Abstract
The ability of an animal to acquire, process and learn from information in their environment is thought to be fundamental to fitness. We currently have a poor understanding of the learning ability of young animals within the first few months of their life, the types of learning they use and the extent of their learning ability. Furthermore, an animal’s developmental environment, such as nest incubation temperature, may profoundly influence motor and cognitive skills. We first tested the ability of hatchling three-lined skinks (Bassiana duperreyi) incubated at ‘hot’ and ‘cold’ temperatures to solve an instrumental (motor) task before assessing their ability to learn colour associations in a multi-stage instrumental task, with a choice reversal. While 53 (88.3 %) lizards successfully completed the training phase, 14 (46.7 %) of the ‘hot’ incubated and none of the ‘cold’ incubated lizards successfully completed the instrumental task. Thirteen of these lizards rapidly learnt to discriminate colours, and this culminated in eight individuals successfully completing a choice reversal. Hatchling B. duperreyi demonstrated surprisingly rapid learning, and these results highlight the potentially important role of cognition during development and ultimately, in fitness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognition—the mechanisms by which animals acquire, process and store information from their environment through perception, learning, memory and decision making (Shettleworth 2009)—is increasingly recognized as being subject to natural selection and therefore integral to fitness (Dukas 2009). Although much of the research on animal cognition has focused on adult individuals, early life experiences are likely to have a significant effect on cognitive ability and potentially fitness (Warkentin and Caldwell 2009). Higher vulnerability to predation during the early stages of life may select for behavioural flexibility and especially learning ability. Even embryos may be capable of reacting to external cues in ways that adaptively trade off development time against survival probability (Sih and Moore 1993; Warkentin and Caldwell 2009; Doody and Paull 2013). The conditions that an embryo experiences during incubation also can influence its cognitive abilities post-emergence. For example, when genetically identical mouse embryos are transferred into same-strain or hybrid uteri, mice that developed in hybrid uteri performed better on a multitude of cognitive tasks (Denenberg et al. 1998). Furthermore, brain development and subsequent cognitive ability in both humans and animals are influenced by a multitude of factors including exposure to androgens, chemical elements, prenatal stress, environmental complexity or enrichment, and social setting (e.g. Rice and Barone 2000; Chapillon et al. 2002; Gobbo and O’Mara 2004; Tomporowski et al. 2008). In many species, an embryo’s development can be influenced by temperature. Theoretically, this could also apply to neural development of the brain although this remains practically unstudied. In the honeybee, incubation temperature affects short-term, but not long-term, memory. Honeybees that were raised at higher temperatures (range tested, 32–36 °C) performed significantly better at short-term memory tests, while there was no significant effect on long-term memory (Jones et al. 2005).
In lizards, incubation temperature can have profound effects on a wide suite of phenotypic traits, from sex through to morphology and locomotor performance (Elphick and Shine 1998; Shine 2004a). Incubation temperature can also influence brain morphology. In the leopard gecko (Eublepharis macularius), incubation temperature determines the volume of the ventromedial nucleus of the hypothalamus and the preoptic area. Incubation temperature also affects the metabolic capacity of a multitude of brain regions (Coomber et al. 1997). To date, only one species of lizard has been tested for cognitive effects as a consequence of incubation temperature. Three-lined skinks (Bassiana duperreyi) incubated at warmer temperatures learnt the location of a safe refuge in the presence of a predatory threat faster than hatchlings incubated at lower temperatures (Amiel and Shine 2012) and also performed better in a Y-maze using a food reward (Amiel et al. 2013).
Typically, animals discriminate among objects using feature or positional cues (Day et al. 2003), depending on sensory modality. For example, some species rely on brightness over hue, or olfactory over visual cues. Lizards have access to a diverse array of sensory cues, reflecting well-developed chemosensory abilities (Halpern 1992) as well as high visual acuity (Fleishman et al. 2011). Diurnal lizards are tetrachromatic (Fleishman et al. 2011), and many species use colour cues to detect food and predators and to evaluate mates and rivals (reviewed in Cooper and Greenberg 1992). Thus, we might expect lizards to be capable of learning to discriminate among colours in response–reinforcer associations.
The three-lined skink (Bassiana duperreyi) is a medium-sized oviparous montane lizard with multifactorial (genetic plus temperature-dependent) sex determination and is widely distributed throughout southeastern Australia (Dubey and Shine 2010). The bright red throat colours of hatchling B. duperreyi, which fade a few weeks later (Shine 2004b), suggest that colour cues may be important in this species. In related studies, we have shown that incubation regimes influence the learning ability of B. duperreyi in a predator evasion task (Amiel and Shine 2012) and a maze task (Amiel et al. 2013). Here, we first assess whether incubation temperature also influences the ability of hatchlings to perform an instrumental task, before testing their ability to learn colour associations in a more complex multi-stage instrumental task, with a choice reversal.
Materials and methods
Collection and incubation of eggs
We collected B. duperreyi eggs from field sites in the Brindabella Range, 40 km west of Canberra in the Australian Capital Territory. The habitat at all sites consisted of grass, low shrubs and loose rock. Female B. duperreyi lay their eggs in communal nests in soft soil under rocks. Oviposition is synchronous and occurs in summer [late November to early December (Elphick and Shine 1998)]. We began surveying for eggs every 2 weeks in mid-November, prior to oviposition, so all the eggs that we collected had been laid only recently (<2 weeks prior to collection). In order to limit the use of full and half-siblings in our study, we collected eggs from different nests, at sites from five different elevations (1,060, 1,080, 1,240, 1,615 and 1,700 m a.s.l.). Some of the eggs we used came from the same communal nests and may have had common parents; however, this is likely to have occurred only rarely, as we randomly selected eggs from several nests at each site (see below for further details on egg selection).
Eggs were removed from natural nests and placed into 70 mL plastic jars filled with moist vermiculite for transport to the University of Sydney. Prior to incubation, we weighed each egg and transferred it to its own 64 mL glass jar filled with moist vermiculite (water potential = −200 kPa). Because egg size can affect offspring size, we randomised eggs to treatments such that mean egg mass did not differ significantly between the ‘cold’ and ‘hot’ treatments (mean ‘cold’, 0.44 ± 0.012 (N = 30); mean ‘hot’, 0.45 ± 0.012 (N = 29); t 57 = −0.34; P = 0.74). Jars were covered with plastic wrap to prevent moisture loss. We randomly divided eggs from each nest among four 10-step Clayson incubators (Brisbane, Queensland, Australia). Two incubators were programmed to mimic ‘hot’ natural nest conditions (diel cycle of 24 ± 5 °C), and two were programmed to mimic ‘cold’ conditions (diel cycle of 18 ± 5 °C). We created these thermal regimes using annual temperature data collected from over 300 natural B. duperreyi nests as part of a long-term study on this species (Shine et al. 1997). We monitored incubator temperatures using iButton thermochrons (Dallas, Texas, USA).
In the summer of 2011–2012, we incubated 609 eggs from 34 nests containing 4–77 eggs (mean ± 1 SE = 18.64 ± 2.83 eggs) for multiple experiments. From these eggs, we used a random subset of 60 hatchlings in the present study, including 30 ‘cold’ (19 males and 11 females) and 30 ‘hot’ (12 males and 18 females) individuals. We measured each hatchling’s snout–vent length (SVL) and total length (including tail) to the nearest 1 mm using a transparent ruler and weighed them using a Sartorius top-loading balance (Goettingen, GER), accurate to ± 0.01 g. We determined each hatchling’s sex by establishing the presence or absence of hemipenes through manual eversion (Harlow 1996).
Husbandry and maintenance
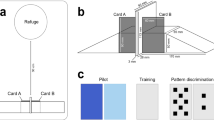
For the duration of the learning trials, we housed hatchlings individually in the same containers (200 × 140 × 60 mm) that we used for cognitive testing to facilitate learning [see Paulissen (2008)]. Each container had a clear plastic lid, paper towel substrate and a refuge at one end made from PVC piping cut in half (100 × 30 × 30 mm; Fig. 1). Room temperature was maintained at approximately 23 °C. There were four 40 W globes spaced evenly 50 cm above containers. These lights were on a timer and provided a 10:14 h light–dark cycle. We misted hatchlings with water once daily to keep them hydrated. Hatchlings were fed during the learning trials (see below). These conditions were maintained for all hatchlings throughout the six stages or until an individual was removed from the experiment. Hatchlings were 10 days old at the start of the learning trials and were given 24–48 h to acclimatize to their containers prior to the first learning trial. To standardize hunger levels, we withheld food for 24 h prior to the first trial.
General experimental design
During behavioural trials, a wooden block with three different feeding wells was placed in each hatchling’s home container (Fig. 1). We then tested the ability of B. duperreyi hatchlings to locate a food reward (cricket: Acheta domesticus) in a series of behavioural tasks (see below and Fig. 2) similar to the methods of Leal and Powell (2012). For each task, including the training phase, hatchlings had to successfully complete five out of six consecutive trials in order to proceed to the next task (i.e., more than one error within that set of six trials meant that the lizard was not classified as having learned the task). Hatchlings had a maximum of 20 trials to successfully complete each task, except that we gave lizards that successfully completed three of their final six trials an additional five trials to complete the task. Trial duration was 2.5 h; any lizard that had not located its food reward within this period failed the trial. If a lizard did not complete five out of six trials on a task within the allotted total number of trials, it was removed from the experiment. We tested hatchlings twice per day with a minimum 45 min rest period between trials. We recorded all trials using security cameras (OzSpy 600 TVL dome cameras) linked to a digital video recorder (OzSpy H.264 Network DVR) and a single researcher (BFC) scored all of the videos.
Schematic of all tasks including training phase 1 and 2, in sequence, with a brief description of each task. The reward was a small cricket placed into the appropriate well. The covers corresponding to the reward were yellow (training phase 2, task 1), blue (task 2 and 3), and green (task 4: choice reversal)
Training phase 1 and 2
Training phase 1 acted as a habituation process, allowing us to introduce the feeding block to the hatchlings. We were also able to assess hatchlings’ ability to access the food and consistently eat from the feeding wells. We placed a cricket in the central feeding well and positioned a yellow cap next to the well, without covering any portion of the opening (Fig. 2). The size of the cricket in relation to the depth of the well prevented the cricket from escaping or being at all visible to the lizard. Successful completion of this task required a hatchling to locate and consume the cricket. Training phase 2 was identical to training phase 1 except that we positioned the cap so that it obscured half of the opening to the feeding well (Fig. 2). This did not restrict the view or access to the crickets (i.e. the cricket could be obtained without the hatchling having to manipulate the cap).
Task 1: novel motor task
This was the first cognition task where the hatchlings had to manipulate an obstacle using some form of motor skill. The cricket remained in the central feeding well but the yellow cap now completely covered the opening to the well (Fig. 2). Individuals could not see the cricket or access it without moving the cap. To successfully complete this task, a hatchling had to maneuver past the obstacle and consume the cricket. This was the first stage where the hatchling encountered a true, novel problem.
Task 2: colour association/spatial discrimination
In this task, we presented hatchlings with three novel obstacles of identical size and shape but different colours. Blue, white and green caps were placed over each of the three feeding wells (Fig. 2). The cricket was placed under the blue cap in either the left or right feeding well (always same location for an individual lizard and balanced for sample size). Thus, hatchlings could not rely on previous colour associations or spatial locations to complete the task. This task was not designed to discriminate between these two possible mechanisms: hatchlings could locate the food reward using colour or spatial cues, or some combination of the two. To successfully complete this task, a hatchling had to remove the blue cap first and consume the cricket. If the green or white caps were removed first, the hatchling failed the trial. In order to minimise disturbance to the lizard colony, the cricket and the caps were removed at the end of a failed trial, after 2.5 h, but the wooden block was left in place. From this task onward, every feeding well was scented with cricket odour and integumentary parts prior to each trial, to control for the use of olfactory cues.
Task 3: colour association
In this task, the colours of the obstacles remained the same (blue, white and green) and the cricket remained under the blue cap. However, the position of the blue cap now rotated among the three feeding wells in a randomized order, never repeating the same location twice in two consecutive trials (Fig. 2). This allowed us to separate the use of colour (hue/brightness) cues from that of spatial cues. As in task 2, hatchlings had to displace the blue cap first and consume the cricket to successfully complete a trial—but the hatchlings had to rely on a single cue (colour hue/brightness) to locate the cricket.
Task 4: choice reversal
In this task, the colours of the obstacles were the same as in tasks 2 and 3 and we continued to randomly rotate the covers as in Task 3. However, the cricket was now located under the green cap (Fig. 2). This task required the hatchlings to form a novel association between the green cap and the food reward. To successfully complete this task, hatchlings had to remove the green cap first and consume the cricket.
Data analysis
We used generalized linear models with a Gaussian error distribution (identity link function) to assess the relationship between incubation treatment and morphology (SVL, mass) while taking sex into account. We also included an interaction between sex and treatment and accounted for SVL in our models testing for treatment effects on mass. In models using mass as a dependent variable, we log transformed SVL and mass prior to analysis. Normality was assessed through visual inspection of residual plots and using Shapiro-Wilk’s normality tests; homogeneity of variance was tested using Levene’s test. We found no evidence for significant heterogeneity of variance (P > 0.05 in all cases). We simplified models using likelihood ratio tests (LRT), excluding non-significant parameters until the full model contained only significant predictors. One data point was excluded from all morphological analyses because it was an extreme outlier as a result of measurement error. We used a Fisher’s exact test to compare the counts of ‘hot’ and ‘cold’ incubated, as well as male and female hatchlings that successfully completed each stage. To test differences in the rate to acquisition (number of trials to complete training phases), we used Wilcoxon rank sum tests, as groups were independent. We examined rates of learning (number of trials to acquisition) in the visual discrimination tasks using Wilcoxon signed ranks (matched pairs) tests (task 1 vs. 2, task 2 vs. 3, task 3 vs. 4). All means are reported ±1 SE.
Results
The proportion of individuals making incorrect choices on a given trial was high across all trials (remaining close to 50 % for most tasks; Fig. 3), suggesting that lizards were learning rather than relying on auditory or chemical cues to find the reward.
Incubation effects on morphology
Hatchlings from the ‘hot’ incubation treatment were larger (SVL) than ‘cold’ hatchlings (‘hot’ mean, 25.7 ± 0.15 mm; ‘cold’ mean, 24.77 ± 0.20 mm; LRT: treatment effect, F = 8.23, P = 0.006, N = 59; Fig. 4) and females were larger than males (LRT: sex effect, F = 19.63, P < 0.001, N = 59). There was no evidence for an interaction between sex and treatment (LRT: sex × treatment effect, F = 0.80, P = 0.37, N = 59) and the top-model only contained main effects (top model: intercept: β = 25.47 ± 0.20, t 1,56 = 127.77, P < 0.001; sex(male): β = −0.93 ± 0.21, t 1,56 = −4.43, P < 0.001; Trt(hot): β = 0.60 ± 0.21, t 1,56 = 2.87, P < 0.01). When controlling for SVL, both incubation treatment (LRT: F = 6.02, P = 0.02, N = 59) and sex (LRT: F = 12.97, P < 0.001, N = 59) significantly affected offspring mass. ‘Hot’ hatchlings were heavier than ‘cold’ hatchlings and males were heavier than females (top model, intercept: β = −7.39 ± 0.84, t 1,55 = −8.79, P < 0.001; log(SVL): β = 1.90 ± 0.26, t 1,56 = 7.34, P < 0.001; sex(male): β = 0.07 ± 0.02, t 1,56 = 3.60, P < 0.001; Trt(hot): β = 0.04 ± 0.02, t 1,56 = 2.45, P = 0.02). Sex and treatment did not have a significant interaction (LRT: F = 0.92, P = 0.34, N = 59).
Incubation effects on motor task
Overall, 53 (88.3 %) hatchlings successfully completed the training (training phase 1 and 2; Figs. 4 and 5). Successful completion of the training phase was unrelated to incubation treatment: training phase 1 (N = 28 hot vs. N = 25 cold; Fisher’s exact test: P = 0.42) or training phase 2 (N = 28 hot vs N = 24 cold; P = 0.47). ‘Hot’ and ‘cold’ incubated lizards required a similar number of trials to complete training phase 1 (‘hot’: 10.39 ± 0.97 vs. ‘cold’ 9.63 ± 0.87: Wilcoxon test, W = 359, P = 0.68) and training phase 2 (‘hot’ 7.54 ± 0.86 vs. ‘cold’ 8.13 ± 0.96: Wilcoxon test, W = 295.5, P = 0.43). However, no ‘cold’ hatchling successfully completed any of the subsequent learning tasks, so we could not statistically compare learning rates on task 1 between incubation treatments. The failure of ‘cold’ incubated lizards to successfully complete the motor task was not explained by body size/condition constraints because there was considerable overlap in both body size and body condition between the ‘cold’ and ‘hot’ treatments (Fig. 4).
Cognition trials
Sex effects on training phase and novel motor task (task 1)
Successful completion of a task was unrelated to sex in both training phase 1 (N = 26 males vs. N = 27 females; Fisher’s exact test: P = 0.43) and training phase 2 (N = 25 males vs. N = 27 females; Fisher’s exact test: P = 0.49) (Fig. 5). Also, males and females required a similar number of trials for acquisition in training phase 1 (males, 9.92 ± 0.94; females, 10.15 ± 0.92; Wilcoxon test: W = 333.5, P = 0.95) and training phase 2 (males, 7.28 ± 0.77; females, 8.30 ± 1.0; Wilcoxon test: W = 291.5, P = 0.37). Of the 28 hatchlings to complete training phase 2, 14 (50 %) completed task 1 (novel motor task) (mean number of trials, 12.07 ± 1.75; Fig. 6) and these were all from the ‘hot’ treatment. Significantly (Fisher’s exact test: P = 0.03) more females (N = 11) completed the novel motor task than did males (N = 3) (Fig. 5).
Task 2: colour association/spatial discrimination
Of the 14 hatchlings that completed task 1 (novel motor task), 13 completed task 2 (mean number of trials: 11.85 ± 1.60; Fig. 6) and the number of attempts to successfully complete task 1 and task 2 did not differ significantly (Wilcoxon signed ranks test: Z = −0.21, P = 0.83).
Task 3: colour association
All 13 hatchlings that completed task 2 also completed task 3 (mean number of trials, 5.92 ± 0.37; Fig. 6), in significantly fewer attempts than they had required for task 2 (Wilcoxon signed ranks test: Z = −2.751, P = 0.01).
Task 4: choice reversal
Of the 13 hatchlings that completed task 3, eight completed task 4 (mean number of trials, 16.63 ± 1.30; Fig. 6), although this required significantly more trials than they had required for task 3 (Wilcoxon signed-rank test: Z = 2.527, P = 0.012).
Discussion
Hatchling lizards from both the ‘hot’ and ‘cold’ incubation temperatures quickly learnt the location of the food reward during the training phase, where the well containing the reward was adjacent to the yellow cap. However, once the yellow cap completely covered the food well (instrumental task), only the ‘hot’ incubated hatchlings were able to displace the cover to access the food. The act of removing the cap was purposeful and often preceded by a circling of the cover. Overall, 13 hatchlings completed the first two visual discrimination tasks (tasks 2 and 3) and eight hatchlings completed the choice reversal (task 4). Therefore, B. duperreyi is capable of learning a visual discrimination task using colour (hue/brightness) cues. Hatchlings located the food reward when we provided them with both colour and spatial cues (task 2) and they learnt to locate the food reward once we began rotating the position of the reward cover (blue cap), eliminating their ability to use spatial cues (Task 3). Furthermore, hatchlings completed task 3 faster than task 2 (Fig. 6). The colour of the reward obstacle did not change between tasks 2 and 3, and thus, the improved performance of the hatchlings suggests that they rapidly learnt to associate the reward with a colour cue rather than a spatial cue and then maintained this colour association in task 3.
Might the hatchlings have used other visual, olfactory or auditory cues to locate the food reward? First, the obstacles (caps) were identical in size and shape, leaving colour as the only visual cue available to the hatchlings. Second, to control for scent cues we scented all of the feeding wells with cricket integumental products. Unfortunately, we did not conduct any trials in which the location of the food reward was switched following successful completion of a task, and therefore, we cannot entirely rule out the possibility of sensory cues influencing decisions by individuals. Nevertheless, we can address whether hatchlings were using sensory cues as opposed to associative learning by assessing performance in the visual discrimination tasks. If lizards were using auditory/visual cues we would predict that they would go directly to the feeding well containing the cricket in each trial. However, in the two tasks where we presented the hatchlings with a novel colour association (tasks 2 and 4), the number of trials they took to locate the reward increased, and they made frequent mistakes during the learning phase. Overall, for the training phases and tasks 1 and 2, there were still a large proportion of individuals who choose incorrectly and this number was consistent across 15 trials (Fig. 3). This is not what we would predict from learners if they were simply honing in on auditory or chemical cues to choose the correct dish. In that scenario, we would expect lizards to make a low number of mistakes consistently across all trials. The patterns in learning that we detected thus strongly suggest that the lizards learnt to discriminate colours and associate particular colours with a food reward.
In other work, we have found that hatchling B. duperreyi from eggs that were incubated at warmer temperatures out-performed conspecifics incubated at lower temperatures in both a predator evasion task (Amiel and Shine 2012) and in a maze learning trial (Amiel et al. 2013). In the current study, incubation temperature regimes did not affect a young lizard’s proficiency at learning the two training phases. However, the ‘cold’ incubated hatchlings were unable to complete the motor task, precluding any comparison of learning rates between our incubation treatments in any of the visual discrimination tasks. Hatchlings from the ‘hot’ treatment were larger (SVL) and heavier-bodied (mass relative to SVL) than were those from the ‘cold’ treatment. Thus, body size may be correlated with learning ability. However, the considerable overlap in body size between individuals from the two incubation treatments (Fig. 4) suggests a direct effect of low incubation temperature on cognition per se, rather than a secondary effect mediated via body size. The inability of cold-incubated lizards to complete the motor task was not due to a physical inability to remove the cap. Indeed, three individuals from the ‘cold’ treatment removed the cap covering the food reward on multiple occasions, but none successfully completed five out of six consecutive trials (and thus did not advance to the subsequent task). Also, most individuals from the ‘cold’ treatment group successfully completed both training phases, suggesting that motivation was not an issue.
In other species of lizards, there is evidence that incubation temperature influences brain morphology although complementary tests of cognitive ability are lacking. For example, in the leopard gecko (E. macularius) incubation temperature has a significant effect on the volume of specific brain regions and also interacts with sex to determine metabolic capacity in a multitude of brain regions. We have also detected key differences in brain morphology in relation to incubation temperature (JJA, RS unpublished data), which may explain functional differences in cognition.
Of the 14 individuals from the ‘hot’ group that successfully completed the novel motor task (task 1), 11 were females and only three were males. This small number of males limited our ability to compare learning rates in males and females beyond the motor task. Properly testing for sex differences in learning colour discrimination might be facilitated by a task that is less dependent on motor skills.
Our results demonstrate that B. duperreyi are capable of learning a novel instrumental task, as well as colour discrimination through associative learning. The tasks we designed became progressively more difficult and we changed the colour of the reward dish twice. To confirm that lizards were forming a colour association and were not simply using spatial cues, we continuously changed the position of the reward dish in relation to two other dishes (Task 3). Finally, of the 13 lizards that completed task 3 (colour association), 8 successfully completed a choice reversal (in which the reward was switched from a blue to a green cover). These results, together with other recent studies of B. duperreyi that demonstrate learning in the presence of a predatory threat (Amiel and Shine 2012) and in a maze (Amiel et al. 2013) are strongly suggestive of behavioural flexibility. Importantly, these are independent studies of different individuals. The criteria for behavioural flexibility include the solving of novel problems or the solving of old problems in a novel way through the modification of an existing behaviour (Leal and Powell 2012). A further indication of behavioural flexibility is proficiency across multiple cognitive tasks. We did not specifically set out to quantify behavioural flexibility, but proficiency in a novel instrumental task and associative learning of a colour cue (including a choice reversal) make for a compelling case.
Comparative cognition seeks to understand the evolution of learning (including perception, decision making and memory) and how animals acquire and process information (Shettleworth 2009). Recent work on lizards suggests that they are capable of rapid learning (e.g. Amiel and Shine 2012; LaDage et al. 2012; Noble et al. 2012; also see review in Burghardt 1977) and behavioural flexibility (this study; Leal and Powell 2012). Methodological differences among studies of lizard cognition preclude quantitative comparisons across taxa, but such comparisons would be of great interest. Notably, B. duperreyi in our study demonstrated rapid learning of a colour association that also carried over to a choice reversal. Such abilities may well prove to be widespread in squamate reptiles—even in (or perhaps, especially in) very young individuals. The current study was based on very young lizards that were exposed to cognition trials within weeks of hatching. The ability to rapidly acquire and process information, particularly during an early and vulnerable life stage, may be an important and underappreciated dimension of any organism’s life, with strong effects on organismal viability and thus evolutionary fitness.
References
Amiel JJ, Lindström T, Shine R (2013) Egg incubation effects generate positive correlations between size, speed and learning ability in young lizards. Anim Cogn. doi:10.1007/s10071-013-0665-4
Amiel JJ, Shine R (2012) Hotter nests produce smarter young lizards. Biol Lett 8:372–374
Burghardt GM (1977) Learning processes in reptiles. In: Gans C, Tinkle DW (eds) Biology of the Reptilia, vol 7, Ecology and Behaviour A. Academic Press, London, pp 555–681
Chapillon P, Patin V, Roy V, Vincent A, Caston J (2002) Effects of pre- and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: A review. Dev Psychobiol 41:373–387
Coomber P, Crews D, Gonzalez-Lima F (1997) Independent effects of incubation temperature and gonadal sex on the volume and metabolic capacity of brain nuclei in the leopard gecko (Eublepharis macularius), a lizard with temperature-dependent sex determination. J Comp Neurol 380:409–421
Cooper W, Greenberg N (1992) Reptilian coloration and behavior. Biol Reptil 18:298–422
Day LB, Ismail N, Wilczynski W (2003) Use of position and feature cues in discrimination learning by the whiptail lizard (Cnemidophorus inornatus). J Comp Psychol 117:440–448
Denenberg VH, Hoplight BJ, Mobraaten LE (1998) The uterine environment enhances cognitive competence. Neuroreport 9:1667–1671
Doody JS, Paull P (2013) Hitting the ground running: environmentally cued hatching in a lizard. Copeia 2013:160–165
Dubey S, Shine R (2010) Evolutionary diversification of the lizard genus Bassiana (Scincidae) across Southern Australia. PLoS ONE 5:e12982. doi:10.1371/journal.pone.0012982
Dukas R (2009) Learning: mechanisms, ecology, and evolution. In: Dukas R, Ratcliffe JM (eds) Cognitive ecology II. Chicago University Press, Chicago, pp 7–26
Elphick MJ, Shine R (1998) Long-term effects of incubation temperatures on the morphology and locomotor performance of hatchling lizards (Bassiana duperreyi, Scincidae). Biol J Linn Soc 63:429–447
Fleishman LJ, Loew ER, Whiting MJ (2011) High sensitivity to short wavelengths in a lizard and implications for understanding the evolution of visual systems in lizards. Proc R Soc Lond B 278:2891–2899
Gobbo O, O’Mara S (2004) Impact of enriched-environment housing on brain-derived neurotrophic factor and on cognitive performance after a transient global ischemia. Behav Brain Res 152:231–241
Halpern M (1992) Nasal chemical senses in reptiles: structure and function. In: Gans C (ed) Biology of the Reptilia: hormones, brain, and behaviour, vol 18, Physiology E. Chicago University Press, Chicago, pp 423–523
Harlow PS (1996) A harmless technique for sexing hatchling lizards. Herpetol Rev 27:71–72
Jones JC, Helliwell P, Beekman M, Maleszka R, Oldroyd BP (2005) The effects of rearing temperature on developmental stability and learning and memory in the honey bee, Apis mellifera. J Comp Physiol A 191:1121–1129
LaDage L, Roth T, Cerjanic A, Sinervo B, Pravosudov V (2012) Spatial memory: are lizards really deficient? Biol Lett 8:939–941
Leal M, Powell BJ (2012) Behavioural flexibility and problem-solving in a tropical lizard. Biol Lett 8:28–30
Noble DWA, Carazo P, Whiting MJ (2012) Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol Lett 8:946–948
Paulissen MA (2008) Spatial learning in the little brown skink, Scincella lateralis: the importance of experience. Anim Behav 76:135–141
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Persp 108(Suppl 3):511–533
Shettleworth SJ (2009) Cognition, evolution, and behavior. Oxford University Press, New York
Shine R (2004a) Incubation regimes of cold-climate reptiles: the thermal consequences of nest-site choice, viviparity and maternal basking. Biol J Linn Soc 83:145–155
Shine R (2004b) Does viviparity evolve in cold climate reptiles because pregnant females maintain stable (not high) body temperatures? Evolution 58:1809–1818
Shine R, Elphick MJ, Harlow PS (1997) The influence of natural incubation environments on the phenotypic traits of hatchling lizards. Ecology 78:2559–2568
Sih A, Moore RD (1993) Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am Nat 142:947–960
Tomporowski P, Davis C, Miller P, Naglieri J (2008) Exercise and children’s intelligence, cognition, and academic achievement. Educ Psychol Rev 20:111–131
Warkentin KM, Caldwell MS (2009) Assessing risk: embryos, information, and escape hatching. In: Dukas R, Ratcliffe J (eds) Cognitive ecology II: the evolutionary ecology of learning, memory, and information use. University of Chicago Press, Chicago, pp 177–200
Acknowledgments
Funding of this study was from Australian Research Council to RS, Natural Sciences and Engineering Research Council of Canada to JJA and DWAN, and a Macquarie University internal grant to BFC and MJW. We thank the reviewers for their constructive criticism of our study.
Ethical standards
All work was carried out under the approval of the Animal Ethics Committee of the University of Sydney (ARA 5361) in agreement with the Animal Ethics Committee of Macquarie University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. J. Downes
Rights and permissions
About this article
Cite this article
Clark, B.F., Amiel, J.J., Shine, R. et al. Colour discrimination and associative learning in hatchling lizards incubated at ‘hot’ and ‘cold’ temperatures. Behav Ecol Sociobiol 68, 239–247 (2014). https://doi.org/10.1007/s00265-013-1639-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1639-x