Abstract
Background
Lower activation of higher threshold (type-II) motor units (MUs) has been suggested in children compared with adults. We examined child–adult differences in discrete MU activation of the flexor carpi radialis (FCR).
Methods
Fifteen boys (10.2 ± 1.4 years), and 17 men (25.0 ± 2.7 years) completed 2 laboratory sessions. Following a habituation session, maximal voluntary isometric wrist flexion torque (MVIC) was determined before completing trapezoidal isometric contractions at 70%MVIC. Surface electromyography was captured by Delsys Trigno Galileo sensors and decomposed into individual MU action potential trains. Recruitment threshold (RT), and MU firing rates (MUFR) were calculated.
Results
MVIC was significantly greater in men (10.19 ± 1.92 Nm) than in boys (4.33 ± 1.47 Nm) (p < 0.05), but not statistically different after accounting for differences in body size. Mean MUFR was not different between boys (17.41 ± 7.83 pps) and men (17.47 ± 7.64 pps). However, the MUFR–RT slope was significantly (p < 0.05) steeper (more negative) in boys, reflecting a progressively greater decrease in MUFR with increasing RT. Additionally, boys recruited more of their MUs early in the ramped contraction.
Conclusion
Compared with men, boys tended to recruit their MUs earlier and at a lower percentage of MVIC. This difference in MU recruitment may explain the greater decrease in MUFR with increasing RT in boys compared with men. Overall, these findings suggest an age-related difference in the neural strategy used to develop moderate–high torque in wrist flexors, where boys recruit more of their MUs earlier in the force gradation process, possibly resulting in a narrower recruitment range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Even after accounting for differences in body size, children are not as strong as adults (e.g., Blimkie 1989; De Ste Croix et al. 1999; Falk et al. 2009). In addition to their smaller body and muscle size, children also have been reported to have smaller muscle pennation angles (Dobrzynska and Celichowski 2016; Kannas et al. 2010; Kumar et al. 2021; Radnor et al. 2020; Weide et al. 2015), a greater percentage of slow twitch (type-I) muscle fibers (Esbjörnsson et al. 2021; Lexell et al. 1992; Metaxas et al. 2019), and more compliant tendons (Lambertz et al. 2003; Oliver and Smith 2010). However, even after accounting for these differences, age-related differences in muscle performance persist (Dotan et al. 2012, 2013; O’Brien et al. 2010). Therefore, there are other factors contributing to age-related differences in muscle performance and metabolism between children and adults.
During maximal voluntary isometric contractions (MVIC), children are characterized by lower volitional muscle activation compared with adults (Woods et al. 2022). This difference in muscle activation can account for many age-related differences in muscle performance, such as children’s lower size-normalized maximal strength (Grosset et al. 2008; O’Brien et al. 2010). In line with the Henneman size principle (Henneman 1957), Dotan et al. (2012) hypothesized that children’s lower volitional activation reflects lesser activation of specifically the higher threshold (primarily type-II) motor units (MUs). This hypothesis has been supported by studies examining age-related differences in the surface electromyographic (sEMG) threshold. The sEMG threshold is suggested to reflect the exercise intensity at which there is accelerated recruitment of type-II MUs (Hug et al. 2003; Moritani and DeVries 1978). During progressive cycling and intermittent isometric contractions, the vastus lateralis sEMG threshold has been found to occur later and at higher relative exercise intensities in children compared with adults (Long et al. 2017; Pitt et al. 2015; Woods et al. 2019, 2020), suggesting that children recruit higher threshold MUs to a lesser extent. However, the traditional sEMG signal does not distinguish between individual MUs and does not allow the determination of MU recruitment and firing rates (Farina et al. 2004). Thus, it is unclear whether the age-related difference in the sEMG threshold is due to children’s lesser type-II MU recruitment or to a lower MU discharge rate.
With advancements in technology, it is now possible to examine discrete MU activation from the surface of the skin. Recently, a few studies have used this technology to examine differences in discrete MU activation between children and adults in the quadriceps (vastus lateralis and vastus medialis) and first dorsal interossei (FDI) muscles (Chalchat et al. 2019; Miller et al. 2019; Parra et al. 2020), with varied results. For example, Chalchat et al., (2019) demonstrated no differences in MU firing rates (MUFR) between boys and men, during knee extensions from 20 to 80%MVIC. On the other hand, during second-digit abduction at 30%MVIC, Miller et al. (2019) observed greater FDI MUFR in boys compared with men. These inconsistent findings may be related to differences in the muscle examined or contraction protocol (i.e., intensity, sustained vs progressive).
Antagonist coactivation has also been suggested to play a role in children’s lower size-normalized maximal strength, neuromuscular efficiency and movement economy (Frost et al. 1997, 2002; Grosset et al. 2008; Lambertz et al. 2003). Specifically, higher antagonist coactivation may result in higher agonist MUFR. It has also been suggested to explain the children’s greater normalized sEMG amplitude during submaximal contractions (Grosset et al. 2008; Miller et al. 2019). On the other hand, we recently found that during isometric contractions, antagonist coactivation is similar in children and adults (Woods et al. 2023). Therefore, the role of antagonist coactivation in age-related differences in MU activation patterns is unclear.
The flexor carpi radalis (FCR) muscle is characterized by a mixed fiber type (50% type-I fibers) and a relatively narrow recruitment range, where most MU’s are recruited by ~ 50%MVIC (Clamann 1993; McIntosh et al. 1985; Wernbom & Aagaard 2019). Compared to muscles with a broader recruitment range, the narrow recruitment strategy utilized by the FCR allows for the examination of a relatively large sample of high-threshold MUs during moderate, sustainable contraction intensities. By comparison, in the vastus lateralis, MUs are recruited up to 80–90%MVIC (DeLuca & Contessa 2012). Therefore, high-threshold MUs can only be identified during relatively high contraction intensities, which are usually not sustainable for a sufficiently long duration for discrete MU analysis. It should also be noted that typical physical training does not usually involve the FCR but does involve other muscles such as the quadriceps or biceps brachii. Thus, examination of MU activation in the FCR in children and adults may reveal maturation-related changes, with minimal effect (if any) of training or long-term use.
The purpose of our study was to examine differences in FCR MU activation between boys and men during isometric wrist flexion. We used a 70%MVIC contraction intensity to maximize MU recruitment (Clamann 1993; McIntosh et al. 1985; Wernbom & Aagaard 2019). Secondly, we aimed to assess the influence of antagonist coactivation on age-related differences in MU activation. We hypothesized that MUFR would be lower in boys compared with men, specifically among higher threshold MUs. Additionally, we hypothesized that coactivation would be low, and not different between boys and men.
Methods
Participants
Participants included in the study were involved in a larger study examining child–adult differences in neuromuscular function. The results of that study have not yet been published. Thirty-four boys (7–12 year-old) and 22 men (18–30 year-old) volunteered to participate in the study. Participants were healthy and recreationally active, not participating in any structured training program > 5 h per week. Participants were excluded if they were taking any medication which may influence neurological function. Both boys and men reported they participated in regular sessions of vigorous, moderate and mild exercise on a weekly basis. Due to COVID-19 restrictions, fitness centres were closed and sports programs were on hold at the time of the data collection. However, many boys had been regularly involved in activities such as hockey, volleyball, baseball, basketball, lacrosse, and jujitsu 4–6 months before they participated in the study. Before participating in the study and signing informed consent and assent forms, a thorough explanation of the study’s purpose, measurement procedures, benefits and potential risks or discomforts were provided to all participants and the boys’ parents/guardians. All study procedures were reviewed and cleared by our university’s Research Ethics Board (REB# 20-066). The participant’s physical characteristics appear in Table 1.
Participants’ data were only included in the final analysis if the following criteria were met: (1) participants were able to execute and sustain 70%MVIC target torque for one of two trials, and (2) the MU data met all the validation criteria (see below). In addition, only boys who self-assessed themselves to be pre- or early-pubertal (Tanner 1962) were included in the analysis. Data from one man were excluded as their MVIC could not be accurately calculated (technical malfunction). Thus, data from 15 boys and 17 men were included in the final analysis. All participants included in the final analysis were right-handed, except for one boy who reported being left-handed and one man who reported being ambidextrous.
Experimental procedures
Participants made two visits to the laboratory, completing a habituation and an experimental session. Participants were asked to refrain from strenuous exercise, and caffeine consumption for 24, and 12 h, respectively prior to all testing sessions. During the first visit anthropometric measures, including height, seated height (boys only), mass, and body composition (see anthropometric measurements section below) were obtained. The dynamometer was then fitted to each participant prior to performing maximal strength tests for wrist flexor and extensor muscle groups. Participants were habituated to maximal and submaximal contraction protocols (see exercise protocols, below). During the second visit, subcutaneous fat thickness underlying the surface electrodes was determined using ultrasound (see anthropometric measurements section below), before the assessment of maximal strength (MVIC). Following a 2-min rest, participants then performed two trials of submaximal contractions at 70%MVIC.
Anthropometric measurements
Standing and sitting (boys only) height were measured to the nearest 0.1 cm (Ellard Instrumentation Ltd. Stadiometer). Body mass and fat percentage were measured to the nearest 0.1 kg and 0.1%, respectively, by bioelectrical impedance analysis (InBody 520, Biospace CO., Ltd., S. Korea). Participants were hydrated upon arrival to the laboratory and asked to void their bladder before measurements.
Ultrasound (B-Mode; Vivid-q; General Electric Healthcare, USA) was used to assess the thickness of subcutaneous fat tissue underlying the FCR and ECR electrode sites (see below). Subcutaneous fat tissue thickness was measured from the deepest aspect of the dermis to the deepest aspect of the subcutaneous tissue layer. During measurement, the probe was placed on the forearm with as little pressure as possible so that the underlying tissues would not be compressed. The median of three measurements was used to characterize subcutaneous fat tissue thickness at each site.
Pubertal status was self-assessed based on secondary sex characteristics of pubertal hair, using drawings, as outlined by Tanner (1962).
Contraction protocols
All exercise protocols were performed using the right arm on a custom, purpose-built dynamometer placed adjacent to a Biodex dynamometer seat. The participant’s right forearm and hand were secured to the dynamometer using custom-made clamps (Fig. 1). Contractions were performed with the wrist and hand in a neutral position and elbow at ~ 120° of flexion. Special care was taken to ensure that contractions were performed only with the wrist flexors, with no movement of the fingers or shoulder. To minimize the use of other muscles during the wrist flexion task, participants were secured to the seat using straps, with hips at 60° of flexion. Before performing the maximal strength assessments participants were guided through a warm-up consisting of three, 5–10 s isometric contractions at progressing intensity from ~ 50 to 80% of maximal perceived effort. In order to determine the maximal strength of the wrist flexors and extensors, participants completed three maximal voluntary isometric contractions (MVIC), separated by 2 min of rest. Maximal strength was then determined as the highest 500 ms of torque achieved during one of the trials. During MVIC trials, participants were provided visual feedback and verbal encouragement to ensure a maximal performance was achieved.
Isometric trapezoidal contractions at 70%MVIC were used to assess submaximal MU activation. Contractions comprised a steady progression of torque development at a rate of 10%MVIC/second to 70%MVIC, an isometric hold at 70%MVIC lasting 12 s and then a steady release of torque back to baseline at a rate of 10%MVIC/second. Two trials were performed with a 2-min rest interval.
Data acquisition
Neuromuscular activity was recorded by Delsys Inc. Trigno Galileo Sensors at 2222 Hz. The sensors comprised four silver electrode pins arranged in a diamond configuration, with an inter-electrode distance of 5 mm. EMG signals were digitized and filtered at 20–450 Hz using a 16-bit A/D converter before being transmitted using Bluetooth to a base station (Delsys Inc., Natick, MA, USA). The skin overlying the FCR and extensor carpi radialis (ECR) was thoroughly prepared by shaving, then performing a series of tape pulls to remove dead skin cells, before lastly cleaning the area using rubbing alcohol. Electrode sites were determined using palpation of the muscles during repeated wrist flexions and extensions. The reference for each sensor was placed on the belly of the biceps brachii muscle (see Fig. 1). Sensors were secured to the skin surface using double-sided adhesives, and taping over the head of the sensor.
Force was measured via a load cell (Interface Force measurement solutions, Pickering ON, Canada) attached in series to where participants applied force on the purpose-built dynamometer (Fig. 1). The force signal was digitized (16-bit A/D) then Bluetooth transmitted by a Delsys Inc. Wireless Trigno Analog Adapter at a rate of 2222 Hz (Delsys Inc), to a base station and filtered at DC-100 Hz. Force signals were all captured in Volts and converted to torque during the initial phases of data processing (see below). Force and EMG signals were synchronized and transmitted simultaneously.
Data proccessing
The force signal was imported into MATLAB (v.2020b, MathWorks Inc., Natick MA, USA) and LabView (National Instruments, Austin, TX, USA) and converted from Volts to Newtons. MVIC was determined as the highest 500 ms of force achieved. The mean submaximal force was assessed during the most stable 3 s period (lowest standard deviation) of the trapezoid contraction. The mean submaximal force was then expressed as a percentage of MVIC force. During the same data window, the Coefficient of variation (CV) of force was calculated using the following formula: (standard deviation of force/mean force) × 100. Force was converted to torque (Nm) by multiplying by the dynamometer’s moment-arm length (0.059 m) (fixed distance). For the submaximal trials, torque was expressed as a percentage of MVIC.
Overall muscle activation and antagonist coactivation were assessed from the sEMG signal collected in the first of the four sEMG channels. All calculations were performed in MATLAB (v.2020b). sEMG root mean squared (EMGRMS) amplitude was calculated for the MVIC and 70%MVIC trials during the highest 500 ms and most stable 3 s of torque (lowest standard deviation), respectively. FCR EMGRMS at 70%MVIC was expressed as a percentage of EMGRMS calculated during the MVIC. Antagonist coactivation was calculated as the ECR EMGRMS, expressed as a percentage of its maximal EMGRMS amplitude when acting as an agonist (Falk et al. 2009; Kellis and Unnithan 1999).
Once collected, sEMG data were decomposed into individual motor-unit action potential trains by Delsys Neuromap software V.4.7.3, using the newest version of precision decomposition software (EMGworks 4.7.6; De Luca and Contessa 2012; De Luca and Hostage 2010). Following decomposition, all MU data were exported from NeuroMap and validated using spike trigger averaging (STA) procedures in LabView (Herda et al. 2020; National Instruments Corp.). NeuroMap-derived motor-unit action potential (MUAP) templates and firing times were used to perform STA analysis on each sEMG channel. MUs were included in the final analysis if the coefficient of variation of the STA derived MUAP P–P amplitude was < 0.3 across all 4 channels and if the correlation coefficient between the P–P amplitude of NeuroMap-derived templates and the STA-determined amplitude was > 0.7. These MUs had a reconstruct-and-test accuracy (Delsys-determined) of 91 and 92% for boys and men, respectively. Additionally, participant’s data were included in the analysis if the recruitment threshold (RT) range was > 1/3 of the contraction intensity, and ≥ 5 MUs met all the above criteria. This resulted in the exclusion of data of 6 boys and 4 men. Only data from one of the two trials was included in the final analysis. The trial with the best performance, largest RT range, and highest number of MUs were selected.
For each MU, mean MUFR (pps) and recruitment threshold (RT) (%MVIC) were computed externally using LabView (Herda et al. 2019, 2020; Miller et al. 2019; Sterczala et al. 2018). Mean MUFR was assessed during the 3 s period during which the torque signal was most stable (lowest standard deviation) and was calculated as the reciprocal of the average inter-spike interval for each MU. Lastly, RT was determined as the average 0.1 ms epoch of torque (average of 4 samples) associated with the first firing time (i.e., discharge) for the MU. MUFRs were then expressed relative to RT to characterize MU activation patterns.
Statistical analysis
All assumptions and statistical analyses were performed using IBM SPSS 28.0 (IBM Corp., Armonk, NY, USA). Normality was assessed by visual examination of the frequency distributions and determined if skewness or kurtosis was less than ± 3. The assumption of normality was met for all measures. Statistical differences were indicated when p < 0.05. The effect size was assessed using d and β, as recommended by (Cohen 1988). Data are presented as means and standard deviations.
T tests were used to assess group differences in physical characteristics, MVIC, normalized MVIC (Nm per kg lean body mass), the performance of the trapezoid contraction, and MU parameters such as MU recruitment range, the number of MUs identified from decomposition, and average MUFR.
Linear regression was used to assess the effect of Group on the relationship between MUFR and RT. For this analysis, MUFR (i.e., dependent variable) was regressed onto Group, RT, and Group*RT predictor variables (i.e., independent variables). The predictor variable Group*RT was used to determine whether the slope of the relationships differs between groups. The predictor variable Group was used to examine differences in y-intercept between boys and men. RT was used to examine the overall relationship between RT and MUFR.
Lastly, the cumulative probability density function was calculated to determine the number of MUs recruited at each recruitment threshold as a percentage of the total number of MUs detected during the ramp increase in force. The focus of the analysis was to characterize the FCR recruitment range in the two groups.
Results
Muscle performance
Group means and standard deviations of the muscle performance assessments are presented in Table 2. Wrist flexor strength was significantly higher in men compared with boys [t(30) = − 5.75, p < 0.001, d = − 2.03]. However, after accounting for differences in body size, wrist flexor strength was not different between groups (p = 0.09). During the submaximal contractions, the ramp speed and relative torque sustained were not different between boys and men (p = 0.11; p = 0.99, respectively). However, torque steadiness was lower (i.e., greater variation, %MVIC) in boys compared with men [t(30) = 5.11, p < 0.001, d = 1.81].
Surface electromyography
Absolute FCR EMGRMS during wrist flexion MVIC was not different between boys (0.19 ± 0.1 mV) and men (0.20 ± 0.09 mV) (p = 0.68). Normalized FCR EMGRMS during high-intensity trapezoidal contractions was also not different between boys (77.9 ± 24.1%MVIC) and men (78.6 ± 27.2%MVIC) (p = 0.93). Lastly, antagonist coactivation was not different between boys and men, but was significantly higher during MVIC compared with 70%MVIC, in both groups [F(1,30) = 25.09, p < 0.001, η2 = 0.46] (Table 2).
Motor-unit decomposition
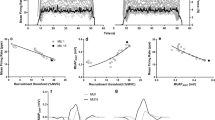
Table 3 describes the number of MUs identified and the RT range for each group. In total, 188 and 222 discrete MUs were identified in boys and men, respectively, with a similar number of MUs per participant (p = 0.48). The initial RT (RT at which the first MU was identified) was not different between groups (p = 0.30), while the final RT (highest RT at which a MU was identified) was significantly higher in men compared with boys [t(30) = − 2.56, p < 0.05, d = − 0.91], resulting in a greater RT range in men compared with boys. Furthermore, boys tended to recruit their MUs earlier in the contraction compared with men, as demonstrated by the cumulative probability plot of MU recruitment (Fig. 2). The mean MUFR, assessed during the 3 s period during which the torque signal was most stable, was not different between boys and men (p = 0.94).
Motor-unit firing rates and recruitment threshold
Figure 3 displays the linear relationship between MUFR and RT in boys and men during trapezoidal contractions at 70%MVIC (Fig. 3). Table 4 provides the results of the regression analysis examining group differences in the MUFR–RT relationship. In both groups, there was a significant negative relationship between MUFR and RT (main-effect RT). The y-intercept of the MUFR–RT relationship was significantly lower in boys compared with men (main-effect Group). Moreover, the slope of the MUFR–RT relationship was significantly steeper (more negative) in boys compared with men (Group*RT).
Discussion
This study is the first to compare MU activation of the FCR muscle between boys and men during isometric contractions. The FCR is characterized by a relatively small recruitment range, where most MUs are assumed to be recruited by around 50% MVIC (Aagaard et al. 2002; DeLuca and Hostage 2010). Unexpectedly, we found no difference in the mean MUFR between groups. However, recruitment timing and MUFR patterns across the active MU pool appear to differ between boys and men. Specifically, we observed that compared with men, boys tended to recruit their MUs earlier, and within a smaller RT range. Additionally, boys demonstrate a greater decrease in MUFR with increasing RT. These findings suggest an age-related difference in the strategy of force gradation during wrist flexion, where boys recruit more of their MUs earlier in the force gradation process, possibly resulting in their narrower recruitment range.
A novel finding of this study is that boys demonstrated a steeper (more negative) MUFR–RT slope (Fig. 3), reflecting a lower discharge rate of their higher threshold MUs, compared with men. The steeper slope may be related to the narrower recruitment range in the boys. During low intensity (30%MVIC) contractions of the FDI, Miller et al. (2019) also reported greater rates of decay (analogous to a steeper slope) of MUFR with increasing MUAP amplitude in boys compared with men, although the difference did not reach satistical significance. It is possible that at higher contraction intensities (> 30%MVIC), as in the present study, the difference between groups would be greater due to the recruitment of larger, higher threshold MUs. It should be noted, that Miller et al. (2019) also observed overall higher, rather than lower MUFR in boys compared with men. The authors suggested that the higher MUFR in the boys may be related to greater antagonist coactivation (not measured). In the present study, antagonist coactivation was not different between boys and men and, therefore, was not likely to contribute to age-related differences in the MUFR–RT relationships.
Interestingly, although MUFR–RT slopes were different between boys and men, the mean MUFR was not. The apparent discrepancy may be explained by the fact that boys recruited more MUs earlier in the contraction and within a smaller recruitment threshold range compared with men. In accordance with the onion skin principle, MUs recruited early in the recruitment range are characterized by high MUFRs (DeLuca and Contessa 2012; DeLuca and Erim 1994; DeLuca and Hostage 2010). The mean RT range was significantly greater in men (~ 1.8–47.7%MVIC), compared with boys (~ 3.1–39.7%MVIC) (Table 3). Additionally, boys demonstrated an asymmetrical distribution of MU recruitment, where more MUs were recruited earlier in the contraction (Fig. 2, Table 5). For example, by ~ 20%MVIC, boys had already recruited ~ 60% of their MUs, whereas the men reached this level of recruitment only at ~ 30%MVIC. These differences in MU recruitment patterns between groups suggest that boys rely on their lower threshold MUs to a greater extent compared with men in order to attain the target force. This suggestion is supported by findings in younger and older rats (1, 2 and 9 months), where twitch and muscle characteristics were found to change with development. Dobrzynska and Celichowski (2016) observed that the twitch-to-tetnus ratio of all MU types was greater in younger compared with older rats. Additionally, the force–frequency relationship of fast-twitch fibers was observed to shift to the right such that, for example, stimulation frequencies associated with 60% of maximal twitch torque were lower in younger compared with older rats. As the authors indicate, the higher twitch-to-tetanus ratio in the developing animals suggests that changes in MUFR play a smaller role and thus, MU recruitment plays a larger role in force development. These findings may explain the greater recruitment of MUs earlier in the contraction, the smaller recruitment range, as well as the steeper MUFR-RT slope in the boys compared with the men.
Differences in MU recruitment between groups may also be related to disparities in torque modulation between boys and men. Despite extensive habituation, boys had more difficulty performing the contraction ramp compared with men (Fig. 4) and displayed lower steadiness during the contraction (Table 2). Lesser torque control during the ramp phase in boys may be related to their greater MU recruitment at a lower %MVIC (RT).
The mechanisms underlying differences in MUFR and recruitment patterns between boys and men are unclear but may be related to the development of the central nervous system. Koh and Eyre (1988) suggested that the maturation of intraneuronal synaptic connections was responsible for lower motor evoked potential latency with increasing age. Indeed, using MRI, it has been observed that between the ages of 8 and 12 years, the nervous tissue undergoes myelination and pruning process to make synaptic connections more efficient (Giedd et al. 1999; Groeschel et al. 2010; Huttenlocher and Dabholkar 1997; Pfefferbaum et al. 1994). While it is unknown whether such developmental changes are related to the observed age-related differences in MUFRs, a possible association between cortical synaptic connectivity and differences in MU recruitment strategy should be considered in future studies.
Limitations
Due to differences in muscle size between boys and men, it is likely that the volume of muscle that was captured by the sEMG sensor was greater in boys compared with men. However, despite these differences in conduction volume, a similar number of motor units was detected in both groups.
Conclusions
Despite similar mean MUFRs in boys and men, the pattern of MUFRs used to modulate torque during submaximal wrist flexion differed between groups. Specifically, boys demonstrated a greater decrease in MUFR with increasing RT. They recruited more MUs earlier in the contraction and their recruitment range was smaller compared with men. It is possible that the boy’s pattern of MU discharge, specifically their steeper MUFR–RT slope, may be related to recruitment compression.
Data availability
Data are available from the corresponding author upon reasonable request.
Abbreviations
- CV:
-
Coefficient of variation
- EMGRMS :
-
Root mean square of the electromyography signal
- ECR:
-
Extensor Carpi Radialis
- FCR:
-
Flexor Carpi Radialis
- FDI:
-
First dorsal interossei
- LBM:
-
Lean body mass
- MU:
-
Motor unit
- MUAP:
-
Motor-unit action potential
- MUFR:
-
Motor-unit firing rates
- MVIC:
-
Maximal voluntary isometric contraction
- P–P:
-
Peak to peak
- PHV:
-
Peak height velocity
- RT:
-
Recruitment threshold
- sEMG:
-
Electromyography
- STA:
-
Spike trigger average
References
Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-poulsen P, Dyhre-Poulsen P (2002) Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol 92:2309–2318. https://doi.org/10.1152/japplphysiol.01185
Blimkie, C. J. R. (1989). Age- and sex-associated variation in strength during childhood: anthropometric, morphologic, neurological, biomechanical, genetic and physical activity correlates. In D. Gisolfi, C., Lamb (Eds.), Perspectives in exercise science and sports, Benchmark, (pp. 99-157).
Chalchat E, Piponnier E, Bontemps B, Julian V, Bocock O, Duclos M, Ratel S, Martin V (2019) Characteristics of motor unit recruitment in boys and men at maximal and submaximal force levels. Exp Brain Res 237:1289–1302. https://doi.org/10.1007/s00221-019-05508-z
Clamann HP (1993) Motor unit recruitment and the gradation of muscle force. Phys Ther 73(12):830–843. https://doi.org/10.1093/ptj/73.12.830
De Ste Croix MBA, Armstrong N, Welsman J (1999) Concentric isokinetic leg strength in pre-teen, teenage and adult males and females. Biol Sport 16(2):75–86
DeLuca C, Contessa P (2012) Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107:178–195. https://doi.org/10.1152/jn.00961.2010.-For
DeLuca C, Erim Z (1994) Common drive of motor units in regulation of muscle force. Trends Neurosci 17(7):299–305
DeLuca C, Hostage E (2010) Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol 104:1034–1046. https://doi.org/10.1152/jn.01018.2009
Dobrzynska, Z., and Celichowski, J. (2016). Changes in contractile properties and action potentials of motor units in the rat medial gastrocnemius muscle during maturation. Journal of Physiology and Pharmacology, 67(1), 139–150. http://jpp.krakow.pl/journal/archive/02_16/pdf/139_02_16_article.pdf
Dotan R, Mitchell C, Cohen R, Gabriel D, Klentrou P, Falk B (2013) Explosive sport training and torque kinetics in children. Appl Physiol Nutr Metab 38(7):740–745. https://doi.org/10.1139/apnm-2012-0330
Dotan R, Mitchell C, Cohen R, Klentrou P, Gabriel D, Falk B (2012) Child—adult differences in muscle activation—a review. Pediatr Exerc Sci 24:2–21
Esbjörnsson M, Dahlström M, Gierup J, Jansson E (2021) Muscle fiber size in healthy children and adults in relation to sex and fiber types. Muscle Nerve 63:586–592. https://doi.org/10.1002/mus.27151
Falk B, Usselman C, Dotan R, Brunton L, Klentrou P, Shaw J, Gabriel D (2009) Child–adult differences in muscle strength and activation pattern during isometric elbow flexion and extension. Appl Physiol Nutr Metab 34(4):609–615. https://doi.org/10.1139/H09-020
Farina D, Merletti R, Enoka RM, Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA (2004) The extraction of neural strategies from the surface EMG Neural Control of Movement The extraction of neural strategies from the surface EMG. J Appl Physiol 96:1486–1495. https://doi.org/10.1152/japplphysiol.01070.2003
Frost G, Bar-Or OO, Dowling J, Dyson K (2002) Explaining differences in the metabolic cost and efficiency of treadmill locomotion in children. J Sports Sci 20(6):451–461. https://doi.org/10.1080/02640410252925125
Frost G, Dowling J, Dyson K, Bar-Or O (1997) Cocontraction in three age groups of children during treadmill locomotion. J Electromyogr Kinesiol 7(3):179–186
Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2(10):861–863. https://doi.org/10.1038/13158
Groeschel S, Vollmer B, King MD, Connelly A (2010) Developmental changes in cerebral grey and white matter volume from infancy to adulthood. Int J Dev Neurosci 28(6):481–489. https://doi.org/10.1016/j.ijdevneu.2010.06.004
Grosset JF, Mora I, Lambertz D, Pérot C (2008) Voluntary activation of the triceps surae in prepubertal children. J Electromyogr Kinesiol 18:455–465. https://doi.org/10.1016/j.jelekin.2006.11.002
Henneman E (1957) Relation between size of neurons and their susceptibility to discharge. Science 126:1345–1347. https://doi.org/10.1126/science.126.3287.1345
Herda TJ, Miller JD, Wray ME, Sterczala AJ, Dimmick HL, Trevino MA (2019) Motor unit firing rates of the first dorsal interosseous differ between male and female children aged 8–10 years. Hum Mov Sci 66:416–424. https://doi.org/10.1016/j.humov.2019.04.011
Herda TJ, Parra ME, Miller JD, Sterczala AJ, Kelly MR (2020) Measuring the accuracies of motor unit firing times and action potential waveforms derived from surface electromyographic decomposition. J Electromyogr Kinesiol 52:102421. https://doi.org/10.1016/j.jelekin.2020.102421
Hug F, Laplaud D, Savin B, Grélot L (2003) Occurrence of electromyographic and ventilatory thresholds in professional road cyclists. Eur J Appl Physiol 90(5–6):643–646. https://doi.org/10.1007/s00421-003-0949-5
Huttenlocher PR, Dabholkar AS (1997) Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 387(2):167–178
Kannas T, Kellis E, Arampatzi F (2010) Medial gastrocnemius architectural properties during isometric contractions in boys and men. Pediatr Exerc Sci 22(1):152–164
Koh THHG, Eyre JA (1988) Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child 63(11):1347–1352. https://doi.org/10.1136/adc.63.11.1347
Kumar N, Oliver JL, Lloyd RS, Pedley JS, Radnor JM (2021) The influence of growth, maturation and resistance training on muscle-tendon and neuromuscular adaptations: a narrative review. Sports 9(59):9050059
Lambertz D, Mora I, Grosset JF, Perot C (2003) Evaluation of musculotendinous stiffness in prepubertal children and adults, taking into account muscle activity. J Appl Physiol 95(1):64–72. https://doi.org/10.1152/japplphysiol.00885.2002
Lexell J, Sjostrom M, Nordlund A, Taylor CC (1992) Growth and development of human muscle: a quantitative morphological study of whole vastus lateralis from childhood to adult age. Muscle Nerve 15:404–409
Long D, Dotan R, Pitt B, McKinlay B, O’Brien TD, Tokuno C, Falk B (2017) The electromyographic threshold in girls and women. Pediatr Exerc Sci 29(1):84–93. https://doi.org/10.1123/pes.2016-0056
McIntosh JS, Ringqvist M, Schmidt EM (1985) Fiber type composition of monkey forearm muscle. Anat Rec 211(4):403–409. https://doi.org/10.1002/ar.1092110405
Metaxas T, Mandroukas A, Michailidis Y, Koutlianos N, Christoulas K, Ekblom B (2019) Correlation of fber-Type composition and sprint performance in Youth Soccer players. J Strength Cond Res 33(10):2629–2634. https://doi.org/10.1519/JSC.0000000000003320
Miller JD, Sterczala AJ, Trevino MA, Wray ME, Dimmick HL, Herda TJ (2019) Motor unit action potential amplitudes and firing rates during repetitive muscle actions of the first dorsal interosseous in children and adults. Eur J Appl Physiol 119(4):1007–1018. https://doi.org/10.1007/s00421-019-04090-0
Moritani T, DeVries H (1978) Re-examination of the relationship between the surface integrated electromyogram (IEMG) and force of isometric contraction. Am J Phys Med 57:263–277
O’Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN (2010) In vivo measurements of muscle specific tension in adults and children. Exp Physiol 95(1):202–210. https://doi.org/10.1113/expphysiol.2009.048967
Oliver JL, Smith PM (2010) Neural control of leg stiffness during hopping in boys and men. J Electromyogr Kinesiol 20(5):973–979. https://doi.org/10.1016/j.jelekin.2010.03.011
Parra ME, Miller JD, Sterczala AJ, Trevino MA, Dimmick HL, Herda TJ (2020) Differences in the firing rate versus recruitment threshold relationships of the vastus lateralis in children ages 7–10 years and adults. Hum Mov Sci 72:102650. https://doi.org/10.1016/j.humov.2020.102650
Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO (1994) A quantitative magnetic resonance imaging study of changes in brain morphology from infance to late adulthood. JAMA Neurol 51(9):874–887. https://doi.org/10.1001/archneur.1994.00540210046012
Pitt B, Dotan R, Millar J, Long D, Tokuno C, O’Brien T, Falk B (2015) The electromyographic threshold in boys and men. Eur J Appl Physiol 115(6):1273–1281. https://doi.org/10.1007/s00421-015-3100-5
Radnor JM, Oliver JL, Waugh CM, Myer GD, Lloyd RS (2020) The influence of maturity status on muscle architecture in school-aged boys. Pediatr Exerc Sci 32(2):89–96. https://doi.org/10.1123/pes.2019-0201
Sterczala AJ, Miller JD, Trevino MA, Dimmick HL, Herda TJ (2018) Differences in the motor unit firing rates and amplitudes in relation to recruitment thresholds during submaximal contractions of the first dorsal interosseous between chronically resistance-trained and physically active men. Appl Physiol Nutr Metab 43(8):759–768. https://doi.org/10.1139/apnm-2017-0646
Tanner JM (1962) Growth at adolescence, 2nd edn. Blackwell Scientific Publications, Oxford
Weide G, Huijing PA, Maas JC, Becher JG, Harlaar J, Jaspers RT (2015) Medial gastrocnemius muscle growth during adolescence is mediated by increased fascicle diameter rather than by longitudinal fascicle growth. J Anat 226:530–541. https://doi.org/10.1111/joa.12306
Wernbom M, Aagaard P (2019) Muscle fibre activation and fatigue with low-load blood flow restricted resistance exercise—an integrative physiology review. Acta Physiol 228(1):1–48. https://doi.org/10.1111/apha.13302
Woods S, Dotan R, Jenicek N, Falk B (2020) Isometric-based EMG threshold in girls and women. Eur J Appl Physiol 120(4):907–914. https://doi.org/10.1007/s00421-020-04331-7
Woods S, Dotan R, Jenicek N, Maynard J, Gabriel D, Tokuno C, Falk B (2019) Isometric-based test improves EMG-threshold determination in boys vs men. Eur J Appl Physiol 119(9):1971–1979. https://doi.org/10.1007/s00421-019-04185-8
Woods S, O’Mahoney C, Maynard J, Dotan R, Tenenbaum G, Filho E, Falk B (2022) Increase in volitional muscle activation from childhood to adulthood: a systematic review and meta-analysis. Med Sci Sports Exerc 54(5):789–799. https://doi.org/10.1249/MSS.0000000000002853
Woods S, O’Mahoney C, Mckiel A, Natale L, Falk B (2023) Child-Adult differences in antagonist muscle coactivation: a systematic review. J Electromyogr Kinesiol 26:102727. https://doi.org/10.1016/j.jelekin.2022.102727
Acknowledgements
We would like to thank all the participants for their time, effort and hard work. Additionally, we would like to thank the parents/guardians for making it all happen, even when faced with COVID-related limitations and hardships.
Funding
This work was supported by the Natural Science and Engineering Research Council of Canada (NSERC grants to B. Falk and to M. Holmes). S Woods and A McKiel were supported by Ontario Graduate Scholarship.
Author information
Authors and Affiliations
Contributions
SW, MH, DG and BF conceived and designed research. SW, AM conducted experiments. SW, TH, DG and BF analyzed data and wrote the manuscript. TH and PK commented on previous versions. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Communicated by Toshio Moritani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woods, S., McKiel, A., Herda, T. et al. Different discrete motor-unit activation patterns in the flexor carpi radialis in boys and men. Eur J Appl Physiol 124, 1933–1942 (2024). https://doi.org/10.1007/s00421-024-05417-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-024-05417-2