Abstract
This article describes a sensitive and selective fluorometric method for the determination of Salmonella enteritidis by exploiting the polymerase activity of the Klenow fragment and dual fluorescence. First, one end of a target-selective aptamer was labeled with the fluorophore 6-carboxyfluorescein (FAM). Once the labeled aptamer binds to graphene oxide (GO) via π-stacking interaction, the fluorescence of FAM is quenched. However, the addition of target (16S rRNA) leads to the restoration of fluorescence due to the binding of probe and target which shifts the FAM fluorophore away from the quenching GO. By using the Klenow fragment and by exploiting the synergistic effect of FAM and the DNA probe SYBR Green I (which is strongly fluorescent in presence of dsDNA only), fluorescence is strongly amplified and sensitivity improved. The analyte 16SrRNA can be determined by this method in the 60 pM to 100 nM concentration range, and the detection limit is 60 pM. It is also shown that Salmonella enteritidis can be determined in milk samples by this method in concentrations between 102 to 105 cfu⋅mL‾1, with a detection limit of 300 cfu⋅mL‾1. This assay displays high sensitivity and selectivity and may possess wide applications in pathogen detection.

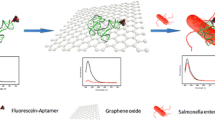
If labeled aptamer against Salmonella enteritidis 16S rRNA binds to graphene oxide GO) by π-interaction, the fluorescence of the label is quenched. The addition of target rRNA leads to the restoration of fluorescence, and this effect can be used to quantify Salmonella enteritidis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonella enteritidis, as a foodborne pathogen, is a major cause of gastroenteritis, diarrhea and pain. Its infections cause morbidity, mortality, and economic burden and are particularly severe in the infant, elderly, or immunocompromised patient. The infections caused by Salmonella enteritidis are steadily increasing in incidence and geographic scope [1]. There are annually 3 million deaths due to Salmonella infections worldwide [2], Salmonella serotype Typhimurium and Salmonella serotype Enteritidis are the most prevalent [3].
Although there are various available detecting techniques for this pathogen, which are culture and colony counting [4], enzyme linked immunosorbent assay [5] and polymerase chain reaction [6, 7], most of them are complex and need lengthy process [8]. Besides, the bacteria may be present in tiny numbers and many related bacteria can be found within the same sample, hence enrichment steps are necessary [9]. The polymerase chain reaction (PCR) has been accepted as gold standard for detection of S. enteritidis [10]. However, it is laborious and time-consuming, and needs sophisticated and expensive equipments and skilled operators. In addition, it may give false positives because of nonspecific amplification during the complicated thermal cycling steps [11]. Recently, many biosensors were developed by combining biological receptor compounds with transducer directing, such as surface plasmon resonance (SPR) devices, modified carbon electrode and glassy carbon electrode. They were able to detect S. enteritidis with high performance, nevertheless, the high cost and complex process of the preparation of materials remained obstacles [12–14]. Therefore, a more rapid, simple, sensitive and selective detection technology to identify this pathogen is desirable.
Fluorescent in situ hybridization (FISH) is based on the specific binding of a nucleic acid probe. With 16S rRNA-targeted probes, this staining technique would allow phylogenetic identification of bacteria without prior cultivation or DNA extraction [15]. rRNA molecules have been used to gain the detecting ability of sensors since they allow the design of taxon-specific oligonucleotides [16]. Accordingly, hybridization using oligonucleotides directly in sensors for 16S rRNA might be a prominent way to detect S. enteritidis much more rapidly than current methods.
Graphene oxide (GO) is a two-dimensional carbon material consists of sp2-bonded carbon with a hexagonal configuration. GO has a large surface area for loading nucleic acids, proteins, and some small inorganics via π-interactions or chemical modification [17–19], it may also serve as an excellent quencher in Dexter mechanism because of its broad absorption spectrum and high quenching efficiency. Consequently, GO is an ideal material for biosensor-based device constructing and has been widely used in detection applications [20, 21].
Several methods to increase detection sensitivity were designed [22, 23]. As an unsymmetrical cyanine dye, SYBR Green I has no fluorescence when single-stranded DNA (ssDNA) was only present. However, the fluorescence signals will be greatly enhanced when it binds to double strand DNA (dsDNA) [24]. The maximum excitation wavelength and maximum emission wavelength of carboxyfluorescein group (FAM) and SYBR Green I are both very close, and the fluorescence peaks of these two dyes overlap completely. The sensitivity of the detection method is greatly improved when it was combined with these two dyes [25].
Here, an aptasensor based on GO and signal amplification for the determination of Salmonella enteritidis was designed, in which one end was labeled with the fluorophore 6-Carboxyfluorescein (FAM). When the FAM-aptamer bound to the GO by π-interaction, fluorescence of FAM was quenched via a Dexter mechanism. The addition of target (16S rRNA) led to the restoration of fluorescence due to the binding of aptamer and target, which keep FAM away from the GO. Additionally, both of Klenow Fragment and SYBR Green I were used to amplify the fluorescence signal. The detection approach not only has a lower limitation and good selectivity, but also can detect its targeted bacteria rapidly. These advantages such as simplicity, sensitivity and high efficiency, make it a promising prospects in pathogen detection.
Material and methods
Chemicals and materials
A specific sequence was chosen from the V3-V6 region of 16S rRNA, and updated from the NCBI gene bank. Oligonucleotides were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China, http://www.sangon.com), as shown in Table 1. The sequences were dissolved in TE buffer to prepare 100 μM stocking solution. Graphene oxide was purchased from Xianfeng Materials Technology (Nanjing, China http://www.xfnano.com). UNlQ-10 Column TRIzol Total RNA Isolation Kit, Klenow Fragment (10 U·μL−1) and 10 × reaction buffer for Klenow Fragment (500 mM Tris–HCl, 50 mM MgCl2, 10 mM DTT, pH 8.0) were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). SYBR Green I was obtained from Fanbo Biochemical Co., Ltd. (Beijing, China). Water was purified before use by a Milli-Q water purification system.
Apparatus
All fluorescence measurements and spectra were performed using a LS45 fluorescence spectrophotometer (Perkin–Elmer, UK). Excitation and emission slits were both set for a 10.0 nm band-pass. The fluorescence was excited at 495 nm and emission spectra were collected from 500 to 700 nm. The water was purified by an Ultra-pure water system (Millipore, Boston, MA, USA).
Preparation of graphene oxide
Graphene oxide powder was dissolved in deionized water and subjected to ultrasonication for 150 min (300 W, 20 % amplitude). The homogeneous GO aqueous dispersion (1 mg·mL−1) was obtained and stored at room temperature prior to use.
Determination of target sequence in water
The analyte of 16S rRNA (10−7 mol) was dissolved in water (1 mL) prior to use. 1 μL of FAM-aptamer suspended in Tris-HCl (pH 7.5) and 10 μL of GO solution were added to a 1.5 mL centrifuge tube. The mixture was left at 37 °C for 10 min to allow the GO absorb the aptamers. Then, Klenow Fragment (0.5 μL), 10 × reaction (2 μL) buffer and SYBR Green I (2 μL) were added, followed by different concentrations of analyte of 16S rRNA with the resulting solution to obtain a final volume of 100 μL. The same reaction mixture without target sequence was used as a control. After the solution was incubated at 37 °C for 30 min to carry out the fluorescence signal amplification reaction, the fluorescence was detected (Reaction solutions were excited at 495 nm and emission spectra were collected from 500 to 700 nm. Excitation and emission slits were both set with a band pass of 10.0 nm).
Application in milk sample
S. enteritidis were mixed with milk (1.0 mL) in different concentrations respectively. After they were equilibrated at room temperature for 10 min, the mixtures were subjected to centrifugation (1700 g) for 10 min and the sediments were transferred into different tubes. Subsequently, the RNA was extracted from the sedimentary cells using UNlQ-10 Column TRIzol Total RNA Isolation Kit. FAM-aptamer suspended in Tris-HCl and GO solution was added. After the mixture was left at 37 °C for 10 min, Klenow Fragment, 10 × reaction buffer and SYBR Green I were added, followed by the extracted RNA of different concentrations of S. enteritidis with the resulting solution to obtain a final volume of 100 μL. The same reaction mixture without extracted RNA was used as a control. Finally, solution was incubated at 37 °C for 30 min and then the fluorescence was measured.
Detection specificity
S. typhimurium, S. paratyphi A and E. coli, ETEC K88 were used to determine the specificity of the method. The bacteria were diluted to 6 × 103 CFU·mL−1 and centrifugated (1700 g) for 10 min. The sediments were transferred into different tubes and their RNA was extracted from the sedimentary cells using UNlQ-10 Column TRIzol Total RNA Isolation Kit respectively. The remaining procedures were as described above.
Sensitivity comparison of different dyeing strategies
To evaluate the synergistic effect of using FAM and SYBR Green I simultaneously, three determination experiments with different dyeing strategies were performed. The first experiment used both FAM and SYBR Green I, the second one was performed without SYBR Green I. The third one was carried out with SYBR Green I only by using an unlabeled aptamer and the rest process was as described above.
Results and discussion
Design of the method
As shown in Fig. 1, the aptamer mainly consisted of a complementary DNA sequence of 16S rRNA (V3-V6 region), in which one end was labeled with FAM and the other one formed a self-assembled short hairpin structure. When the FAM-aptamer bound to GO by π-interaction, fluorescence of FAM was quenched via a Dexter mechanism. The addition of target (16S rRNA) led to restoration of fluorescence, because the binding of the aptamer and target sequence unzipped the connection between the aptamer and GO since the aptamer was energetically more stable when hybridized with 16S rRNA than when attached to GO. As the Klenow Fragment will extend the 3’end of the self-assembled short hairpin structure to form a double strand DNA and displace the target (16S rRNA) at the same time, the displaced target will bind to those remaining aptamers on GO again. SYBR Green I bind to the dsDNA and the fluorescence intensity is significantly enhanced. Conventional assays are generally based on a recognition interaction between the target and the sensor with a 1:1 ratio, which means that each target can only bind to one fluorescent probe to generate a signal [26]. In this method, each target strand may go through many cycles by using Kelnow Fragment, leading to cleavage of numerous aptamers and a lower limit of detection (LOD) than other methods [27, 28]. Moreover, the synergy effect of FAM and SYBR Green I will further improve the sensitivity of this method. Figure 2 displayed the fluorescence emission spectra of FAM-aptamer at different conditions. Its spectrum without GO exhibited higher than others (curve 5). Over 95 % fluorescence signal was quenched when GO presented (curve 2). As expected, the fluorescence restored again (curve 3) after the addition of target (200 nM of analyte of 16S rRNA), and increased continually with the help of Klenow Fragment (curve 4). Collectively, these results demonstrated the feasibility of the approach for the rapid determination of target.
Fluorescence emission spectra of FAM-aptamer at different conditions: (1) Blank; (2) FAM-aptamer + GO; (3) FAM-aptamer + GO + target; (200 nM); (4) FAM-aptamer + GO + target + SYBR Green I; (5) FAM-aptamer. FAM-aptamer concentration: 50 nM. GO concentration: 0.1 mg·mL−1. Excitation: 495 nm. Emission spectra were collected from 500 to 700 nm
Optimization of assay conditions
The ratio of fluorescence quenching agent and aptamer is a critical factor for detection efficiency. Therefore, it is necessary to ascertain how many aptamer molecules can be loaded onto GO. Figure 3a showed the fluorescence quenching situation of FAM-aptamer (50 nM) at different concentrations of GO. The relative fluorescence intensity (F0-F/F0) increased quickly with the GO concentration from 0 to 0.08 mg·mL−1. When the concentrations of GO reached 0.1 mg·mL−1, over 95 % fluorescence intensity was quenched, and the relative fluorescence intensity was not increased anymore with the increment of GO. Accordingly, 0.1 mg·mL−1 of GO was used in the next experiment. Moreover, as illustrated in Fig. 3b, the fluorescence signals reduced swiftly. After 10 min, over 95 % fluorescence signal was quenched and no more decrement was observed (curve 1). Hence, 10 min was chosen as the incubation time for GO and FAM-aptamer. When the target and Klenow Fragment were added, the fluorescence signal recovered remarkably in 30 min (curve 2). After 30 min, the fluorescence did not increase over time. Thus, 30 min was chosen as the reaction time of detection.
Analysis of target sequence in water
To investigate the LOD of this method, we prepared a series of different concentrations of target sequence. Fig. S1 shows the relationship between fluorescence intensity and target concentrations from 0 to 100 nM. Fluorescence intensity increased gradually as the concentration of target increased, indicating that aptamers were detached from GO increasingly by the oligonucleotide-specific hybridization of target and aptamer. Additionally, the inset of Fig. S1 illustrated the linear dependence of fluorescence intensity and target concentration from 0 to 1 nM (y = 25.4 x + 29.69, R2 = 0.98581). The LOD of this method was 60 pM. The sensitivity of this method is higher than that of other existing homogeneous assays [29–31]. The improved detection limit can be attributed to the prominent quenching effect of GO, the high affinity of aptamer to target, the signal amplification reaction using Klenow Fragment and the synergistic effect of FAM and SYBR Green I.
Detection specificity
The fluorescence signal intensity of this method mainly depends on the release of aptamers from GO. Accordingly, its specificity is determined by the oligonucleotide-specific hybridization of target and aptamer. Parallel experiments were performed to evaluate the detection selectivity, three other typical food-borne pathogens S. typhimurium, S. paratyphi A and E. coli K88 were used and compared with the results obtained for S. enteritidis under the same experimental conditions (6 × 103 CFU·mL−1). As is shown in Fig. 4, the addition of target bacteria induced significant fluorescence increase, whereas no obvious fluorescence restoration was observed in other bacteria. Thus we can conclude that this aptamer has a high selectivity.
Milk sample test
With respect to the applicability of this method in real sample detection, we used the aptamer to determinate S. enteritidis in milk samples. Fig. S2 shows the performance of the method in real sample test. Fluorescence intensity rose with the concentration of the target from 0 to 105 CFU·mL−1 and showed good correlation (y = 0.034 x + 24.02, R2 = 0.95482) from 0 to 4000 CFU·mL−1. The fluorescence of the control sample was low because the aptamer can only be detached in the presence of target. The LOD of this assay was 3 × 102 CFU·mL−1. The results demonstrated the potential applicability of this aptamer in real samples detection. To highlight the merits of this aptasensor, the analytical properties of this method were compared with those of other methods. As Table 2 shows, this method has a potential in reducing detection limit and is more convenient, cheap and easy to prepare than others.
Comparison of sensitivity of different dyeing strategies
As illustrated in Fig. 5, different concentrations (from 1 nM to 50 nM) of target sequences were determined with 3 distinct dyeing strategies, respectively. In these three dyeing strategies, the fluorescence signal restored with the addition of target, and the signal intensity rose up as the concentration of target increased. Besides, the signal intensity was significantly higher when using both FAM and SYBR Green I than when using only one of them. This result proved that the synergistic effect is present when using the FAM and SYBR Green I simultaneously, which further improved the sensitivity of this assay.
Conclusion
An aptasensor based on GO using double signal amplification has been successfully developed for the rapid, sensitive, and selective detection of S. enteritidis. This method can detect target with a detection limit of 102 CFU·mL−1 in 2 h. Its sensitivity is much higher than that of other methods on account of the dual fluorescence signal amplification by Klenow enzyme and the synergistic effect of FAM and SYBR Green I. GO, as a nano material, has inherent advantages such as higher fluorescence quenching efficiency, ease of manufacturing and low cost, provide us an ideal platform for this method. Since RNA is vulnerable and easy to lose in the extraction process, which may not precisely represent the concentration of bacteria, the performance of the detection method will be further improved through overcoming this deficiency. It has great potential in application for bio-detection if we change the target by bacterial, certain DNA sequences or other proteins. In summary, this aptasensor is an effective and efficient technique to detect S. enteritidis, and can be expanded to be a rapid and feasible strategy for the detection of many other pathogens in food, environment and clinical diagnosis.
References
Rodrigue DC, Tauxe RV, Rowe B (1990) International increase in salmonella enteritidis: a new pandemic? Epidemiol Infect 105:21–27
Pui CF, Wong WC, Chai LC, Tunung R, Jeyaletchumi P, Noor Hidayah MS, Ubong A, Farinazleen MG, Cheah YK, Son R (2011) Salmonella: a foodborne pathogen. International Food Research Journal 18:465–473
Herikstad H, Motarjemi Y, Tauxe RV (2002) Salmonella surveillance: a global survey of public health serotyping. Epidemiol Infect 129:1–8
Allen MJ, Edberg SC, Reasoner DJ (2004) Heterotrophic plate count bacteria-what is their significance in drinking water? Int J Food Microbiol 92(3):265–274
Uyttendaele M, Vanwildemeersch K, Debevere J (2003) Evaluation of real-time PCR vs automated ELISA and a conventional culture method using a semi-solid medium for detection of salmonella. Lett Appl Microbiol 37:386–391
Martin B, Garriga M, Aymerich T (2012) Pre-PCR treatments as a key factor on the probability of detection of Listeria monocytogenes and salmonella in readyto-eat meat products by real-time PCR. Food Control 27(1):163–169
Sanvicens N, Pastells C, Pascual N, Marco MP (2009) Nanoparticle-based aptasensors for detection of pathogenic bacteria. Trends Anal Chem 28(11):1243–1252
Sanvicens N, Pascual N, Fernández-Argüelles MT, Adrián J, Costa-Fernández JM, Sánchez-Baeza F, et al. (2011) Quantum dot-based array for sensitive detection of Escherichia coli. Anal Bioanal Chem 399(8):2755–2762
Lungu B, Waltman WD, Berghaus RD, Hofacre CL (2012) Comparison of a real-time PCR method with a culture method for the detection of Salmonella enterica serotype enteritidis in naturally contaminated environmental samples from integrated poultry houses. J Food Prot Apr. 75(4):743–747. doi:10.4315/0362-028X.JFP-11-297
Sokolov DM, Sokolov MS (2013) Rapid methods for the genus salmonella bacteria detection in food and raw materials. Vopr Pitan 82:33–40
Zhi Y, Sasai D, Okubo Y, Shinozaki M, Nakayama H, Yamagata Murayama S, Wakayama M, Ide T, Zhang Z, Shibuya K (2013) Comparison between the effectiveness of polymerase chain reaction and in situ hybridization in detecting the presence of pathogenic fungi by using the preserved DNA in formalin-fixed and paraffin-embedded tissues. Jpn J Infect Dis 66:173–179
Lei P, Tang H, Ding S, et al. (2015) Determination of the invA gene of salmonella using surface Plasmon resonance along with streptavidin aptamer amplification. Microchim Acta 182:289–296
Jia F, Duan N, Wu S, Dai R, Wang Z, Li X (2015) Impedimetric salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta. doi:10.1007/s00604-015-1649-7
Fei J, Dou W, Zhao G (2015) A sandwich electrochemical immunosensor for salmonella pullorum and salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Microchim Acta. doi:10.1007/s00604-015-1573-x
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Harvey I, Cormier Y, Beaulieu C, Akimov VN, Meriaux A, Duchaine C (2001) Random amplified ribosomal DNA restriction analysis for rapid identification of thermophilic actinomycete-like bacteria involved in hypersensitivity pneumonitis. Syst Appl Microbiol 24:277–284
Robinson JT, Perkins FK, Snow ES, Wei ZQ, Sheehan PE (2008) Reduced graphene oxide molecular sensors. Nano Lett 8:3137–3140
Wu J, Wang YS, Yang XY, Liu YY, Yang JR, Yang R, Zhang N (2012) Graphene oxide used as a carrier for Adriamycin can reverse drug resistance in breast cancercells. Nanotechnology 23:355101. doi:10.1088/0957-4484/23/35/355101
Yin D, Li Y, Lin H, Guo B, Du Y, Li X, Jia H, Zhao X, Tang J, Zhang L (2013) Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology 24:105102. doi:10.1088/0957-4484/24/10/105102
Pérez-López B, Merkoçi A (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1–16
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of salmonella typhimurium based on a graphene oxide platform. Microchim Acta 181:647–653
Xiang DS, Zhou GH, Luo M, Ji XH, He ZK (2012) Dual color fluorescence quantitative detection of specific single-stranded DNA with molecular beacons and nucleic acid dye SYBR green I. Analyst 137:3787–3793
Cordeiro M, Giestas L, Lima JC, Baptista PJ (2013) Coupling an universal primer to SBE combined spectral codification strategy for single nucleotide polymorphism analysis. Biotechnol. 168(1):90–94
Singer VL, Lawlor TE, Yue S (1999) Comparison of SYBR green I nucleic acid gel stain mutagenicity and ethidium bromide mutagenicity in the salmonella/mammalian microsome reverse mutation assay (ames test). Mutat Res 439(1):37–47
Xiang D, Zhai K, Xiang W, Wang L (2014) Highly sensitive fluorescence quantitative detection of specific DNA sequences with molecular beacons and nucleic acid dye SYBR green I. Talanta 129:249–253
Song Y, Li WK, Duan YF, Li ZJ, Deng L (2014) Nicking enzyme-assisted aptasensor for salmonella enteritidis detection based on fluorescence resonance energy transfer. Biosens Bioelectron 55:400–404
Li S, Li Y, Chen H, Horikawa S, Shen W, Simonian A, Chin BA (2010) Direct detection of salmonella typhimurium on fresh produce using phage-based magnetoelastic aptasensors. Biosens Bioelectron 26:1313–1319
Ohk S-H, Bhunia AK (2013) Multiplex fiber optic aptasensor for detection of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella enterica from ready-to-eat meat samples. Food Microbiol 33:166–171
Duan N, Wu S, Yu Y, Ma X, Xia Y, Chen X, Huang Y, Wang Z (2013) A dual-color flow cytometry protocol for the simultaneous detection of vibrio parahaemolyticus and salmonella typhimurium using aptamer conjugated quantum dots as labels. Anal Chim Acta 804:151–158
Wu X, Xu C, Tripp RA, Huang YW, Zhao Y (2013) Detection and differentiation of foodborne pathogenic bacteria in mung bean sprouts using field deployable label-free SERS devices. Analyst 138:3005–3012
Wu WH, Li M, Wang Y, Ouyang HX, Wang L, Li CX, Cao YC, Meng QH, Lu JX (2012) Aptasensors for rapid detection of Escherichia coli O157:H7 and salmonella typhimurium. Nanoscale Res Lett 7:658
Nguyen P-D, Tran TB, Nguyen DTX, et al. (2014) Magnetic silica nanotube-assisted impedimetric immunosensor for the separation and label-free detection of salmonella typhimurium. Sensors Actuators B Chem 197:314–320
Wu W, Li J, Pan D, Li J, Song S, RongM LZ, Gao J, Lu J (2014) Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of salmonella typhimurium. ACS Appl Mater Interfaces 6(19):16974–16981
Acknowledgments
This work was financially supported by the National Natural Science Foundation (81271660), Doctoral Program of Higher Education from the Ministry of Education (20114306110006).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 305 kb)
Rights and permissions
About this article
Cite this article
Liu, K., Yan, X., Mao, B. et al. Aptamer-based detection of Salmonella enteritidis using double signal amplification by Klenow fragment and dual fluorescence. Microchim Acta 183, 643–649 (2016). https://doi.org/10.1007/s00604-015-1692-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1692-4