Abstract
Background
Children have been hypothesized to utilize higher-threshold (type-II) motor units (MUs) to a lesser extent than adults. Two recent studies, using a cycling-based EMG-threshold (EMGTh) protocol, supported the hypothesis, showing children’s EMGTh intensities to be higher than adults’. Conclusions, however, were hampered by children’s low EMGTh detection rates. Insufficiently high contractile forces at exhaustion were postulated as the reason for non-detection, predominantly in children. An intermittent isometric contraction test (IICT) protocol facilitates higher contractile forces prior to exhaustion and was shown effective in EMGTh testing of adults.
Purpose
Determine whether an IICT protocol would enhance EMGTh detection in children, and consequently increase the magnitude of the previously observed child–adult EMGTh differences.
Methods
18 boys and 21 men completed one-repetition-maximum (1RM) isometric knee-extension test. The IICT protocol followed, commencing at 25%1RM and comprising five isometric contractions per load, incremented by ~ 3%1RM to exhaustion. Vastus lateralis surface EMG was recorded and EMGTh, expressed as %1RM, was defined as the onset of the EMG-response’s steeper segment.
Results
EMGTh was detected in 88.9% of boys and 95.2% of men, and occurred at higher relative intensities in boys (56.4 ± 9.2%1RM) than in men (46.0 ± 6.8%1RM). This 10.4% difference was 57% greater than the corresponding, previously reported cycling-based age-related difference.
Conclusions
With the boys’ detection rate nearly on par with the men’s, the IICT protocol appears to overcome much of the intensity limitation of cycling-based protocols and provide a more sensitive EMGTh detection tool, thus extending the previously observed boys‒men difference. This difference adds supports to the notion of children’s more limited type-II MU recruitment capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Children have been shown to activate a smaller fraction of their motor-unit pool, compared with adults, during maximal volitional contractions (O'Brien et al. 2010), i.e., children have a greater activation deficit during maximal contractions. Based on Henneman’s size principle (Henneman et al. 1965) and glycogen depletion studies (Vollestad and Blom 1985; Vollestad et al. 1984), it has been suggested that this activation deficit is due to children’s lower recruitment capacity of the higher-threshold, type-II motor units (MUs) (Dotan et al. 2012). This proposed age- or maturation-related difference in MU activation has been argued to be the common factor underlying numerous well-known child–adult differences in muscle performance, such as in maximal strength and power (Asai and Aoki 1996; De Ste Croix et al. 1999), muscle substrate metabolism (Riddell et al. 2008), muscle oxygen-uptake kinetics (Fawkner and Armstrong 2004), as well as muscular endurance (Armatas et al. 2010; Ratel et al. 2015; Zafeiridis et al. 2005) or rate of recovery (Falk and Dotan 2006; Hebestreit et al. 1993). Moreover, the differential MU activation pattern may be important in gaining insight into neuromotor development and can possibly have implications for youth physical training and therapy guidelines.
Investigating MU activation patterns is highly challenging, whether invasively or non-invasively. The assessment of the electromyographic threshold (EMGTh) employs surface EMG to non-invasively determine what many researchers of this topic consider to be the onset of accelerated recruitment of high-threshold MUs (Chwalbinska-Moneta et al. 1998; Hug et al. 2003; Maestu et al. 2006; Miyashita and Kanehisa 1980; Moritani and deVries 1978). It has been studied extensively in adults, mainly in cycling ergometry (Camic et al. 2010, 2011; Candotti et al. 2008; Hug et al. 2003, 2004, 2006b; Jurimae et al. 2007; Lucia et al. 1999; Maestu et al. 2006; Moritani et al. 1993; Taylor and Bronks 1996). Importantly, the EMGTh is independent of absolute torque or power, relative muscle size, fiber size, or EMG amplitude. The EMGTh is expressed as the relative (%max) exercise intensity where a change in the EMG slope (threshold) occurs and can thus be directly compared between groups of varying sizes and performance levels, such as children and adults.
In two recent studies using progressive cycling, EMGTh tended to occur later in children than in adults (at ~ 6% higher exercise intensity) (Long et al. 2017; Pitt et al. 2015). Based on the above assumption (EMGTh reflecting the onset of accelerated recruitment of type-II MUs), children’s higher EMGTh is taken to reflect their lower recruitment capacity of type-II MUs. However, children’s EMGTh detection rates were 17−23% lower than those of their adult counterparts and child−adult EMGTh differences were shown statistically significant only when un-detected thresholds were assumed to have occurred at exhaustion. This was based on the assumption that children in whom the EMGTh could not be detected, reached exhaustion prior to attaining sufficiently high contractile intensity for the EMGTh to be manifested. This assumption stems from earlier findings in adults, showing that exhaustion in progressive cycling tasks may take place when the force applied to the pedals is only ~ 50% of the maximal volitional contractile force (MVC) for the given pedalling cadence (Greig et al. 1985; Sargeant et al. 1981). Thus, if an EMGTh was to occur at higher contractile intensities, e.g., 55% MVC, it would not be detectable in the cycling test. Children are known to have lower size-normalized maximal strength/force (De Ste Croix et al. 1999; Falk et al. 2009), higher muscle endurance (Armatas et al. 2010; Ratel et al. 2015; Zafeiridis et al. 2005), and their EMGTh appears to occur at higher relative intensities (Long et al. 2017; Pitt et al. 2015). It could thus be expected that children would require higher relative exercise intensities for their EMGTh to manifest itself and that exercise tasks, in which exhaustion sets in before sufficiently high local muscular force is reached (e.g., cycling), would result in lower EMGTh manifestation, i.e., lower detection rates.
De Ruiter et al. recently reported a progressive isometric knee-extension protocol that successfully detected EMGTh in adults (De Ruiter et al. 2016). Using that protocol, participants were able to reach ~ 67% MVC, suggesting that such an approach could facilitate EMGTh detection in individuals, such as children, whose higher EMGTh could not be detected in a cycling-based test.
The additional detection, presumably afforded by the higher contractile forces attainable in an isometric protocol, can be presumed to consist of children of higher end (higher intensity) thresholds that could not be detected by the cycling-based protocol. Consequently, the children’s mean EMGTh can be expected to increase relative to adults. Thus, the aim of the present study was to examine whether the use of an intermittent, isometric contraction test protocol (IICT) would bring children’s EMGTh detection rate on par with that of adults and whether children’s extra-detection would expand the previously observed, cycling-based, child−adult EMGTh difference.
Methods
Participants
Twenty-two boys and 22 men volunteered for the study. Three of the boys were eliminated from analysis due to poor compliance with the IICT protocol. One boy and one man were eliminated due to corruption of the EMG data files. Participant characteristics are presented in Table 1. The boys participated in more leisure activities, but both groups had similar structured sports and physical training (17 of the boys were soccer players and most of the men engaged in general fitness and resistance exercise training).
The study was cleared by Brock University’s Research Ethics Board. A thorough explanation of the study’s purpose, measurement procedures, benefits and potential risks or discomforts were provided to all participants and the children’s parents/guardians. All signed an informed consent form prior to testing.
Experimental procedure
All tests and procedures were completed at the Applied Physiology Laboratory at Brock University. Following an explanation of all procedures, anthropometric measurements (height, weight, body composition) were completed (see “Measurements”, below). Training history and pubertal stage (children only) were also determined. Participants were then seated onto the dynamometer (see below) in a standardized position (hip at 90º, knee at 125–130º), with a strap tightened across the hips and two across the chest, in ‘X’ fashion, to minimize unwanted movements. All tests were conducted on the right leg. The participants performed a warm-up consisting of repeated sub-maximal knee-extensions (i.e., 4 sets of 10, 8, 5 and 3 repetitions, with increasing weights), followed by a one-repetition maximum test (1RM; see below). Following a rest period, the participants were familiarized and habituated to the IICT protocol, by performing several sub-maximal stages (see below). Following 10−15 min rest, participants performed the IICT protocol until volitional exhaustion, or failure to lift the applied load. The 1RM test was repeated on a subsequent visit to the laboratory, 2–14 days later.
Dynamometer
While common dynamometers (e.g., Biodex, Cybex) have an isometric option at any desired joint angle, they cannot control the applied torque, which, therefore, depends on the participant and can fluctuate considerably. To address this problem, a custom dynamometer was built in which a knee-extension lever was connected via a pulley system to a weight rack. Single weights or combinations thereof could be added or removed to produce discrete torque settings. The dynamometer’s torque output was calibrated between 16 and 183 Nm against known weights to determine its weight-to-torque conversion factor. The correlation coefficient through that range was 0.999. This dynamometer was used for both the 1RM and EMGTh tests.
Measurements
Anthropometric measures included standing and sitting (children only) height to the nearest 0.1 cm (Ellard Instrumentation Ltd. Stadiometer), body mass and fat percentage measured to the nearest 0.1 kg and 0.1%, respectively, by a bioelectrical impedance analysis apparatus (InBody 520, Biospace CO., Ltd., S. Korea). Participants were hydrated upon arrival to the laboratory and asked to void their bladder prior to measurements. Pubertal status was self-assessed by the boys, based on secondary sex characteristics (i.e., pubic hair) as outlined by Tanner (1962).
Habitual physical activity was assessed using the Godin–Shephard Leisure-Time Exercise Questionnaire (Godin and Shephard 1985). Current and past history of sports training was determined by a questionnaire.
Exercise protocols
The 1RM was determined, in 2–8 trials with ~ 1-min rest intervals, as the highest knee-extension torque a participant could produce once. The 1RM was used to determine the starting load (~ 25% 1RM) and loading progression (~ 3% 1RM) for the IICT protocol. The highest 1RM value of the two visits was used as the reference for the EMGTh test loads, as well as the EMGTh value (as %1RM).
The IICT protocol comprised repetitive isometric contractions at 125–130º knee-extension. Starting at 25% 1RM participants performed five 5-s isometric contractions interspersed by 3-s recovery intervals. A 30-s rest followed the last contraction of each set before the next load increment (~ 3%1RM) was applied for the subsequent set. The test was terminated upon volitional exhaustion after the participants received verbal encouragements to continue as long as possible. Since loading was attained by discrete weights, the smallest of which were 0.25 lb (113 g), some of the smallest/weakest boys load increments had to be set closer to 4% to avoid excessively long tests to exhaustion due to the use of the next smaller increments. For this reason, the mean load incrementation was 2.98 ± 0.25%1RM for the men, but 3.25 ± 0.41%1RM for the boys ( p < 0.05).
Data acquisition
Muscle activation was recorded using surface EMG double-differential tripolar electrodes (Delsys Inc., Boston, MA, USA). The 20 × 30 mm rectangular electrode casing comprised three Ag/Ag contact bars, 10 mm apart. The electrode was placed according to SENIAM guidelines, specifically, at 2/3 the distance between the superior border of the patella and the anterior superior spina iliaca. A reference electrode was placed over the 7th cervical vertebra. EMG data were sampled and recorded at 1000 Hz, using a computer-based oscillograph and data acquisition system (EMGworks Acquisition, Delsys Inc., Boston, MA, USA) and band-pass filtered at 20‒450 Hz, using the Bagnoli-4 bioamplifier (Delsys Inc., Boston, MA, USA). The electrode skin site (vastus lateralis) was shaved if necessary and thoroughly cleaned with alcohol and NuPrep skin preparation gel (Weaver and Company, CO, USA). The muscle- and reference-electrodes were placed according to SENIAM guidelines for the vastus lateralis.
An electro-goniometer (S700/S720 Joint Angle SHAPE SENSOR, Measurand Inc., Fredericton, NB, Canada) was attached to the dynamometer across the lever’s pivot axis. It was verified by a mechanical goniometer and used to continually record knee angle and changes thereof. Those were used for EMG data reduction (see below).
Data reduction and EMG-threshold determination
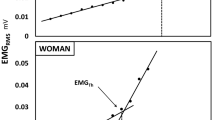
Data reduction was carried out separately for each participant, using MATLAB v.2017a. The function ‘Peakfinder’ (Yoder 2011) was applied to the goniometer position data to identify the start of each isometric contraction. The root mean square (RMS) of each contraction’s EMG signal (EMGRMS) was then calculated over a 2-s window where knee position was the most stable (based on the goniometer data).
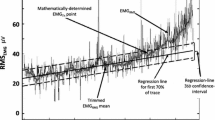
Five EMGRMS values were calculated for each load (Fig. 1a) and then averaged (EMGRMS5). An EMGRMS point was eliminated from analysis if it exceeded ± 3SD from the mean of the other four values, in which case the EMGRMS5 would comprise only 4 contractions. This was necessary in only ~ 0.8 and 0.2% of all contractions, for the boys and men, respectively. The 5 or 4 points were then averaged to produce a single value per load (EMGRMS5) (Fig. 1b). The point of the bi-segmental least sum of squares (LSS) was determined, using Prism GraphPad software (Ver.7; GraphPad Software, San Diego, CA, USA) (Fig. 1b). Using the bi-segmental model, previously employed by others (Hug et al. 2006a; Lucia et al. 1999), the EMGTh was then calculated as the exercise intensity (%1RM) at the intersection of the two resulting regression lines (Fig. 1c), as follows. The bi-segmental regressions were plotted separately in Microsoft Excel for EMGTh determination and defined as the intersection of the two regression lines. If necessary, the following was performed ahead of the final EMGTh determination: (1) An EMGRMS5 point, at or next to the LSS, was moved from one regression line to the other, if that improved the overall fit of the data points to the bi-segmental model; (2) Outlying data points were removed from analysis if one, two, or three points deviated ≥ ± 2SD, ≥ ± 3SD, or ≥ ± 4SD, respectively, from the regression line of the remaining regression points. The procedure was performed only for refining EMGTh determination. When this step was necessary, the EMGTh was re-calculated from the regression lines determined from the remaining points. The resulting final EMGTh was never allowed to deviate more than ± 1 load (~ ± 3%) from the initial LSS-determined EMGTh.
Since there is always a mathematical LSS point in any bi-segmental regression analysis of real-life data, the criterion for accepting an EMGTh was that at least one point of the second (steeper) regression had to extend above the extrapolated + 3SD line of the first regression.
Statistical analysis
All statistical analysis was performed using SPSS v.23. Data are presented as means ± 1SD. All group data were normally distributed. An independent t test was used to assess group differences in physical characteristics, and EMGTh. Chi squared statistics were used to compare the detection rates of EMGTh between the cycling protocol, as reported by Pitt et al. (2015) and the IICT protocol. The acceptance level for statistical significance for all tests was set at p < 0.05.
Results
Figure 2 illustrates representative EMGTh determinations in a man and a boy. The study’s results are presented in Table 2 and Fig. 3. EMGTh could not be detected in 2 of the 18 boys and 1 of the 21 men. The boys’ detection rate (88.9%) was 13.5% higher than previously reported for a cycling-based protocol (78.3%; Pitt et al. 2015) [(88.9–78.3)/78.3 × 100], although this difference was not statistically significant (□2(chi squared) = 0.783). The mean EMGTh was significantly higher in boys than in men (Fig. 3). The boys completed, on average, more stages than the men, but due to their slightly larger load increments, this difference did not reach statistical significance. Nevertheless, upon exhaustion at the end the EMGTh test, the boys’ fractional load (%1RM) was significantly higher than the men’s (Table 2).
Discussion
The main finding of the current study is that, using IICT, the boys–men EMGTh difference was markedly higher (Δ = 10.4%, p < 0.001, ES = 1.30) than the previously observed cycling-based difference (Pitt et al. 2015; Δ = 6.6%, p < 0.060, ES = 0.68). Given that the boys’ mean torque at exhaustion was 76.8%1RM, this finding can presumably be ascribed to the higher attained contractile forces in the current protocol, compared with cycling. The high contractile forces at exhaustion can facilitate detection of higher thresholds that would be undetectable by a cycling-based test protocol. This was manifested by the 13.5% (88.9 vs. 78.3) greater detection rate than that previously attained using the cycling protocol (Pitt et al. 2015). It is noteworthy that the men’s detection rate was identical in the IICT and the cycling protocol, namely 95.2%. These findings suggest the IICT protocol to be more sensitive and effective in testing for EMGTh or MU recruitment, particularly in children. The boys’ EMGTh was shown to be higher than that of adults, lending further support to the notion that children are less capable of high-threshold MU recruitment than adults.
Extending our previous cycling studies (Long et al. 2017; Pitt et al. 2015), the boys demonstrated higher mean EMGTh values than men, which serves as further support for our hypothesis that children are less able than adults to activate their higher-threshold/type-II MUs (Dotan et al. 2012). It may be worth emphasizing that our observed EMGTh values were not affected by the large child‒adult differences in 1RM or peak torque since they are expressed as percentages thereof. Thus, the 57% higher observed boys−men EMGTh difference, compared with 6.6% in the cycling study (Pitt et al. 2015), supports our assumption that the higher observed age-related difference is due to the isometric protocol facilitating higher contractile forces at exhaustion, thus allowing the detection of higher thresholds, which are more typical of children than adults.
Indeed, considering the different percentage ranges in the two determination protocols, the relative boys‒men EMGTh difference is even larger in the currently described IICT protocol: 22.6% in the IICT protocol [(56.4−46.0)/46.0 × 100], but only 8.3% in the cycling protocol [(86.4−79.7)/79.7 × 100]. This further highlights the IICT protocol’s higher sensitivity than that of the cycling-based protocol for detecting and differentiating EMGTh, particularly in pediatric populations. It is noteworthy that our participant samples included two apparent outliers (a man with 3.7 SD higher EMGTh, and a boy 5.8 SD heavier who had the lowest EMGTh value, relative to their respective peers). Without them, the absolute boys−men EMGTh difference would have been 12.3%1RM and a relative difference 27.3% (p < 0.0001).
The EMGTh detection rates, previously observed in our cycling EMGTh studies (Long et al. 2017; Pitt et al. 2015), were 17−23% lower in boys and girls, respectively, compared with their adult counterparts. These differences suggested that an obstacle to EMGTh detection specifically affected children. While the men maintained the same 95.2% (20/21) detection rate as in our corresponding cycling study (Long et al. 2017; Pitt et al. 2015), the corresponding boys’ rate rose from 78.3 to 88.9% in the present study. Despite our relatively large group sizes (Men’s = 21, Boys = 18), we did not have the power to establish a statistically significant difference between the two detection rates (sample sizes would have to be more than quadrupled). Nevertheless, we consider this 57% (10.4 vs. 6.6%) detection rate difference both physiologically and practically significant.
Only few studies, other than ours, reported EMGTh detection rates, and only in men. Two studies reported 100% detection, but only with 6−8 participants (Hug et al. 2003, 2006b). With 15 participants, de Ruiter et al. (De Ruiter et al. 2016) reported 93.3% detection, also in an isometric protocol. Only Jürimäe et al. reported detection rates in a large study (n = 49), which reached 98% in the vastus lateralis and lower rates in other muscles (Jurimae et al. 2007). Thus, it appears that, although under-reported, a level of EMGTh undetectability is part and parcel of current EMGTh determination techniques. The reason for this is unknown, but the phenomenon may possibly be due to the inherently high variability of EMG activity.
We cannot dismiss a possible existence of an additional factor that still handicaps boys’ EMGTh detection compared with the men’s. However, as some level of undetectability has been reported in most previous studies of adults, we suggest that a difference of a single detection [the failed-detection difference between our men (1/21) and boys (2/18)] could be considered immaterial. That is, we suggest that the men’s and boys’ detection rates should be viewed as qualitatively similar and that the main obstacle to EMGTh detection was largely or fully eliminated. The fact that the IICT protocol facilitated the attainment of 76% of the boys’ 1RM prior to exhaustion, supports our position that insufficient contractile intensity was the main obstacle to EMGTh detection in the cycling protocol, where contraction intensity at exhaustion has been shown to be closer to 50% of maximal force (Greig et al. 1985; Sargeant et al. 1981). The fact that the elevated contractile intensity affected only the boys’ detection rate is congruent with the observation that 4 boys exceeded EMGTh of 65%1RM, while among the men, one reached 64.6%1RM, while the rest were no higher than 54.0%1RM.
The boys’ LBM-corrected 1RM was only 73.8% that of the men (Table 2), which can be partly explained by children’s ~ 12% lower relative muscle mass (Malina 1969) and boys’ ~ 11% greater activation deficit relative to men (O'Brien et al. 2010). However, low maximal force in and of itself cannot explain the necessity of higher relative contractile intensities for EMGTh detection. According to the known MU recruitment hierarchy and the Henneman size principle (Henneman et al. 1965), activation deficit consists mostly of higher-threshold/type-II MUs. This supports the contention that children’s functional type-II MU pool is smaller than that of adults, and suggests that children require higher relative intensities to reach their more limited type-II MU pool and demonstrate an EMGTh.
On average, the boys completed 2.1 more stages in the EMGTh test and reached 16.7% higher relative intensity at exhaustion, compared with the men (Table 2). These findings are congruent with the boys’ higher EMGTh, as well as with children’s previously observed higher relative muscle endurance (Hatzikotoulas et al. 2014), also reflected by higher lactate- and ventilatory-thresholds (Klentrou et al. 2006; Simon et al. 1981; Tanaka and Shindo 1985), compared with men. These findings, along with the boys’ lower LBM-normalized 1RM, reinforce the observations that children cannot utilize their total MU pool to the same extent as adults (Asmussen and Heeboll-Nielsen 1955; O'Brien et al. 2010). The combination of the boys’ lower relative force, but higher EMGTh, suggests that the ‘inaccessible’ portion of their MU pool is predominantly made up of higher-threshold, type-II MUs.
The apparent greater sensitivity of our isometric-based protocol for the detection of the EMGTh may be important not only when testing children, or comparing children to adults, but possibly also in the elderly and in various clinical populations with any form of muscular impairment that might prevent them from attaining sufficiently high intensity in cycling-based testing. Moreover, IICT’s higher sensitivity, as manifested by the 57% greater boys‒men EMGTh difference (relative to cycling), also suggests that the observed 10.4% higher EMGTh in the boys is a better representation of the boys–men true physiological difference.
Limitations and future research
In the present study, we showed 13.5% higher detection rate among the boys, using the IICT protocol, compared with boys in our previous, cycling-based study. However, since these were two separate samples, a more robust comparison should test both the cycling-based and IICT protocols on the same participants. Further insight into the factors determining the manifestation and magnitude of the EMGTh could be gained by examining the same individuals in different exercise modalities or muscle groups.
While considerable amount of time and work was invested in the development and pilot testing of the IICT protocol, it is not necessarily the optimal protocol. Further experimentation with factors such as contraction types, number of contractions per load, recovery intervals, or load incrementation, could possibly result in a more sensitive test protocol and could shed more light on the relative contribution of fatigue or other factors, to the EMGTh and possibly, higher-threshold MU recruitment, as well. Additionally, future intervention studies are needed to examine the effect of training on the EMGTh.
Conclusions
Facilitating the attainment of higher contractile intensities prior to exhaustion, the isometric contraction protocol appears to have resulted in detecting the higher intensity, otherwise undetectable thresholds, amongst the boys. The boys’ increased EMGTh detection rate, associated with a greater boys–men EMGTh difference relative to that previously derived by a cycling-based protocol, suggests that the IICT is a more sensitive tool for determining EMGTh in pediatric populations. For the same reasons, this also suggests that the larger, IICT-based boys–men EMGTh difference is more representative of the true biological difference.
Abbreviations
- EMG:
-
Electromyography
- EMGTh :
-
Electromyographic threshold
- EMGRMS :
-
Root mean square of each contraction’s EMG signal
- EMGRMS5:
-
Average of EMGRMS values for each load
- IICT:
-
Intermittent isometric contraction test
- LBM:
-
Lean body mass
- LSS:
-
Least sum of squares
- MU:
-
Motor units
- MVC:
-
Maximal volitional contraction
- RM:
-
Repetition maximum
- RMS:
-
Root mean square
References
Armatas V, Bassa E, Patikas D, Kitsas I, Zangelidis G, Kotzamanidis C (2010) Neuromuscular differences between men and prepubescent boys during a peak isometric knee extension intermittent fatigue test. Pediatr Exerc Sci 22:205–217
Asai H, Aoki J (1996) Force development of dynamic and static contractions in children and adults. Int J Sports Med 17:170–174
Asmussen E, Heeboll-Nielsen K (1955) A dimensional analysis of physical performance and growth in boys. J Appl Physiol 7:593–603
Camic CL, Housh TJ, Johnson GO, Hendrix CR, Zuniga JM, Mielke M, Schmidt RJ (2010) An EMG frequency-based test for estimating the neuromuscular fatigue threshold during cycle ergometry. Eur J Appl Physiol 108:337–345
Camic CL, Housh TJ, Hendrix CR, Zuniga JM, Bergstrom HC, Schmidt RJ, Johnson GO (2011) The influence of the muscle fiber pennation angle and innervation zone on the identification of neuromuscular fatigue during cycle ergometry. J Electromyogr Kinesiol 21:33–40
Candotti CT, Loss JF, Melo Mde O, La Torre M, Pasini M, Dutra LA, de Oliveira JL, de Oliveira LP (2008) Comparing the lactate and EMG thresholds of recreational cyclists during incremental pedaling exercise. Can J Physiol Pharmacol 86:272–278
Chwalbinska-Moneta J, Kaciuba-Uscilko H, Krysztofiak H, Ziemba A, Krzeminski K, Kruk B, Nazar K (1998) Relationship between EMG blood lactate, and plasma catecholamine thresholds during graded exercise in men. J Physiol Pharmacol 49:433–441
De Ruiter CJ, Hamacher P, Wolfs BG (2016) A short submaximal test to determine the fatigue threshold of knee extensors in young men. Med Sci Sports Exerc 48:913–919
De Ste Croix MBA, Armstrong N, Welsman JR (1999) Concentric isokinetic leg strength in pre-teen, teenage and adult males and females. Biol Sport 16:75–88
Dotan R, Mitchell C, Cohen R, Klentrou P, Gabriel D, Falk B (2012) Child-adult differences in muscle activation—a review. Pediatr Exerc Sci 24:2–21
Falk B, Dotan R (2006) Child-adult differences in the recovery from high-intensity exercise. Exerc Sport Sci Rev 34:107–112
Falk B, Usselman C, Dotan R, Brunton L, Klentrou P, Shaw J, Gabriel D (2009) Child-adult differences in muscle strength and activation pattern during isometric elbow flexion and extension. Appl Physiol Nutr Metab 34:609–615
Fawkner SG, Armstrong N (2004) Longitudinal changes in the kinetic response to heavy-intensity exercise in children. J Appl Physiol 97:460–466
Godin G, Shephard RJ (1985) A simple method to assess exercise behavior in the community. Can J Appl Sport Sci 10:141–146
Greig C, Sargeant AJ, Vollestad NK (1985) Muscle force and fibre recruitment during dynamic exercise in man. J Physiol Lond 371:176P
Hatzikotoulas K, Patikas D, Ratel S, Bassa E, Kotzamanidis C (2014) Central and peripheral fatigability in boys and men during maximal contraction. Med Sci Sports Exerc 46:1326–1333
Hebestreit H, Mimura K, Bar-Or O (1993) Recovery of muscle power after high-intensity short-term exercise: comparing boys and men. J Appl Physiol 74:2875–2880
Henneman E, Somjen G, Carpenter DO (1965) Functional Significance of Cell Size in Spinal Motoneurons. J Neurophysiol 28:560–580
Hug F, Laplaud D, Savin B, Grelot L (2003) Occurrence of electromyographic and ventilatory thresholds in professional road cyclists. Eur J Appl Physiol 90:643–646
Hug F, Decherchi P, Marqueste T, Jammes Y (2004) EMG versus oxygen uptake during cycling exercise in trained and untrained subjects. J Electromyogr Kinesiol 14:187–195
Hug F, Laplaud D, Lucia A, Grelot L (2006a) A comparison of visual and mathematical detection of the electromyographic threshold during incremental pedaling exercise: a pilot study. J Strength Cond Res 20:704–708
Hug F, Laplaud D, Lucia A, Grelot L (2006b) EMG threshold determination in eight lower limb muscles during cycling exercise: a pilot study. Int J Sports Med 27:456–462
Jurimae J, von Duvillard SP, Maestu J, Cicchella A, Purge P, Ruosi S, Jurimae T, Hamra J (2007) Aerobic-anaerobic transition intensity measured via EMG signals in athletes with different physical activity patterns. Eur J Appl Physiol 101:341–346
Klentrou N, Nishio M-L, Plyley M (2006) Ventilatory breakpoints in boys and men. Pediatr Exerc Sci 18:216–225
Long D, Dotan R, Pitt B, McKinlay B, O'Brien TD, Tokuno C, Falk B (2017) The Electromyographic Threshold in Girls and Women. Pediatr Exerc Sci 29:84–93
Lucia A, Sanchez O, Carvajal A, Chicharro JL (1999) Analysis of the aerobic-anaerobic transition in elite cyclists during incremental exercise with the use of electromyography. Br J Sports Med 33:178–185
Maestu J, Cicchella A, Purge P, Ruosi S, Jurimae J, Jurimae T (2006) Electromyographic and neuromuscular fatigue thresholds as concepts of fatigue. Journal of strength and conditioning research / National Strength & Conditioning Association 20:824–828
Malina RM (1969) Quantification of fat, muscle and bone in man. Clin Orthop Relat Res 65:9–38
Miyashita M, Kanehisa H (1980) Correlation between efficiency in cycling and maximal power of human extensor muscles. J Sports Med Phys Fitness 20:365–370
Moritani T, deVries HA (1978) Reexamination of the relationship between the surface integrated electromyogram (IEMG) and force of isometric contraction. Am J Phys Med 57:263–277
Moritani T, Takaishi T, Matsumoto T (1993) Determination of maximal power output at neuromuscular fatigue threshold. J Appl Physiol (1985) 74:1729–1734
O'Brien TD, Reeves ND, Baltzopoulos V, Jones DA, Maganaris CN (2010) In vivo measurements of muscle specific tension in adults and children. Exp Physiol 95:202–210
Pitt B, Dotan R, Millar J, Long D, Tokuno C, O'Brien T, Falk B (2015) The electromyographic threshold in boys and men. Eur J Appl Physiol 115:1273–1281
Ratel S, Kluka V, Vicencio SG, Jegu AG, Cardenoux C, Morio C, Coudeyre E, Martin V (2015) Insights into the mechanisms of neuromuscular fatigue in boys and men. Med Sci Sports Exerc 47:2319–2328
Riddell MC, Jamnik VK, Iscoe KE, Timmons BW, Gledhill N (2008) Fat oxidation rate and the exercise intensity that elicits maximal fat oxidation decreases with pubertal status in young male subjects. J Appl Physiol 105:742–748
Sargeant AJ, Hoinville E, Young A (1981) Maximum leg force and power output during short-term dynamic exercise. J Appl Physiol Respir Environ Exerc Physiol 51:1175–1182
Simon G, Berg A, Simon-Alt A, Keul J (1981) Determination of the anaerobic threshold depending on age and performance potential. Dtsch Z Sportsmed 32:7–14
Tanaka H, Shindo M (1985) Running velocity at blood lactate threshold of boys aged 6–15 years compared with untrained and trained young males. Int J Sports Med 6:90–94
Tanner JM (1962) Growth at Adolescence. Blackwell Scientific Publications, Oxford
Taylor AD, Bronks R (1996) Effect of acute normobaric hypoxia on quadriceps integrated electromyogram and blood metabolites during incremental exercise to exhaustion. Eur J Appl Physiol Occup Physiol 73:121–129
Vollestad NK, Blom PC (1985) Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand 125:395–405
Vollestad NK, Vaage O, Hermansen L (1984) Muscle glycogen depletion patterns in type I and subgroups of type II fibres during prolonged severe exercise in man. Acta Physiol Scand 122:433–441
Yoder NC (2011) “Peak Finder,” Matlab program. http://www.mathworks.com/matlabcentral/fileexchange/25500-peakfinder
Zafeiridis A, Dalamitros A, Dipla K, Manou V, Galanis N, Kellis S (2005) Recovery during high-intensity intermittent anaerobic exercise in boys, teens, and men. Med Sci Sports Exerc 37:505–512
Acknowledgements
The authors wish to thank all participants for their hard work and dedication and to the parents or guardians for bringing the boys and making it all possible.
Funding
The study was funded by a Brock University internal grant.
Author information
Authors and Affiliations
Contributions
SW, RD and BF conceived and designed research. SW, NJ, RD and JM conducted the experiments. RD designed and built the custom-made ergometer. SW and RD analyzed the data. SW, RD and BF wrote the manuscript. DG and CT contributed to the design of the experiments and the analysis approach. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Additional information
Communicated by Toshio Moritani.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Woods, S., Dotan, R., Jenicek, N. et al. Isometric-based test improves EMG-threshold determination in boys vs. men. Eur J Appl Physiol 119, 1971–1979 (2019). https://doi.org/10.1007/s00421-019-04185-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-019-04185-8