Abstract
Learning is a change in state resulting from new experiences enabling behavioural responses to be adjusted in alignment with external cues. Individuals differ in the speed and accuracy at which they learn. Personality has been postulated as being a major influence on learning ability in terms of attention and encounter rates of environmental cues. This link forms the basis of the cognitive style hypothesis (CSH), predicting that an individual’s cognitive style will occur along a fast–slow behavioural gradient. Fast types are characterised as being active, neophilic, and bold individuals who sample their environment rapidly, yet superficially, enabling learning to occur at a higher speed, but at the cost of accuracy. Slow types have the opposite suite of personality traits resulting in them being more accurate flexible learners. Greater level of learning flexibility is thought to help promote invasions success. Here, we test the predictions of the CSH in an invasive lizard (Lampropholis delicata) to determine if personality dictates learning performance in a two-phase associative task. Results indicated that the delicate skink was capable of learning an associative task but only provided partial support for the CSH. Personality was found to influence learning accuracy, however, the direction of that relationship was opposite to that predicted. Instead, fast lizards made fewer mistakes when learning to associate a colour to a goal. These findings highlight the need to further investigate the CSH across taxa and consider its potential as an underlying mechanism of the invasion process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals in the wild are confronted with a multitude of external stimuli. These cues are relied upon to maximise foraging efficiency, reproductive output, and predator avoidance (Dukas 2004; Shettleworth 2001). How the information transmitted by a given cue is applied is highly dependent upon an animal’s cognitive ability. In other words, variation in perception, information processing, and memory will dictate the ultimate behavioural response employed (Raine and Chittka 2008). Learning is a change in state resulting from new experiences that enables behavioural responses to be adjusted in alignment with external cues (Burghardt 1977; Shettleworth 1998). Having the capacity to perform this process is, therefore, considered to be adaptive in that it enables critical information about the environment to be acquired and responded to appropriately (Grieco et al. 2002; Tebbich et al. 2012).
Nonetheless, the fitness advantage of learning is not only mediated through its effect on the perception of environmental cues themselves, but also through its effect on the perception of changes in those cues (Guenther et al. 2013). Detecting such fluctuations in cue reliability and meaning requires an animal to maintain an elevated level of attention. In doing so, the animal can then have the capacity to modify its behavioural response in an adaptive way. Given the costs, not all species or populations exhibit learning flexibility. Instead, a flexible learning style tends to emerge under variable or novel environmental conditions and is thought to be particularly important during species invasions (Amiel et al. 2011; Bezzina et al. 2014; Lowry et al. 2013; Sol et al. 2008, 2013). When establishing in new environments, invaders are confronted with the challenge of navigating through unfamiliar habitats to locate and identify key resources while also avoiding novel predators. Cues which were once relied upon in the native range may therefore no longer hold the same meaning, necessitating new associations to be formed (Sih and Del Giudice 2012).
Studies investigating learning have primarily done so at the species level (Mery 2013; Sih and Del Giudice 2012; Thornton and Lukas 2012; Titulaer et al. 2012). However, there is mounting evidence indicating that cognition in general, and learning in particular, can vary among individuals (Niemela et al. 2013; Sol et al. 2013). Several studies have suggested that learning within a species can be influenced by factors such as sex, age, social status, and developmental conditions (Amiel et al. 2013; Amiel and Shine 2012; Carazo et al. 2014; Clark et al. 2014; Jones et al. 2005; Noble et al. 2014). An additional factor that has recently been postulated to moderate learning ability is personality (Amy et al. 2012; Carazo et al. 2014; Guenther et al. 2013; Sih and Del Giudice 2012; Tebbich et al. 2012). Personality, defined as repeatable individual differences in behaviour, may affect performance in cognitive tasks in that individual variation in attention and encounter rates of environmental stimuli act to either facilitate or constrain learning (Carere and Locurto 2011; Griffin et al. 2015; Sih et al. 2004). This link forms the basis of the cognitive style hypothesis (CSH) which predicts that an individual’s cognitive style, as mediated by its personality type, will occur along a fast–slow gradient (Carere and Locurto 2011; Sih and Del Giudice 2012). At one end of the spectrum are fast types that are characterised as having active, neophilic, and bold personalities. Such individuals are thought to pay less attention to environmental cues and instead sample their environment rapidly, yet superficially, enabling decision-making and learning of new activity-based tasks to occur at a higher speed (Guenther et al. 2013; Sih and Del Giudice 2012). However, this may come at the cost of learning accuracy and flexibility, as fast types may be less responsive to environmental changes (Sih and Del Giudice 2012). In contrast, slow types have the opposite suite of personality traits (e.g., inactive, neophobic, and shy) resulting in them moving through their environment at a slower rate but paying more attention to external cues (Mamuneas et al. 2014). For instance, Guillette et al. (2011) found that slow-exploring black-capped chickadees (Poecile atricapillus) were more flexible in learning a vocal discrimination task compared to fast-exploring birds. Those that visited fewer trees in a novel environment required less trials to extinguish a previously learned response to a B note and instead respond to the newly relevant C note. Likewise, in cavies (Cavia aperea), activity was strongly related to learning speed where individuals who engaged in greater levels of locomotor behaviour were also faster at associating a symbol with a food reward (Guenther et al. 2013).
High cognitive function, particularly learning flexibility, has been invoked as a major contributor to invasion success, as has personality (Carvalho et al. 2013; Chapple et al. 2012; Sol et al. 2002; Wright et al. 2010). Much support has indeed demonstrated that among and within species, those that were invasive exhibited higher levels of innovation (Sasvári 1985), associative learning ability (Hazlett et al. 2002), as well as exploratory behaviour (Carvalho et al. 2013; Chapple et al. 2012). For example, the invasive green crab (Carcinus maenas) was found to be faster in remembering the location of a hidden food item than the native blue crab (C. sapidus)(Roudez et al. 2008). It can be envisioned then that faster behavioural and cognitive types would be more capable of finding and using novel resources such as food, refuges, and mates than slower types, thereby, facilitating their invasion success.
Despite much support of the CSH, there are still studies which provide evidence against its predictions (Amy et al. 2012; Carazo et al. 2014; Dugatkin and Alfieri 2003; Griffin et al. 2015; Guenther et al. 2013; Tebbich et al. 2012). Thus, examining the role that personality plays in the learning process is critical to our understanding of the mechanisms driving inter-individual variation in cognitive function. Invasive species offer a promising system with which to examine this relationship as the functional integration of personality and learning should enable invaders to meet the challenges of the novel environments into which they become established (Amiel et al. 2013). Accordingly, we tested the predictions put forth by the CSH by investigating the relationship between personality and learning ability in the invasive delicate skink (Lampropholis delicata). This species is an ideal model for addressing these questions as it exhibits consistent inter-individual variation in activity, exploratory behaviour, boldness, and sociability with all behaviours except boldness being structured into a behavioural syndrome (Melki-Wegner 2015; Michelangeli et al. 2016b; Moule et al. 2016). These behaviours have also been shown to enhance its invasion success (Chapple et al. 2012). We aimed to determine if: (1) the delicate skink is capable of learning an associative task; (2) variation in personality among delicate skink individuals influences learning speed and accuracy. We expected that fast individuals, in having greater levels of activity, exploratory behaviour, and boldness would have higher learning speed at the cost of accuracy. Slow types, on the other hand, were instead expected to take a greater number of trials to become trained, but in being slower and more attentive, would exhibit higher levels of accuracy and flexibility.
Methods and materials
Study species and field collection
The delicate skink is a small [35–55 mm adult snout–vent length (SVL)] heliotherm that is locally abundant and geographically widespread in eastern Australia (Chapple et al. 2011). Its native distributional range spans 26° of latitude from north Queensland to southern Tasmania (Chapple et al. 2011). In addition, this species has been introduced to Hawaii, New Zealand, and Lord Howe Island (Chapple et al. 2013, 2014; Tingley et al. 2016). Within both its native and introduced range delicate skinks occur in moist habitats, including rainforests, wet sclerophyll forests, woodlands, and heaths but are also able to utilise urban settings as well (Chapple et al. 2014).
Fifty adult male lizards with complete tails were collected from the Sydney region (New South Wales, Australia: 27°38S 153°05E) in Oct-2013 and Apr-2014. Each were individually marked with a unique Visible Implant Elastomer (Northwest Marine Technology) colour code and transported back to the animal housing facility at Monash University (Clayton, Victoria, Australia). Lizards were maintained at 20 °C with a 14-h light: 10-h dark cycle (0600–2000 h) and fed crickets (Acheta domesticus). Experimental trials were conducted when lizards were in a post-absorptive state (24 h without food). All applicable institutional and/or national guidelines for the care and use of animals were followed.
Behavioural measurements
The personality of each lizard was scored based on the methodology described in Michelangeli et al. (2016a, b). Briefly, behaviour was evaluated in four contexts: activity, exploration, boldness and sociability. However, as boldness was shown not to be repeatable, it was omitted from the present study. Activity was measured by placing lizards individually into an arena (550 × 320 × 240 mm) marked with 20 equal grid squares. Activity was scored based on the number and rate of transitions between squares. Exploration was measured by presenting lizards with an obstacle which divided an arena into two compartments. Latency to reach the goal compartment was used as a measure of exploratory behaviour. Sociability was measured by placing lizards in an arena divided into three zones: social, asocial, and a neutral zone. The social zone was comprised of a basking site divided in half by a clear Perspex™ partition running the length of the arena, with three stimulus lizards placed behind the partition. The asocial zone located at the opposite end of the arena was identical but contained no stimulus lizards. The amount of time spent basking with conspecifics was a measure of sociability. Each lizard was exposed to each assay twice.
Associative learning
Apparatus and general design
To investigate associative learning ability, we used a standard Y-maze (Orchid Scientific & Innovative India Pty Ltd) as this is an established design for testing learning in a range of taxa, including the delicate skink (Amiel et al. 2013; Bezzina et al. 2014; Burger et al. 1991; Waldman 1985). Mazes were constructed from opaque white plastic with each arm (L: 37.5 × W: 6.5 × H: 13 cm) connecting to a central decision point (CDP). The start arm was fitted with a guillotine door and the two remaining arms were designated as decision arms. Both decision arms were painted with either blue or yellow horizontal stripes. These colours were selected as a lizard’s visual system is capable of distinguishing between them (Clark et al. 2014; Fleishman et al. 2011). A shelter (D: 57 mm × H: 43 mm) was placed at the end of each decision arm with the shelter matching the colour of the arm. Lizards were observed from behind a black curtain and recorded using a Panasonic HC-V130 video camera. All equipment was cleaned between trials with soap and water to remove olfactory cues.
Associative learning involves visual discrimination between two alternative stimuli presented simultaneously. The stimuli used in the present study were a “safe” refuge and an “unsafe” refuge. The safe refuge was that in which lizards were permitted to remain inside without being disturbed while lizards were removed from the unsafe refuge. A visual cue (e.g., colour) was provided to enable lizards to discriminate between the two stimuli and to reinforce the association between cue and stimuli (positive or negative).
Here, lizards were exposed to a two-phase learning paradigm. Each phase differed in cue relevance to assess the lizard’s ability to associate a colour with a goal, in this case the safe refuge. The first phase (training phase) served to train lizards to associate a specific colour with safety using negative reinforcement while the second phase (associative learning) was meant to determine if the colour association was established and used to locate the safe refuge. Because refuges utilised in the study were similar to those provided within the lizard’s housing containers, no habituation was conducted to familiarise lizards with their use.
Phase 1: training
Prior to the onset of the training phase, experimentally naïve lizards were randomly assigned a safe goal colour that was held constant for the entire experiment. Goal allocation was counter-balanced for both colour and side to control for possible colour or side biases. The spatial location (i.e., left or right) of the goal remained constant for all trials during the training phase thereby enabling lizards to use position and/or colour cues to navigate to the goal. Lizards were subjected to up to two trials per day for a total of 15 trials or until the training the criterion of five correct trials within six consecutive trials was met (Clark et al. 2014; Noble et al. 2014). Lizards not reaching criterion were considered untrained and removed from the experiment, whereas all trained lizards continued onto the learning phase.
At the start of each trial, lizards were individually placed behind the guillotine door for 5 min of acclimation. The door was then lifted allowing them to move freely throughout the maze. Each lizard was given up to 10 min to enter half of its body into the goal shelter. Lizards remaining motionless for 15 continuous seconds were gently tapped on their pelvic girdle with a small paint brush to instigate movement. If the lizard entered the incorrect (unsafe) shelter, it was immediately placed back into the decision arm. If the goal was not reached within 10 min the lizard was then chased into the goal shelter as reinforcement. Trials were only scored as correct if the lizard initially turned into the goal arm and entered half of its body into the goal shelter without leaving that arm, with all other choices scored as incorrect (correct/incorrect turn). The number of correct trials served as a measure of accuracy. The trial at which the criterion was achieved was designated as the training trial and served as a measure of training speed. Additional metrics recorded during each trial were the number of incorrect turns into non-goal arms from the CDP (# incorrect turns) as another measure of accuracy (Mamuneas et al. 2014), latency to the goal (Burghardt 1977), as well as the first side and colour the lizard turned towards to investigate potential side and colour biases. Lizards not entering the goal by the end of the trial were assigned 10 min as their latency.
Phase 2: associative learning
In phase 2, the general methods, learning criterion, and metrics remained the same as that of the training phase described above. However, the number of trials increased to 20 and the maze colour layout alternated between trials. By changing the position of the goal, the use of colour versus spatial cues could, thus, be deciphered. Lizards correctly reaching the criterion were considered to have learned the associative task while those that did not meet the criteria were non-learners. The trial at which the criterion was reached was designated as the associative learning trial and served as a measure of learning speed.
Statistical analyses
Data were analysed using R (R Core Team 2013) and SPSS version 20.0 (SPSS Inc. 2011), with statistical significance assigned at α = 0.05. Data were checked for normality and homogeneity of variance. Data not meeting these assumptions were log transformed.
To be incorporated into a behavioural syndrome, behavioural traits must show repeatability (test–retest reliability) and between-individual variation (Bell 2007). Accordingly, repeatability between trials for each trait was quantified using intra-class correlation coefficients (ICC) (‘rptR’ package: (Nakagawa and Schielzeth 2010); ‘ICC’ package (Wolak et al. 2011)). Repeatability implies that individual behaviour is consistent over multiple trials. Between-individual variation for each trait was also quantified through the ‘ICC’ package, with significance assessed using one-way ANOVAs. Traits with significant between-individual variation and repeatability were then considered in tests assessing the presence and structure of a behavioural syndrome. Pairwise correlation tests were first completed followed by a principal components analysis (PCAs) with varimax rotation. This technique summarises correlations between multiple variables and characterises the traits which make up the syndrome.
Learning metrics were assessed separately for each phase. Spearman’s rank correlation tests were used to determine whether an individual’s training performance was predictive of its learning ability. Pearson’s correlation tests were conducted to examine the overall relationship between personality and training trial number as well as personality and learning trial number. The effect of personality on the probability of passing each phase was assessed with a Kruskal–Wallis rank sum test and generalised linear models (GLM). Learning curves for the number of incorrect turns, within-trial latency, and the probability of getting the trial correct were constructed and examined independently of personality using generalised linear mixed effect models (GLMM) or linear mixed effect models (LMM). Trial number was specified as a fixed effect and Skink ID as a random effect. LMMs were performed to determine if personality influenced learning metrics, including within-trial latency, the number of incorrect turns, and correct/incorrect trial. Trial number, batch, and individual personality scores derived from the PCA were specified as fixed effects and Skink ID as a random effect. The presence of colour and side biases was determined using Chi-squared tests.
Results
Behavioural syndrome structure
Activity, exploratory behaviour, and sociability were repeatable with significant between-individual variation for each of these traits (Table 1). Consequently, all three traits were included in the PCA. Pairwise correlations did not reveal any significant relationships between behaviours (Table S1). By contrast, the PCA analysis showed a shared pattern of behavioural variation. A first major axis (PC1) meeting the Kaiser–Guttman criterion (eigenvalue > 1) explained most of the variation (44.5%) and was positively correlated with all three traits. Specifically, more active lizards passed over an obstacle more quickly, and spent more time basking with other lizards. PC2 explained comparatively less of the variance (30.9%) and did not meet the Kaiser–Guttman criterion for retention (eigenvalue = 0.93). Thus, only the individual scores for PC1 were used as a measure of personality in the learning analyses.
Learning ability in the delicate skink
Twenty-eight of the 50 lizards (56%) passed the training phase and 16 of the 28 trained lizards (57%) went on to become learners. The probability of correctly completing five of six consecutive trials by chance alone was low (Phase 1 = 0.18; Phase 2 = 0.25). Lizards took 5–15 trials inclusive (mean ± SE: 9.82 ± 0.65, n = 28) to become trained (Phase 1) and trained lizards took 6-20 trials inclusive (mean ± SE: 12.88 ± 1.16, n = 16) to learn the associative task (Phase 2).
Chi-squared tests indicated that within trials lizards preferentially turned right but did not exhibit a colour bias (χ 2 = 4.20, df = 1, P = 0.040; χ 2 = 0.015, df = 1, P = 0.901; respectively). However, when comparing the performance of lizards assigned either goal side or colour, the number of learners did not vary (Phase 1: χ 2 = 0.14, df = 1, P = 0.706; Phase 2: χ 2 = 0.25, df = 1, P = 0.617; Phase 1: χ 2 = 0.14, df = 1, P = 0.706; Phase 2: χ 2 = 1, df = 1, P = 0.317; respectively).
The Spearman’s rank correlation test indicated that during the training phase, both the number of incorrect turns as well as latency to reach the safe refuge were associated with the number of trials to reach the training criterion (Table S2). Similarly, the number of incorrect turns was related to the number of trials to reach the learning criterion. However, the time to reach the goal was not associated with the number of trials to learning criterion nor did training performance predict learning performance (Table S2; Figure S1).
Analysis of learning curves for the training phase independent of personality revealed that both the number of incorrect turns and latency to reach the goal decreased with successive trials (GLMM: estimate ± SE = − 0.06 ± 0.01, z = − 5.21, P < 0.001; LMM: estimate ± SE = − 0.03 ± 0.01, t = − 3.11, P < 0.001; respectively) (Figure S2a and S3a) whereas the probability of getting the trial correct was unaffected by trial number (GLMM: estimate ± SE = 0.04 ± 0.02, z = 1.85, P = 0.065). In the associative learning phase, overall learning performance improved as indicated by a decrease in the number of incorrect turns across trials (GLMM: estimate ± SE = − 0.04 ± 0.01, z = − 3.95, P < 0.001; Figure S2b) and an increase in the probability of getting the trial correct (GLMM: estimate ± SE = 0.04 ± 0.02, z = 2.27, P = 0.02). However, latency to reach the goal during the learning phase did not exhibit a significant decrease over successive trials (LMM: estimate ± SE = − 0.01 ± 0.01, t = −1.82, P = 0.069; Figure S3b).
Influence of personality on learning
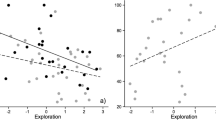
Personality had little influence on learning metrics in either phase. During the training phase, no relationship was detected between personality and the latency to reach the goal (Table 2; Fig. 1a, b), whether a trial was correct or incorrect, or the number of trials to reach training criterion (GLM: χ 2 = 0.07, df = 1, P = 0.79; Fig. 1c). However, for the number of incorrect turns, personality did interact with trial number suggesting that faster types (e.g., more active and exploratory individuals) decreased the number of turns into the non-goal arm over consecutive trials (F = 1.85, df = 15, P = 0.03) relative to slow types. Additionally, within the associative learning phase, personality was not associated with either the number of incorrect turns (Table 2; Fig. 1d, e), latency, or learning speed (GLM: χ 2 = 2.82, df = 1, P = 0.09; Fig. 1f). Whether a trial was correct or incorrect was, on the other hand, influenced by the combined effect of trial and personality (F = 1.82, df = 19, P = 0.02; Table 2) where fast personality types increased accuracy over trials at a higher rate than slow types. The Kruskal–Wallis rank sum test further supported the lack of influence of personality on learning in that personality of untrained, trained, non-learners, and learners did not vary (χ 2 = 0.96, df = 2, P = 0.620).
Discussion
Overall, delicate skinks were able to learn to perceive and identify a colour cue as it related to a safe shelter when presented with an associative learning paradigm. Nearly equal numbers of lizards passed the training and learning phase, indicating that even when position cues became unreliable more than half of the trained lizards had the capacity to perceive the change in position cue reliability and associate the safe shelter based solely on the visual cue. However, in testing the CSH, we found that personality had little influence on an individual’s learning accuracy, learning speed, or the probability of learning. This is the first study to our knowledge evaluating the impact of personality on aspects of learning in an invasive species, suggesting that aspects of cognition may have the potential to contribute to an invader’s ability to successfully establish outside its native range.
Our results indicate that the delicate skink is capable of learning an associative task. This was evidenced by the high proportion (> 50%) of lizards successfully completing both the training and learning phases. Furthermore, the same learning criterion and similar maximum trial numbers have been used in previous studies of associative learning suggesting that the level of learning success demonstrated here was not inflated as a result of having a greater opportunity to reach the criterion (Clark et al. 2014; Noble et al. 2014).
When considering the four learning metrics measured, learning performance differed between the training and learning phases. During the first phase (training phase), lizards decreased the number of incorrect turns and latency to reach the goal with successive trials, but the number of correct first turns did not improve from one trial to the next. By contrast, during the learning phase, an increase in learning accuracy was observed whereas latency to reach the goal did not decrease over successive trials. Varying results among the two phases may be due to a number of factors. Firstly, stable cue reliability may have promoted the use of a ‘rule-of-thumb’ strategy during the training phase whereby lizards employed the same behavioural response across trials (e.g., always turn right). This could explain the decrease in latency without a corresponding increase in the number of correct first turns (Carazo et al. 2014). Secondly, lizards had the opportunity to utilise both position and colour as a cue in their decision-making process during this initial phase while only colour was available during the learning phase. By having to rely entirely on the visual cue while extinguishing the spatial information, the second phase may have required greater processing time, thus causing latency to reach the goal to increase (Day et al. 2003). Finally, overtraining effects may also have contributed to the lack of latency reduction during this phase. Since lizards completed up to 35 trials over the entire experiment, they may have lost motivation to complete the task or become habituated to the perceived threat of the paint brush (Carazo et al. 2014).
The cognitive performance demonstrated here is consistent with that of other species of skinks (Clark et al. 2014; Day et al. 2003; Leal and Powell 2012; Noble et al. 2014). For example, in the only other study on delicate skink cognition, latency to reach a food reward in a Y-maze decreased while the number of correct turns did not improve (Bezzina et al. 2014). Similarly, three-lined skinks (Bassiana duperreyi) decreased their latency under the same testing paradigm (Amiel et al. 2013). However, these authors did not examine the number of incorrect turns across successive trials; thus, this metric could be compared. Together these findings suggest that some level of learning appears to be a common trait among this group of animals, potentially due to common foraging, reproductive, or predator avoidance strategies.
Influence of personality on learning
The CSH states that behavioural types will fall along a fast–slow continuum whereby fast types are more active and exploratory than slower types, and that this individual variation in personality will in turn correspond to an individual’s position along the fast–slow cognitive style axis (Sih and Del Giudice 2012). This purported link between personality and cognitive style arises through a shared speed–accuracy trade-off. By exhibiting a higher level of activity and exploration, fast personality types sample their environment more rapidly, albeit, more superficially. These behavioural tendencies should, therefore, enable fast types to learn novel tasks more quickly, but at the cost of accurate decision-making and responsiveness to changes in the meaning of cues. Slow types, by contrast, should instead have a reduced learning speed but express more learning flexibility through their greater attention towards cue relevancy, increasing their accuracy under variable environmental conditions.
Accordingly, we expected a greater proportion of lizards exhibiting a high level of the activity–exploration–sociability syndrome (fast type) to successfully reach the training criterion and do so more rapidly but with potentially more errors relative to those expressing a low level of the syndrome (slow type). Moreover, we further expected that slow lizards would achieve the learning criterion at a faster rate and with greater accuracy than fast lizards. In testing these predictions, we found that personality had little influence on learning in the delicate skink, with most metrics having no relationship with personality. Neither learning ability nor speed was affected by a lizard’s personality. In other words, the expectation that fast lizards would disproportionately achieve the training criterion at a faster rate while slow lizards would achieve the learning criterion more rapidly was not observed. By contrast, personality did influence learning accuracy as stated by the CSH but these effects were in the opposite direction. It was expected that fast personality types, with their superficial sampling and inaccurate cognitive style, would not perceive the change in cue meaning during this phase and thus would make greater number of mistakes when associating the colour with the goal. However, lizards exhibiting slower personality types were less accurate countering the CSH. Similar results were reported by Mamuneas et al. (2014#1163) where bolder fish (Gasterosteus aculeatus) were no less accurate than shy fish when tested in a standard food reward experimental paradigm.
Contradictory results have been demonstrated in several other studies as well (Ducatez et al. 2015; Guillette et al. 2015). For example, exploration was found to be positively, rather than negatively, correlated with reversal learning ability in two species of Darwin’s finches (Camarhynchus parvulus and Cactospiza pallida) (Tebbich et al. 2012). Similarly, an individual’s level of exploratory behaviour did not affect either associative learning or reversal learning success in great tits (Parus major) nor were the expected speed–accuracy trade-offs observed (Amy et al. 2012). In other words, there was no difference in the number of errors (e.g., accuracy) between fast and slow explorers during either experimental phase.
The lack of full support of the CSH demonstrated here could indeed be a true reflection of the limited influence personality has on the learning process of the delicate skink. However, individual variation in motivation and/or habituation may instead be causing differences in the latency to reach the safe refuge. Each behavioural type (e.g., fast or slow) may have different levels of motivation to explore and be active as each has different underlying energetic demands. Likewise, the ability to habituate to novel cognitive problems may also vary as a function of personality. Such differences may then result in fast lizards having higher encounter rates with environmental cues than slow lizards, effecting the time an individual takes to employ an appropriate behavioural response. This has been suggested in several empirical as well as theoretical studies (Biro and Stamps 2010; Careau and Garland 2012; Shettleworth 2001; Sol et al. 2013; Stamps and Groothuis 2010).
An alternative explanation could be the fact that we investigated the effect of a suite of correlated behaviours (e.g., behavioural syndrome) rather than each one individually. It may be that only some, or one, of the constituent behaviours making up the personality syndrome in the delicate skink is functionally related to its learning. For example, both activity and exploration have been shown to positively affect cognitive traits (Guenther et al. 2013; Guillette et al. 2015; Titulaer et al. 2012), but it is less clear how sociability may be involved. Furthermore, it may also be possible that learning and personality interact more strongly in a different cognitive trait. It has been shown that an individual’s performance in one learning task may not necessarily be indicative of that individual’s performance in a different task (Leal and Powell 2012). Indeed, to fully evaluate whether the CSH holds for a species, learning ability must be assessed over a variety of cognitive traits such as navigation or problem-solving (Griffin et al. 2015; Sih and Del Giudice 2012).
Our study is the first of its kind to investigate the link between personality and learning in an invasive species while also considering personality-mediated speed–accuracy trade-offs within the context of the CSH. Our results indicate that the delicate skink has the capacity to learn, and that previously learnt associations between a visual cue and a behavioural response can be modified. These findings contrast with a previous investigations of general cognitive function in this species, and add to the growing literature demonstrating reptilian learning ability (Bezzina et al. 2014). Moreover, personality only influenced a single aspect of learning, whereby lizards with faster personality types had lower latencies to reach a goal than slower personality types. However, the speed–accuracy trade-offs predicted by the CSH were not supported. Nonetheless, our results will serve to contribute to the paucity of research into reptilian cognition in general, and personality-mediated learning ability specifically.
The learning ability of the invasive delicate skink, as demonstrated here, may act to facilitate its capacity to become established outside of its native range. Having the capacity to use cues as a means of evading novel predators and identifying key resources such as food, mates, and refuges could serve to promote its invasibility. Furthermore, having the flexibility to perceive changes in cue meaning and/or reliability would also assist in adapting to the challenges of a variable environment (Sih and Del Giudice 2012). How personality interacts with cognitive function and how their interplay influences invasion success is, however, still unclear. Our results hint at a potential link that may increase establishment success in the delicate skink. Further investigations comparing the personality-mediated learning abilities between invasive and non-invasive species or populations would, therefore, be warranted to determine if indeed cognitive style is a mechanism underlying the invasion process.
References
Amiel JJ, Shine R (2012) Hotter nests produce smarter young lizards. Biol Lett 8:372–374
Amiel JJ, Tingley R, Shine R (2011) Smart moves: effects of relative brain size on establishment success of invasive amphibians and reptiles. PLoS One 6:e18277
Amiel JJ, Lindstrom T, Shine R (2013) Egg incubation effects generate positive correlations between size, speed and learning ability in young lizards. Anim Cogn 17:337–347. doi:10.1007/s10071-013-0665-4
Amy M, van Oers K, Naguib M (2012) Worms under cover: relationships between performance in learning tasks and personality in great tits (Parus major). Anim Cogn 15:763–770
Bell AM (2007) Future directions in behavioural syndromes research. Proc R Soc Biol Sci Ser B 274:755–761
Bezzina CN, Amiel JJ, Shine R (2014) Does invasion success reflect superior cognitive ability? A case study of two congeneric lizard species (Lampropholis, Scincidae). PLoS One 9:e86271
Biro PA, Stamps JA (2010) Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol Evol 25:653–659. doi:10.1016/j.tree.2010.08.003
Burger J, Boarman W, Kurzava L, Gochfeld M (1991) Effect of experience with pine (Pituophis melanoleucus) and king (Lampropeltis getulus) snake odors on Y-maze behavior of pine snake hatchlings. J Chem Ecol 17:79–87
Burghardt GM (1977) Learning processes in reptiles. Biol Reptil 7:555–681
Carazo P, Noble DWA, Chandrasoma D, Whiting MJ (2014) Sex and boldness explain individual differences in spatial learning in a lizard. Proc R Soc Biol Sci Ser B 281:20133275
Careau V, Garland T Jr (2012) Performance, personality, and energetics correlation, causation and mechanism. Physiol Biochem Zool 85:43–571. doi:10.1086/666970
Carere C, Locurto C (2011) Interaction between animal personality and animal cognition. Curr Zool 57:491–498
Carvalho CF et al (2013) Personality traits are related to ecology across a biological invasion. Behav Ecol 24:1081–1091. doi:10.1093/beheco/art034
Chapple DG, Hoskin CJ, Chapple SN, Thompson MB (2011) Phylogeographic divergence in the widespread delicate skink (Lampropholis delicata) corresponds to dry habitat barriers in eastern Australia. BMC Evol Biol 11:191–208. doi:10.1186/1471-2148-11-191
Chapple DG, Simmonds SM, Wong B (2012) Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64
Chapple DG, Whitaker AH, Chapple SN, Miller KA, Thompson MB (2013) Biosecurity interceptions of an invasive lizard: origin of stowaways and human-assisted spread within New Zealand. Evol Appl 6:324–339
Chapple DG, Miller KA, Chaplin K, Barnett L, Thompson MB, Bray RD (2014) Biology of the invasive delicate skink (Lampropholis delicata) on Lord Howe Island. Aust J Zool 62:498–506. doi:10.1071/ZO14098
Clark BF, Amiel JJ, Shine R, Noble DWA, Whiting MJ (2014) Colour discrimination and associative learning in hatchling lizards incubated at ‘hot’ and ‘cold’ temperatures. Behav Ecol Sociobiol 68:239–247
Day LB, Ismail N, Wilczynski W (2003) Use of position and feature cues in discrimination learning by the whiptail lizard (cnemidophorus inornatus). J Comp Psychol 117:440–448
Ducatez S, Audet JN, Lefebvre L (2015) Problem-solving and learning in Carib grackles: individuals show a consistent speed–accuracy trade-off. Anim Cogn 18:485–496
Dugatkin LA, Alfieri MS (2003) Boldness, behavioral inhibition and learning. Ethol Ecol Evol 15:43–49
Dukas R (2004) Evolutionary biology of animal cognition. Annu Rev Ecol Evol Syst 35:347–374
Fleishman LJ, Loew ER, Whiting MJ (2011) High sensitivity to short wavelengths in a lizard and implications for understanding the evolution of visual systems in lizards. Proc R Soc Biol Sci Ser B. doi:10.1098/rspb.2011.0118
Grieco F, van Noordwijk AJ, Visser ME (2002) Evidence for the effect of learning on timing of reproduction in blue tits. Science 296:136–138
Griffin AS, Guillette LM, Healy SD (2015) Cognition and personality: an analysis of an emerging field. Trends Ecol Evol. doi:10.1016/j.tree.2015.01.012
Guenther A, Brust V, Dersen M, Trillmich F (2013) Learning and personality types are related in cavies (Cavia aperea). J Comp Psychol 128:74. doi:10.1037/a0033678
Guillette LM, Reddon AR, Hoeschele M, Sturdy CB (2011) Sometimes slower is better: slow-exploring birds are more sensitive to changes in a vocal discrimination task. Proc Biol Sci R Soc 278:767–773. doi:10.1098/rspb.2010.1669
Guillette LM, Hahn AH, Hoeschele M, Przylupski A-M, Sturdy CB (2015) Individual differences in learning speed, performance accuracy and exploratory behaviour in black-capped chickadees. Anim Cogn 18:165–178
Hazlett BA, Acquistapace P, Gherardi F (2002) Differences in memory capabilities in invasive and native crayfish. J Crustac Biol 22:439–448
IBM SPSS (2011) Statistics for Windows, Version 20.0., IBM Corp, Armonk, NY
Jones JC, Helliwell P, Beckman M, Maleszka R, Oldroyd BP (2005) The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J Comp Physiol A 191:1121–1129
Leal M, Powell BJ (2012) Behavioural flexibility and problem-solving in a tropical lizard. Biol Lett 8:28–30
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549
Mamuneas D, Spence AJ, Manica A, King AJ (2014) Bolder stickleback fish make faster decisions, but they are not less accurate. Behav Ecol. doi:10.1093/beheco/aru160
Melki-Wegner B (2015) The interaction between tail loss, personality and behaviour in the delicate skink, Lampropholis delicata. Honours Thesis, Monash University, Victoria
Mery F (2013) Natural variation in learning and memory. Curr Opin Neurobiol 23:52–56
Michelangeli M, Chapple DG, Wong BBM (2016a) Are behavioural syndromes sex specific? Personality in a widespread lizard species. Behav Ecol Sociobiol. doi:10.1007/s00265-016-2197-9
Michelangeli M, Wong BB, Chapple DG (2016b) It’sa trap: sampling bias due to animal personality is not always inevitable. Behav Ecol 27:62–67
Moule H, Michelangeli M, Thompson M, Chapple D (2016) The influence of urbanization on the behaviour of an Australian lizard and the presence of an activity–exploratory behavioural syndrome. J Zool 298:103–111
Nakagawa S, Schielzeth H (2010) Repeatability for Gaussian and non-Gaussian data: a practical guide for biologists. Biol Rev 85:935–956
Niemela PT, Vainikka A, Forsman JT, Loukola OJ, Kortet R (2013) How does variation in the environment and individual cognition explain the existence of consistent behavioral differences? Ecol Evol 3:457–464. doi:10.1002/ece3.451
Noble DWA, Byrne RW, Whiting MJ (2014) Age-dependent social learning in a lizard. Biol Lett 10. doi: 10.1098/rsbl.2014.0430
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raine NE, Chittka L (2008) The correlation of learning speed and natural foraging success in bumble-bees. Proc R Soc B Biol Sci 275:803–808
Roudez RJ, Glover T, Weis JS (2008) Learning in an invasive and a native predatory crab. Biol Invasions 10:1191–1196
Sasvári L (1985) Keypeck conditioning with reinforcements in two different locations in thrush, tit and sparrow species. Behav Processes 11:245–252
Shettleworth S (1998) Cognition, evolution, and behavior. Oxford University Press, New York
Shettleworth SJ (2001) Animal cognition and animal behaviour. Anim Behav 61:277–286. doi:10.1006/anbe.2000.1606
Sih A, Del Giudice M (2012) Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc Lond B Biol Sci 367:2762–2772. doi:10.1098/rstb.2012.0216
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. doi:10.1016/j.tree.2004.04.009
Sinervo B et al (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328:894–899. doi:10.1126/science.1184695
Sol D, Timmermans S, Lefebvre L (2002) Behavioural flexibility and invasion success in birds. Anim Behav 63:495–502
Sol D, Bacher S, Reader SM, Lefebvre L (2008) Brain size predicts the success of mammal species introduced into novel environments. Am Nat 172:S63–S71
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112. doi:10.1016/j.anbehav.2013.01.023
Stamps J, Groothuis TG (2010) The development of animal personality: relevance, concepts and perspectives. Biol Rev 85:301–325
Tebbich S, Stankewitz S, Teschke I (2012) The relationship between foraging, learning abilities and neophobia in two species of Darwin’s finches. Ethology 118:135–146. doi:10.1111/j.1439-0310.2011.02001.x
Thornton A, Lukas D (2012) Individual variation in cognitive performance: developmental and evolutionary perspectives. Philos Trans R Soc Lond B Biol Sci 367:2773–2783
Tingley R, Thompson MB, Hartley S, Chapple DG (2016) Patterns of niche filling and expansion across the invaded ranges of an Australian lizard. Ecography 39:270–280. doi:10.1111/ecog.01576
Titulaer M, van Oers K, Naguib M (2012) Personality affects learning performance in difficult tasks in a sex-dependent way. Anim Behav 83:723–730. doi:10.1016/j.anbehav.2011.12.020
Waldman B (1985) Olfactory basis of kin recognition in toad tadpoles. J Comp Physiol A 156:565–577
Wolak ME, Fairbairn D, Paulsen YR (2011) Guidelines for estimating repeatability. Methods Ecol Evol 3:129–137
Wright TF, Eberhard J, Hobson E, Avery ML, Russello M (2010) Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol Ecol Evol 22:393–404
Acknowledgements
We thank H. Moule and M. Bertram for assistance during fieldwork and H. Kang, D. Littlewood, and S. Walsh for help with lizard captive husbandry. R. San Martin, I. Stewart, and P. Arnold provided access to the animal housing facility and construction of experimental equipment. The project was conducted in accordance with our Monash University Animal Ethics Committee approvals (BSCI/2012/17, BSCI/2013/19, BSCI2014/11, BSCI/2014/26, BSCI/2015/04), associated scientific research permits (NSW: SL101203; VIC: 10006866, 10006867), and under special permission from Lane Cove National Park. Financial support was provided to CTG by the ANZ Trustees Foundation-Holsworth Wildlife Research Endowment and to DGC and BBMW by the Australian Research Council Discovery Project Grant (DP170100684).
Author information
Authors and Affiliations
Contributions
MC participated in the conception and design of the study, carried out the laboratory work, provided new methods, conducted data analysis, and manuscript revisions; MM and BM carried out the field work and assisted in the laboratory work; DC and BW participated in the conception and design of the study and contributed substantially to manuscript revisions; CG conception and design of the study and wrote the first draft of the manuscript. All authors have given final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
We have no competing interests.
Additional information
Communicated by Hannu J. Ylonen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chung, M., Goulet, C.T., Michelangeli, M. et al. Does personality influence learning? A case study in an invasive lizard. Oecologia 185, 641–651 (2017). https://doi.org/10.1007/s00442-017-3975-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3975-4