intracranial lesions are described as artificial spaces in which the continuity of the brain parenchyma is interrupted [5].
It has been suggested that the use of intraoperative ultrasound may ease intraoperative delineation and facilitate the extent of resection of various intracranial lesions [6,7,8,9,10,11,12].
Intraoperative imaging has considered as one of the most important adjuncts in neurosurgery, especially in the surgical treatment of gliomas. Navigation and intraoperative magnetic resonance imaging have limitations, and intraoperative ultrasonography (IOUS) has emerged as a versatile and multifaceted alternative [8].
Ultrasound imaging is an effective method in many situations, such as locating tumors, cysts, and necrosis, defining borders and guiding the surgeon to the target and identifying possible tumor remnants [13,14,15,16].
In this study, we reviewed the ultrasound uses in brain surgery as a method of neuro-navigation. We discussed the advantage of intraoperative ultrasound in the detection of residual brain lesions and its help in achieving radical resection and evacuation of brain pathologies.
When the transmitted ultrasound pulse meets boundaries between tissues or structures, for example, between normal brain tissue and a tumor, the pulse is partially reflected backward and partially transmitted forward. Variations in acoustic impedance (Z) between two materials cause such reflection of the ultrasound, i.e., echo back toward the probe. The acoustic impedance (Z) is given by mass density (ρ) and the sound velocity (c):
If ultrasound propagates to tissue with higher acoustic impedance than the surroundings, such as most brain tumors, the magnitude of back-scattered signals makes the lesion appear brighter than the surrounding tissue, so-called hyperechoic. If ultrasound spread through interfaces to soft tissues with lower acoustic impedance, such as cysts or necrotic tumor tissue, such lesions appear darker than the surrounding brain tissue, i.e., hypoechoic. If ultrasound spreads through a lesion with the same acoustic impedance as the adjacent tissue, no delineation of the lesion will be possible as the lesion is isoechoic [17].
Quick and accurate localization of impalpable pathology in critical anatomic areas may decrease anxiety over iatrogenic damage and increase confidence in craniotomy and durotomy placement. An additional impact of the technique was found in its ability to assess for residual tumor at the presumed conclusion of dissection by comparing pre- and post-resection appearance, and we were able to find echogenic material consistent with a residual tumor [18].
To clarify this issue, we studied the value of IOUS “Intraoperative ultrasonography” in guiding us for total excision and evacuation of brain lesions and identifying any residual intraoperative and comparing its accuracy with postoperative CT and MRI.
In a small series of 7 patients, intraoperative 3D ultrasound was compared to 0.2 Tesla intraoperative MRI. Detection of metastases and high-grade gliomas and intraoperative delineation of tumor remnants were comparable in the two imaging modalities. In one instance with low-grade glioma, ultrasonography improved visualization. Even yet, it was determined that intraoperative MR is better than intraoperative ultrasonography in terms of resection control in glioma surgery because intraoperative observations following resection were difficult to interpret [19].
In our study, we performed craniotomy in all cases, however, the difference in their pathological nature, to apply the probe directly to the dura with continuous irrigation with saline. US-guided localization via a bayonet-shaped ultrasound probe through burr holes is used in Shimizu et al. study; this probe was not available at our institute [20].
In El Beltagy et al. study, they had a radiologist to perform the US imaging intraoperative. This was not the case in our study, and the neurosurgeon performed the US imaging himself [21].
In our study, we used two ultrasound probes, while Velthoven et al. used multiple probes, and on the other hand, Erdogan et al. depended on a single probe assessment [6, 22].
Our study included only 20 patients, in comparison with other studies such as Theophilo et al. where they studied only 4 cases, El Beltagy et al. studied 20 patients, and Erdogan et al. studied 32 patients, Strowitzki et al. studied 100 cases, and this was an average sample size considering time table [6, 21, 23, 24].
Despite male predominance, there was no observable difference in the prognosis between males and females. The difference in pathological entities didn’t affect the study outcome in our study or other studies.
Our study involved a wide variety of different-sized lesions from small to huge brain lesions; however, some studies focused on certain sized lesions as in James et al. which were concerned with small-sized lesions up to 10 cm [18].
The postoperative assessment revealed that 5 cases of positive intraoperative US residues and also 11 cases with negative residues were confirmed through postoperative CT/MRI and 4 cases (31%) revealed positive postoperative CT/MRI residues; however, negative intraoperative US residue sounds that IOUS is a good sensitive procedure.
Conclusions
We conclude that ultrasound-based neuro-navigation is an easy-to-use, fast, and safe technique of real-time imaging for various neurosurgical procedures.
The range of intracranial conditions for which intraoperative sonography may be useful requires still further evaluation, but the following advantages of the procedure are already apparent: It provides accurate identification and localization of brain masses of widely varying types, with a definite reduction in the degree of brain exploration and size of dural incisions and, thereby, presumably in surgical morbidity.
It offers instantaneously visible and accepted guidance for achieving radical brain lesions excision and evacuation in the absence of complex radiological devices. It features portability, low cost, lack of ionizing radiation, and the use of equipment already available in many centers. In the hands of an operator with experience in US image acquisition and interpretation, it can be a useful tool for the radical surgical excision of brain lesions.
In terms of independence, affordability, and adaptation to various clinical settings, IOUS offers a clear advantage over the other intraoperative aids for image guiding in brain surgery.
Availability of data and material
Available upon request.
Abbreviations
- IOUS:
-
Intraoperative ultrasonography
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- IV:
-
Intravenous
- Mg:
-
Milligrams
- Kg:
-
Kilograms
- Gm:
-
Grams
- CSF:
-
Cerebrospinal fluid
- SPSS:
-
Statistical Package of Social Science
- ROC:
-
Receiver operating characteristic
- ICH:
-
Intracerebral hematoma
- US:
-
Ultrasonography
- 3D:
-
Three-dimensional
- MR:
-
Magnetic resonance
References
Unsgaard G, Ommendal S, Muller T, Gronningsaeter A, Hernes TAN. Neuronavigation by intraoperative three-dimensional ultrasound: initial experience during brain tumor resection. Neurosurgery. 2002;50(4):804–12.
Almenawer SA, Badhiwala JH, Alhazzani W, Greenspoon J, Farrokhyar F, Yarascavitch B, Algird A, Kachur E, Cenic A, Sharieff W, Klurfan P, Gunnarsson T, Ajani O, Reddy K, Singh SK. Murty NK: biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and metaanalysis. Neuro Oncol. 2015;17(6):868–81.
Zhang ZZ, Shield LBE, Sun DA, Zhang YP, Hunt MA, Christopher BS. The art of intraoperative glioma identification. Front Oncol. 2015;5(175):1–7.
Gronningsaeter A, Kleven A, Ommedal S, Aarseth TE, Lie T, Lindseth F, Lango T, Unsgaard G. Sono Wand: an ultrasound-based neuronavigation system. Neurosurgery. 2000;47(6):1373–80.
Reddy JS, Mishra AM, Behari S, Husain M, Gupta V, Rastogi M, Gupta RK. The role of diffusion-weighted imaging in the differential diagnosis of intracranial cystic mass lesions: a report of 147 lesions. Surg Neurol. 2006;66:246–51.
Erdogan N, Tucer B, Mavili E, Menku A, Kurtsoy A. Ultrasound guidance in intracranial tumor resection: correlation with postoperative magnetic resonance findings. Acta Radiol. 2005;46:743–9.
Gulati S, Berntsen EM, Solheim O, Kvistad KA, Haberg A, Selbekk T, Torp SH, Unsgaard G. Surgical resection of high-grade gliomas in eloquent regions guided by blood oxygenation level dependent functional magnetic resonance imaging, diffusion tensor tractography, and intraoperative navigated 3D ultrasound. Minim Invasive Neurosurg. 2009;52:17–24.
Yeole U, Singh V, Mishra A, Shaikh S, Shetty P, Moiyadi A. Navigated intraoperative ultrasonography for brain tumors: a pictorial essay on the technique, its utility, and its benefits in neuro-oncology. Ultrasonography. 2020;39(4):394–406.
Sastry R, Bi WL, Pieper S, Frisken S, Kapur T, Wells W 3rd, Golby AJ. Applications of ultrasound in the resection of brain tumors. J Neuroimaging. 2017;27(1):5–15.
Lindner D, Trantakis C, Renner C, Arnold S, Schmitgen A, Schneider J, Meixensberger J. Application of intraoperative 3D ultrasound during navigated tumor resection. Minim Invasive Neurosurg. 2006;49:197–202.
Unsgaard G, Gronningsaeter A, Ommedal S, Nagelhus Hernes TA. Brain operations guided by real-time two-dimensional ultrasound: new possibilities as a result of improved image quality. Neurosurgery. 2002;51:402–11.
Woydt M, Krone A, Becker G, Schmidt K, Roggendorf W, Roosen K. Correlation of intra-operative ultrasound with histopathologic findings after tumour resection in supratentorial gliomas. A method to improve gross total tumour resection. Acta Neurochir (Wien). 1996;138:1391–8.
Chacko AG, Kumar NK, Chacko G, Athyal R, Rajshekhar V. Intraoperative ultrasound in determining the extent of resection of parenchymal brain tumours–a comparative study with computed tomography and histopathology. Acta Neurochir (Wien). 2003;145(9):743–8.
Mursch K, Scholz M, Brück W, Behnke-Mursch J. The value of intraoperative ultrasonography during the resection of relapsed irradiated malignant gliomas in the brain. Ultrasonography. 2017;36(1):60–5.
Zhang G, Li Z, Si D, Shen L. Diagnostic ability of intraoperative ultrasound for identifying tumor residual in glioma surgery operation. Oncotarget. 2017;8(42):73105–14.
Sutcliffe JF. Review article: the value of intraoperative ultrasound in neurosurgery. Br J Neurosurg. 1991;5:169–78.
Rygh OM, Selbekk T, Torp SH, Lydersen S, Hernes TA, Unsgaard G. Comparison of navigated 3D ultrasound findings with histopathology in subsequent phases of glioblastoma resection. Acta Neurochir (Wien). 2008;150:1033–41.
Šteňo A, Buvala J, Babková V, Kiss A, Toma D, Lysak A. Current limitations of intraoperative ultrasound in brain tumor surgery. Front Oncol. 2021;22(11):659048.
Tronnier VM, Bonsanto MM, Staubert A, Knauth M, Kunze S, Wirtz CR. Comparison of intraoperative mr imaging and 3d-navigated ultrasonography in the detection and resection control of lesions. Neurosurg Focus. 2001;10:E3.
Shimizu S, Mochizuki T, Osawa S, Kumabe T. Intraoperative ultrasonography during drainage for chronic subdural hematomas: a technique to release isolated deep-seated hematomas. Neurol Med Chir. 2015;55(9):761–5.
El Beltagy MA, Aggag M, Kamal M. Role of intraoperative ultrasound in resection of pediatric brain tumors. Child Nerv Syst. 2010;26:1189–93.
Velthoven V, Auer L. practical application of intraoperative ultrasound. ACTA Neurochir. 1991;10:5–13.
Strowitzki M, Moringlane JR, Steudel WI. Ultrasound-based navigation during intracranial burrhole procedures: experience in a series of 100 cases. Surg Neurol. 2000;54(2):134–44.
Theophilo F, Burnett A, Jucá Filho G, Adler A, Miranda S, Theophilo L, Carvalho M, Lopes J. Ultrasound-guided brain abscess aspiration in neonates. Child Nerv Syst. 1987;3(6):371–4.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors shared with operating patients and data collection.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been revised and approved by the ethical committee of the Faculty of Medicine, Cairo University. The consent to participate has been taken and approved from all participants in this study.
Consent for publication
Consent for publication has been obtained and approved from all individuals included in this study. All authors confirm the ethical committee approval, obtained consent to participate, and obtained consent for publications. Mahmoud Ahmed Gomaa, M.D Mohamed Ahmed Hussein, M.D Mohamed Abdellatif Hussein, M.D Ashraf Abdellatif Osman, M.D The authors confirmed the above section to be sent to JDE and, if appropriate, added to the methods section wherever suitable.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gomaa, M.A., Hussein, M.A., Hussein, M.A. et al. Role of intraoperative cranial ultrasonography in detection of residual brain lesions during surgery. Egypt J Neurosurg 39, 60 (2024). https://doi.org/10.1186/s41984-024-00315-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-024-00315-3