Abstract
Despite ample evidence that females are weaker and possess smaller muscle cross-sectional areas (CSAs) compared to males, it remains unclear if there are sex-related differences in the properties of motor units (MU). Eleven males (age 22 ± 3 years) and 12 females (age 21 ± 1 years) performed isometric trapezoid muscle actions at 10% and 70% of maximal voluntary contraction (MVC). Surface electromyography signals were recorded and decomposed into MU action potential (AP) waveforms and firing instances. Average MUAP amplitudes (MUAPAMPS), mean firing rates (MFRs), initial firing rates (IFRs), and recruitment thresholds (RT) were calculated for the 10% MVC, while MUAPAMPS, IFRs, and MFRs were regressed against RT for the 70% MVC. Ultrasonography was used to measure CSA of the first dorsal interosseous (FDI). Males had greater CSAs (p < 0.001; males 2.34 ± 0.28 cm2, females 1.82 ± 0.18 cm2) and MVC strength (p < 0.001; males 25.9 ± 5.5 N, females 16.44 ± 2.5 N). No differences existed for MUAPAMPS, IFRs, MFRs, or RTs (p > 0.05) during the 10% MVC. For the 70% MVC, the y-intercepts from the MUAPAMPS vs. RT relationships were greater (p < 0.05) for the males (males − 0.19 ± 0.53 mV; females − 0.78 ± 0.75 mV), while the inverse was true for the MFR vs. RT relationships (males 31.55 ± 6.92 pps, females 38.65 ± 6.71 pps) with no differences (p > 0.05) in the slopes. Therefore, smaller CSAs and weaker MVCs are likely the result of smaller higher-threshold MUs for females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The firing rates of motor units (MU) are orderly in correspondence with recruitment threshold during submaximal isometric muscle actions. Earlier recruited (lower-threshold) MUs possess greater firing rates than the later recruited (higher-threshold) MUs regardless of recording methods (Kamen et al. 1995; Carpentier et al. 2001; Farina et al. 2009; Holobar et al. 2009). Only recently has it been speculated that females may possess different MU firing rates than males (Tenan et al. 2013; Peng et al. 2018). Accounting for recruitment threshold as a measure of orderly firing rate behavior, Peng (2018) reported females possessed significantly greater initial firing rates (IFR; firing rates at recruitment) during a 75% maximal voluntary contraction (MVC) of the leg extensors. These findings contradict earlier work by Tenan (2013) who reported no differences for IFRs during a lower-intensity contraction (30% MVC) of the leg extensors. This discrepancy may be due to examined recruitment thresholds of MUs during the 30% MVC. Tenan et al. (2013) observed MUs possessing recruitment thresholds < 11% MVC, unlike the 70% MVC (higher-intensity contraction intensity) where the mean recruitment thresholds of observed MUs were > 11% MVC and up to 70% MVC (Peng et al. 2018). Sex-related differences in IFR, therefore, may be related to recruitment threshold or the intensity of the contraction. Analyzing firing rates at a targeted force rather than at recruitment may provide further insight into possible sex-related differences. Further examination and comparison of firing rates at recruitment and targeted force in males and females during lower- and higher-intensity contractions is warranted.
The Henneman size principle (Henneman 1957) indicates that MUs are recruited in an orderly fashion according to size, with lower-threshold MUs possessing smaller action potential (AP) amplitudes recruited earlier, followed by the recruitment of higher-threshold MUs that possess greater AP amplitudes (Milner-Brown and Stein 1975; Goldberg and Derfler 1977; Martinez-Valdes et al. 2018). Therefore, increases in excitatory synaptic input to the MU pool result in an orderly recruitment of larger MUs (De Luca and Erim 1994). These larger MUs possess larger MUAPAMP (Milner-Brown et al. 1973a; Tanji and Kato 1973) and greater twitch forces (Goldberg and Derfler 1977) than the earlier recruited smaller MUs. The higher-threshold MUs are reported to differ in AP amplitude between males and females for the leg extensors (Trevino et al. 2018). Specifically, Trevino (2018) reported that sex-related differences in the cross-sectional area of the vastus lateralis was explained by the rate of increase in MUAPAMP in relation to recruitment thresholds during a 40% MVC and the type II myosin heavy chain isoform composition (muscle fiber typing) of the muscle. The authors concluded the greater diameter of muscle fibers that primarily expressed type II characteristics accounted for the greater increase in MUAPAMPS relative to recruitment thresholds. Therefore, the diameters of muscle fibers comprising higher-threshold MU primarily explain muscle cross-sectional areas regardless of sex. In addition, Trevino (2018) suggested that the AP amplitudes may be of similar size for the lowest-threshold MUs between males and females, because research has indicated that diameters of fibers that primarily express type I characteristics are more similar in size (Staron et al. 2000) and are believed to encompass lower-threshold MU. The concurrent examination of firing rates and AP amplitudes during lower- and higher-intensity contractions may provide insight on sex-related differences in properties of lower- and higher-threshold MUs.
Sex-related differences in muscle cross-sectional area (CSA) and strength are well documented within the literature for large muscle groups, such as the vastus lateralis and biceps brachii (Maughan et al. 1983; Miller et al. 1993; Hubal et al. 2006). Although untested, sex-related differences in CSA and strength of the first dorsal interosseous (FDI), a mixed fiber type muscle (Johnson et al. 1973), would likely exist similar to the leg extensors. The FDI is the only muscle involved in the abduction of the index finger (Thomas et al. 1986) and the majority of MUs are recruited prior to 50% MVC with a few more recruited up to 70% MVC (Thomas et al. 1986; Kamen et al. 1995; De Luca and Hostage 2010). Therefore, the examination of the higher-threshold MUs is more conducive for the FDI than large limb muscles that have greater recruitment threshold ranges, such as the vastus lateralis where difference in muscle size and strength are more prominent. Examination of the FDI could provide further understanding of the potential sex-related differences in the properties of the lowest- and highest-threshold MUs of a mixed fiber-type muscle. We hypothesize that MU action potential amplitudes and firing rates during a low-intensity contraction will be similar between males and females, but will differ during a high-intensity contraction. Therefore, the purposes of the present study were to examine differences in CSA and strength of the FDI between males and females and, subsequently, to determine if the amplitudes and firing rates of the lower- and higher-threshold MUs recorded during 10% and 70% MVCs differ between sexes. Superimposition of action potential amplitudes does not allow for the recording of the smallest lower-threshold MUs during the 70% MVC and, therefore, the 10% MVC was also included in the study to examine the smallest lower-threshold MUs.
Methods
Participants
Eleven males (mean ± SD: age 22 ± 3 years, height 181.1 ± 6.0 cm, weight 72.9 ± 9.4 kg, BMI 22.2 ± 1.8 kg/m2) and 12 females (age 21 ± 1 years, height 164.2 ± 3.7 cm, weight 63.6 ± 5.3 kg, BMI 23.6 ± kg/m2) volunteered for this investigation. None of the participants reported any current or ongoing neuromuscular disease or musculoskeletal injury. Each participant completed a pre-exercise health history questionnaire and signed a written informed consent document. The participants reported participation in recreational activities and were not competitive athletes. These individuals may best be classified as moderately active or recreationally trained. This study was approved by the university’s institutional review board for human subjects.
Research design
Participants were asked to visit the laboratory twice, separated by at least 24 h. The first visit consisted of familiarization to procedures, which included practising each of the isometric trapezoidal muscle actions several times. During the experimental visit, participants completed the isometric muscle actions as well as ultrasound scans of the FDI. For the isometric muscle actions, the subject’s right forearm was pronated and positioned on a table with the hand open. The forearm, wrist and third through fifth fingers were immobilized with a Velcro strap. The thumb was resisted with a Velcro strap that would allow for a 90° angle between the index finger and the thumb during the isometric contractions. The muscle action of the FDI was isolated and measured during abduction of the index finger against a small flat piece of metal connected to the force transducer (MB-100; Interface, Inc. Scottsdale, AZ, USA), amplified with the Bangnoli-16 EMG Amplifier Unit (Delsys Inc., Natick, MA, USA), and converted digitally with the NI PCI-6220 (National instruments, Austin, TX, USA). Subjects performed three maximal voluntary contractions (MVC) that were 5 s in duration, with 2 min of rest between each contraction. Strong verbal encouragement was given during each MVC. Target force for the 10% and 70% MVCs was determined from the peak force from the three MVCs. The 10% and 70% MVCs were completed in random order. Each participant completed one 10% and one 70% tracing. Subjects were asked to complete an additional contraction if tracings were not satisfactory. Satisfactory tracings were considered when the force output was within 2% of the template as reviewed by an experienced investigator (MEP). For each isometric trapezoidal muscle action (Fig. 1), force was increased at a rate of 10% MVC/second to the target force which was held for 10 s, then decreased at a rate of 10% MVC/second back to baseline. Therefore, the 10% and 70% MVCs lasted 12 and 24 s, respectively. Five minutes rest was given between each submaximal isometric trapezoidal muscle action to ensure potentiation had diminished (Miller et al. 2017a, b). During each contraction, participants maintained their force output as close as possible to the force template displayed digitally on a computer monitor. Subjects were required to refrain from caffeine consumption on the day of the experimental trial.
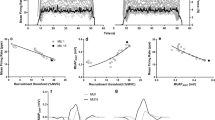
a An example of the individual firing instances of motor units of the first dorsal interosseous during an isometric trapezoidal contraction at 70% maximal voluntary contraction (MVC) for a male participant. The black line is the real-time force signal during the trial. The solid gray vertical lines indicate the individual firing times for each individual motor unit recorded during the contraction. b–d The three relationships analyzed for that subject from the 70% MVC are presented. b IFR vs. recruitment threshold [RT, expressed as percentage of MVC (%MVC)]. c MFR vs. RT. d MU action potential amplitude (MUAPAMP) vs. RT
Electromyography recording
During the trapezoid muscle actions, surface EMG signals were recoded from the FDI using a five-pin surface array sensor (Delsys, Inc, Natick, MA, USA). The sensor array consisted of 0.5 mm pins placed at the corners of a 5 × 5 mm square, with a fifth pin in the center of the square. The surface of the skin was prepared by shaving, removing superficial dead skin with adhesive tape, and sterilizing with an alcohol swab before sensor placement. Dead skin layers were removed by using hypoallergenic tape (3 M, St. Paul, MN, USA) which was repeatedly applied to the skin, then peeled back to remove contaminants (Delsys, Inc., dEMG User Guide). The sensor was then placed over the belly of the FDI muscle and secured with adhesive tape, and a reference electrode was placed over the elbow.
EMG decomposition
Action potentials were extracted into single firing events of MUs from the four separate EMG signals, sampled at 20 kHz, via the Precision Decomposition (PD) III algorithm as described by Deluca (2006). This algorithm was designed for decomposing surface EMG signals into their individual MUAP trains. The accuracy of the decomposed firing instances was initially tested with the reconstruct and test produce (Nawab et al. 2010). In addition, a secondary validation of the accuracy of the MU firing times and AP waveforms generated by the PD III algorithm was performed via spike-trigger average (STA) protocol. Briefly, the PD III algorithm derived firing times were used as triggers for averaging the four raw EMG signals recorded from the surface sensor array (Hu et al. 2013a, b; McManus et al. 2016; Herda et al. 2019; Miller et al. 2019). A window length of 4 s was used and shifted over the surface EMG signal using a step size of 1 s (Hu et al. 2013a, b). For a MU to be included for further analyses, it must have met the 90% accuracy level from the reconstruct and test procedure and met the following criteria from the STA procedure: (1) a high correlation (r > 0.70) between the PD III algorithm and STA-derived AP waveforms and (2) a low coefficient of variation (< 0.30) of the STA-derived AP peak-to-peak amplitude across the contraction time (Hu et al. 2013a).
For each MU, five parameters were extracted from the firing rate data: (1) the recruitment threshold expressed as a percentage of MVC, (2) MUAPAMP (mV), (3) MUAPDUR (ms), (4) initial firing rate (IFR) at recruitment [pulses per second (pps)], and (5) the mean firing rate (MFR) at the targeted contraction level (pps) via custom written software (LabVIEW 2015, National instruments, Austin, TX, USA). The RT was the average of 0.01 s epoch of force that began at the first discharge of the MU. Four unique action potential waveform templates were derived from the four EMG channels. The average of the peak-to-peak amplitude (MUAPAMP) and average duration (MUAPDUR) from the four unique action potential waveforms were used for further analysis (Fig. 2). The IFR was the average of the first three interpulse intervals (Trevino et al. 2016). The MFR was calculated as the average value of the instantaneous discharge rates during the 10 s epoch at the targeted force level. For the 10% MVC, an average of the RT, IFR, MFR, MUAPAMP, and MUAPDUR for all MUs for each subject were used for further statistical analysis. The IFR, MFR, MUAPAMP, and MUAPDUR vs. RT relationships could not be calculated for the 10% MVC as a result of the small range of RTs for each subject. For the 70% MVC, linear regressions were applied to the IFR, MFR, MUAPAMP, and MUAPDUR vs. RT and relationships with the slope and y-intercept values used for statistical analysis (Fig. 2). In the present study, the recorded MU RT range from the 70% MVC for each subject exceeded > 19% MVC. A small recorded RT range of MUs for a subject could result in an outlier or physiologically irrelevant coefficients from the regressions. After close inspection of the data by experienced investigators (MEP and TJH), it was deemed there were no outlier or physiological irrelevant coefficients from the regressions.
The four unique action potential waveforms of the selected MUs from Fig. 1. The lower-threshold MU (MU 1) possesses smaller peak-to-peak action potential amplitudes (MUAPAMPS) in all four channels
Ultrasound measurement
Ultrasound images were taken of the right FDI to determine the muscle cross-sectional area using a LOGIQe ultrasound-imaging device (GE Healthcare UK, Ltd., Chalfont, Buckinghamshire, UK) (Herda et al. 2016; Trevino et al. 2016; Miller et al. 2017a, 2018; Sterczala et al. 2018). Subjects were examined while sitting at a table with the right hand lying open and pronated on top of foam pads formed into an L-shape. The foam pads were to standardize the thumb in a relatively neutral position at 70°. For each scan ultrasound brightness mode (B-mode), the musculoskeletal preset, and a GE 12L-RS Linear Ultrasound Transducer (5–13 MHz), with a 42 × 7 mm footprint were used. The scan depth was set to 2 cm, gain was 38 dB, and transducer frequency was 12 MHz. The origin and insertion of the FDI were located by longitudinally scanning the muscle. The origin and insertion were marked and measured. The midway point between the two was used as the site of the CSA measurement. Once the mid site was determined, a cross section of the muscle belly was scanned with the length of the probe head oriented perpendicular to the second metacarpal. The second metacarpal was used as a reference for the orientation of the probe as the FDI runs along its lateral side. Much care was given to ensure adequate amounts of gel were used to create uniform pressure with the skin and that the probe was perpendicular to the surface of the skin. An image was captured and saved when the muscle was properly focused. Images were saved for each subject and exported as a jpg format image to a personal computer for subsequent analysis. Muscle CSA (cm2) and subcutaneous fat thickness (mm) were determined using the image analysis program ImageJ (National Institutes of Health, Bethesda, MD, USA) (Fig. 3). The scale of each image was calibrated using the centimeter marks inlaid in the image. Subcutaneous fat thickness was measured using the center of the cross section of the muscle as a reference point. The periphery of the muscle was carefully outlined using the polygonal tool and the CSA.
Signal processing
Channel 1 of the four bipolar EMG channels from the five-pin surface array sensors was selected for amplitude analysis. The force (N) and the EMG (mV) signals from channel 1 were recoded with an NI cDAQ (National instruments, Austin, TX, USA) during each isometric muscle action. Data were stored on a personal computer (Dell Optiplex 9010; Dell, Inc., Round Rock, TX, USA) for further analysis. The sampling frequency was 2000 Hz for all signals. The EMG signals were band-pass filtered (zero-phase fourth-order Butterworth filter) at 10–500 Hz, while the force signal was low-pass filtered with a 10-Hz cutoff (zero-phase fourth-order Butterworth filter). All EMG amplitude calculations were performed on the filtered signals. The EMGRMS values during the 10% and 70% MVCs were normalized (%max) to the amplitude (N-EMGRMS) recorded during the highest 0.25 s average of force (N) that occurred during the three MVCs.
Statistical analysis
Independent samples t tests were performed to examine potential sex-related differences in BMI, CSA, sFAT, and MVC force. For MU data from the 10% MVC, the average of IFRs, MFRs, MUAPAMPS, MUAPDURS and RTs were calculated for each subject and independent samples t tests were used to examine potential differences between sexes. For MU data from the 70% MVC, possible sex-related differences in the calculated slopes and y-intercepts from the IFR, MFR, MUAPAMP, and MUAPDUR vs. RT relationships were examined with independent samples t tests. In addition, a two-way mixed factorial ANOVA (sex [male vs. female] × contraction intensity [10%MVC vs. 70%MVC]) was used to examine possible differences in N-EMGRMS during the 10 s epoch at steady force where MFRs were selected between groups and contraction intensities.
Results
Voluntary force and ultrasound
Independent samples t test indicated significant differences between males and females for MVC force (p < 0.001; males 25.9 ± 5.5 N, females 16.6 ± 2.6 N) and CSA (p < 0.001; males 2.34 ± 0.28 cm2, females 1.83 ± 0.19 cm2). However, there were no significant differences between groups for sFAT thickness (p = 0.168, males 0.167 ± 0.03 cm, females 0.182 ± 0.03 cm) or BMI (p = 0.174; male 22.26 ± 2.82 kg/m2, female 26.03 ± 8.64 kg/m2).
Motor units
Descriptive information regarding the number and RTs for recorded MUs from the 10% and 70% MVCs is presented in Table 1. During the 70% MVC, RTs were observed from 18–70% MVC; however, only MUs with RTs from 36 to 42% MVC were recorded for every subject. Therefore, predicted MU data presented in Figs. 4 and 5 reflect this common RT range among all subjects. For the 70% MVC, each subject’s IFR vs. RT (r = 0.85 ± 0.09), MFR vs. RT (r = − 0.90 ± 0.08), and MUAPAMP vs. RT (r = 0.80 ± 0.19) relationships were significant; however, only 2 of 11 and 1 of 12 MUAPDUR vs. RT relationships were significant for the males and females, respectively.
The individual values and mean (SD) for the slopes and y-intercepts from the a initial firing rate (IFR) vs. recruitment threshold [RT, expressed as a percentage of maximal voluntary contraction (%MVC)] and b mean firing rate (MFR) vs. RT relationships. The c IFRs and d MFRs expressed as a function of RT from the 10% (left) and 70% MVCs (right). For the 70% MVC, MU recruitment thresholds were observed for every subject within the presented RT range (36–42% MVC). The three data points represent predicted firing rates for each subject within the range of RTs that was observed for all subjects. Means (SDs) for each group were developed from each subject’s linear regressions and displayed as bar graphs. Asterisk indicates a significant difference for the y-intercepts between the males and females
a Individual values and mean (SD) for the slopes and y-intercepts from the motor unit action potential amplitudes (MUAPAMP) vs. recruitment threshold [expressed as a function of percent maximal voluntary contraction (%MVC)] relationships. b MUAPAMPS from the 10% (left) and 70% MVCs (right). For 70% MVC, MUs were observed for every subject within the presented RT range (36–42% MVC). The three data points represent predicted MUAPAMPS for each subject within the range of RTs that was observed for all subjects. Means (SDs) for each group were developed from each subject’s linear regressions. Asterisk indicates a significant difference for the y-intercepts between the males and females
10% MVC
Independent samples t tests indicated no significant differences between the males and females for average RTs (p = 0.165), IFRs (p = 0.625), MFRs (p = 0.328), MUAPAMPS (p = 0.152), and MUAPDURS (p = 0.176) from the 10% MVC (Fig. 6).
The individual values and mean (SD) for males and females for the 10% maximal voluntary contraction (MVC) for a mean firing rate (MFR, pulses per second, pps), b recruitment threshold (RT, %MVC), c motor unit action potential amplitude (MUAPAMP, mV), d motor unit action potential duration (MUAPDUR, ms) and e initial firing rate (IFR, pps)
70% MVC
For the IFR vs. RT relationships, there were no significant differences between sexes for the slopes (p = 0.603) or the y-intercepts (p = 0.132) (Fig. 4). For the MFR vs. RT relationships, there were no significant differences between sexes for the slopes (p = 0.211); however, there was a significant difference for the y-intercepts (p = 0.021). The y-intercepts of the MFR vs. RT relationships were greater for females (38.65 ± 6.71 pps) than males (31.55 ± 6.92 pps) (Fig. 5).
An additional statistical test was performed on the IFRs to better correspond with the data presented in Peng et al. (2018). A separate two-way mixed factorial ANOVA (sex [male vs. female]) × RT [36% vs. 40% vs. 42% MVC]) calculated on the IFRs presented in Fig. 5 did indicate there was a main effect for sex (p = 0.035) with females having greater IFRs than males, which does tentatively provide support to the findings of Peng et al. (2018).
For the MUAPAMP vs. RT relationships, there was no significant difference (p = 0.323) for the slopes; however, the y-intercepts were significantly different (p = 0.044) between the males and females. The males (− 0.19 ± 0.53 mV) had less negative y-intercepts than the females (− 0.78 ± 0.76 mV) (Fig. 5). The negative y-intercepts were a result of not observing lower-threshold MUs (i.e., < 18% MVC) in combination with the rapid rise of APAMPS of the higher-threshold MUs. Figure 7 illustrates the MUAPAMP vs. RT relationships for a male and female subject. Of note, the y-intercepts are negative despite all action potential amplitudes being positive.
The plotted motor unit action potential amplitude (MUAPAMP) vs. recruitment threshold [RT, expressed as percentage of maximal voluntary contraction (%MVC)] relationships for a male and female participant. The dark circles represent recorded MUs for the male subject and the gray boxes represent recorded MUs for the female subject with corresponding linear equations
For a clearer interpretation, predicted MUAPAMPS were calculated for each individual for MUs within the observed RT ranges for every subject (36–42% MVC) (Fig. 5). It is evident that the males had greater MUAPAMPS within the recorded RT ranges from 36 to 42% MVC, unlike at lower RT ranges during the 10% MVC (Fig. 5).
For the MUAPDUR vs. RT relationships, statistical procedures were performed despite few significant relationships. There were no significant differences between the male and females for the slopes (p = 0.300) or y-intercepts (p = 0.153). Therefore, it is likely that the depth of the recorded MUs within the muscle did not contribute to differences in the amplitude of MUs recorded at the surface of the skin (Fuglevand et al. 1992; Hu et al. 2013a).
N-EMGRMS
There was no significant contraction intensity × sex interaction (p = 0.432), nor was there a significant main effect for sex (p = 0.430). However, there was a significant main effect for contraction intensity (p < 0.001) and, as expected, greater N-EMGRMS was observed at steady force during the 70% MVC (87.1 ± 19.6%) in comparison to 10% MVC (10.7 ± 3.6%).
Discussion
Maximal voluntary contraction force and anatomical CSA were significantly greater for males, which is similar to large mixed fiber-type limb muscles (Miller et al. 1993; Staron et al. 2000). A noteworthy finding in the present study was that the MUs with RTs 1.72–2.76% MVC had similar characteristics (IFRs, MFRs, and MUAPAMPS) for males and females; however, the higher-threshold MUs (RTs > 18% MVC) recruited during the 70% MVC possessed smaller MUAPAMPS and greater MFRs when expressed relative to RT for the females. Therefore, sex-related differences in firing rates at the targeted force and MUAPAMPS were dependent on recruitment threshold and/or intensity of the contraction. There were few significant MUAPDUR vs. RT relationships as expected (Hu et al. 2013a; Miller et al. 2018; Sterczala et al. 2018) and tentatively indicates that variations in MU depth were not a confounding variable in the interpretations of the MUAPAMPS in relation to RT. In addition, there were no differences in MUAPDURS between sexes and suggests that recording distance did not arbitrarily lead to larger action potential amplitudes of the higher-threshold MUs for the males.
Low-intensity contraction
Females had smaller CSAs; however, there were no sex-related differences in the MUAPAMPS for the lowest-threshold MUs (RTs from 1.72 to 2.76% MVC) recorded during the 10% MVC, unlike for MUs recorded during the 70% MVC. Muscular contractions were performed at relative forces; therefore, the absolute targeted force for the females was lower. Despite this lower targeted force, the recorded MUs for the females had similar characteristics (IFRs, MFRs, and MUAPAMPS) to the males at the lower intensity. Previously, the MUAPAMP has been reported to be associated with the force generation capability of the MU (Milner-Brown and Stein 1975; Goldberg and Derfler 1977). It may be expected that females would create similar forces to males if the sizes and the firing rates of recruited MUs during the 10% MVC were equivalent. However, due to a very homogenous population (similar RTs and APAMPS) of MUs recorded during the 10% MVC, linear regressions could not be performed in a manner similar to the MU data from the 70% MVC. Nonetheless, the lowest-threshold MUs (< 3% MVC) recorded in this study possessed similar amplitudes despite differences in muscle CSA between the males and females.
Previously, Tenan et al. (2013) reported no sex-related differences for IFRs during 30% MVCs regardless of menstrual phase. However, the authors indicated that females had higher IFRs during the later phases of the menstrual cycle (ovulation, mid luteal, and late luteal) in comparison to the earlier phases (early follicular and late follicular) for the vastus medialis and vastus medialis oblique muscles. One limitation of this study is we did not record menstrual cycles of the females. Nonetheless, the results of the present study support the findings of Tenan (2013) that firing rates of lower-threshold MUs did not differ between sexes during a low contraction intensity. Together, the MUAPAMPS and firing rates displayed in the present study suggest similar MU control strategies at lower intensities as a result of active MUs being of similar action potential amplitudes and strength (Milner-Brown et al. 1973b; Milner-Brown and Stein 1975; Goldberg and Derfler 1977) for the females and males.
High-intensity contraction
The y-intercepts of the MUAPAMPS vs. RT relationships from the 70% MVC were significantly different between males and females, specifically indicating that the MUAPAMPS of the higher-threshold MUs were smaller for females than males. These results contradict the MUAPAMPS of the lowest-threshold MUs (1.72–2.76% MVC) observed during the 10% MVC (Fig. 5). Therefore, sex-related differences in MUAPAMPS existed as a function of RT and contraction intensity.
It is well understood that males and females differ in the fiber-type characteristics of mixed fiber-type muscles, such as the vastus lateralis and biceps brachii (Simoneau et al. 1985; Miller et al. 1993; Staron et al. 2000; Trevino et al. 2018). Males are reported to possess larger fiber diameters of type I and II muscle fibers with a greater disparity in the diameters of the type II fibers (Miller et al. 1993; Staron et al. 2000). Miller et al (1993) reported the total number of MUs or the number of fibers per MU for the biceps brachii and vastus medialis did not differ between males and females and that fiber diameter and area of fiber type are correlated with CSA and force-generating capabilities of large muscle groups.
Muscle fibers that comprise a MU do not strictly express the characteristics of one fiber type (I vs. II), but rather co-express type I and II characteristics (Carroll et al. 2005; Rowan et al. 2012). It is believed, however, that fibers expressing primarily type II characteristics tend to comprise higher-threshold more so than lower-threshold MUs (Burke et al. 1973; Canepari et al. 2010; Trevino et al. 2018). Therefore, the smaller MUAPAMPS of the higher-thresholds MU for the females in the present study might be the result of smaller diameter and smaller area of fibers that express II characteristics as reported by Trevino (2018). However, similar MUAPAMPS of the lowest-threshold MUs could indicate these MUs consist of fibers that primarily express type I characteristics, which do not differ in size between females and males to the same extent as type II fibers. Future research should include contraction intensities between 10 and 70% MVC to provide further resolution on the potential sex-related differences in MUAPAMPS of the FDI.
The IFR vs. RT relationships from the 70% MVC were strong (r = − 0.85 ± 0.09) for the FDI, unlike that previously reported for the IFR vs. RT relationships of the leg extensors (Trevino et al. 2016; Peng et al. 2018). The negative slopes indicated that the later recruited and larger MUs had lower IFRs as in the present study. As expected, the IFRs for the FDI in the present study were higher than that reported for the leg extensors (De Luca and Contessa 2012; Tenan et al. 2013; Trevino et al. 2016; Peng et al. 2018). There was no difference in the y-intercepts or slopes of the IFR vs. RT relationships between sexes. Peng et al. (2018) provided evidence from 75% MVCs that females possessed greater IFRs than males for the vastus medialis and vastus medialis oblique (pooled data) muscles during the late follicular phase of the menstrual cycle when IFRs were previously reported to be more similar between sexes (Tenan et al. 2013). Subsequently, sex-related differences in IFRs reported by Peng et al. (2018) could be a function of the contraction intensity and/or a function of the recorded MUs (lower- vs. higher-threshold MUs). It should be noted that in the present study, the IFRs during the 70% MVC tended to be higher for the females than males and reflected the sex-related differences for the MFR vs. RT relationships (Fig. 5).
The y-intercepts from the MFRs vs. RT relationships were significantly different between males and females. Females possessed greater MFRs than males for the MUs with RTs ranging from 36 to 42% MVC. The mechanism that would result in greater MFRs for females than males during higher-contraction intensities is unclear. Although speculative, the females might be at a greater operating point of excitation to the motoneuron pool (Contessa and Luca 2012) to maintain the 70% MVC. Sustaining an isometric submaximal contraction at a greater operating point of excitation would lead to increases in MU recruitment and MFRs. Since the majority of MUs would be recruited prior 50% MVC (De Luca and Contessa 2015), the rightward shift in the operating point of excitation would result in significant increases in MFRs along with recruitment of the few remaining MUs. Therefore, performing the 70% MVC at a greater operating point of excitation for the females might provide an explanation for the significantly higher MFRs of the MU pool (i.e., greater y-intercepts).
A potential explanation is that saturation of the firing rates differed between males and females. Previous studies report that firing rates reach a plateau while voluntary force increases linearly (Bigland and Lippold 1954; Dasgupta and Simpson 1962; Clamann 1970; Milner-Brown et al. 1973b; Monster and Chan 1977). The firing rates of the MU pool may have reached the plateau earlier for the males than females and, therefore, firing rates were higher for the females during the 70% MVC. This could also explain why firing rates were not different between sexes during the 10% MVC.
It is reported that females tend to have greater resistance to fatigue during isometric muscle actions; however, there is less disparity between sexes at higher-contraction intensities (Hunter 2016). Considering the majority of MUs are likely recruited prior to 50% MVC for males and females, the greater MFRs during the 70% MVC for females could increase the neural cost of the contraction and minimize the benefits of possessing a greater percentage of fibers that express type I characteristics in regards to fatigue resistance (Hunter 2016; Trevino et al. 2018). Therefore, the greater MFRs for females might provide an explanation for the more similar levels of resistance to fatigue at higher-contraction intensities between sexes in comparison to lower-intensity contractions.
In summary, MUAPAMPS of the lower-threshold MUs with RTs from < 3% MVC were similar in size between males and females, unlike the higher-threshold MUs where males had greater MUAPAMPS. MFRs, and potentially IFRs, were elevated for females during the 70% MVC, which may be the result of performing the contraction at a higher operating point of excitation of the motoneuron pool. Therefore, the non-uniform differences in MUAPAMPS (Fig. 8), or twitch forces, might partially explain alterations in the overall MU control strategies to perform a high-intensity contraction, but not a lower-intensity contraction. Future research should examine the implications of sex-related differences in MUAPAMP and firing rates of the higher-threshold MUs, such as, resistance to fatigue and functional performance (Lee et al. 2017).
Plotted are the motor unit action potentials (MUAPAMP, mV) vs. recruitment thresholds [RT, expressed as percentage of maximal voluntary contraction (%MVC)] relationships for the males and females. RTs were set at 1.7% (female) and 2.7% (male) MVC and 42% MVC from the 70% MVC. These RTs were chosen as it was the average RTs from the 10% MVC and each subject had MUs recorded with RTs up to 42% MVC during the 70% MVC. Linear regressions were used to estimate the APAMP of the last theoretically recruited MU at 67% MVC for the males and females. The vertical dash lines are placed at the %MVC of the lower- and higher-contraction intensity to provide an indication of the sizes of MUs that participated in the respective contraction. It is evident that similar size MUs for males and female were active during the 10% MVC with an overall greater disparity in the sizes of active MUs during the 70% MVC
References
Bigland B, Lippold OCJ (1954) Motor unit activity in the voluntary contraction of human muscle. J Physiol 125:322–335. https://doi.org/10.1113/jphysiol.1954.sp005161
Burke RE, Levine DN, Tsairis P, Zajac FE (1973) Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234:723–748. https://doi.org/10.1113/jphysiol.1973.sp010369
Canepari M, Pellegrino MA, D’Antona G, Bottinelli R (2010) Skeletal muscle fibre diversity and the underlying mechanisms. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1748-1716.2010.02118.x. Accessed 14 Feb 2019
Carpentier A, Duchateau J, Hainaut K (2001) Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol 534:903–912. https://doi.org/10.1111/j.1469-7793.2001.00903.x
Carroll CC, Gallagher PM, Seidle ME, Trappe SW (2005) Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehabil 86:224–229. https://doi.org/10.1016/j.apmr.2004.03.035
Clamann HP (1970) Activity of single motor units during isometric tension. Neurology 20:254. https://doi.org/10.1212/WNL.20.3.254
Contessa P, Luca CJD (2012) Neural control of muscle force: indications from a simulation model. J Neurophysiol 109:1548–1570. https://doi.org/10.1152/jn.00237.2012
Dasgupta A, Simpson JA (1962) Relation between firing frequency of motor units and muscle tension in the human. Electromyography 2:117–128
De Luca CJ, Contessa P (2012) Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107:178–195. https://doi.org/10.1152/jn.00961.2010
De Luca CJ, Contessa P (2015) Biomechanical benefits of the onion-skin motor unit control scheme. J Biomech 48:195–203
De Luca CJ, Erim Z (1994) Common drive of motor units in regulation of muscle force. Trends Neurosci 17:299–305
De Luca CJ, Hostage EC (2010) Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol 104:1034–1046. https://doi.org/10.1152/jn.01018.2009
De Luca CJ, Adam A, Wotiz R et al (2006) Decomposition of surface EMG signals. J Neurophysiol 96:1646–1657. https://doi.org/10.1152/jn.00009.2006
Farina D, Holobar A, Gazzoni M et al (2009) Adjustments differ among low-threshold motor units during intermittent, isometric contractions. J Neurophysiol 101:350–359. https://doi.org/10.1152/jn.90968.2008
Fuglevand AJ, Winter DA, Patla AE, Stashuk D (1992) Detection of motor unit action potentials with surface electrodes: influence of electrode size and spacing. Biol Cybern 67:143–153. https://doi.org/10.1007/BF00201021
Goldberg LJ, Derfler B (1977) Relationship among recruitment order, spike amplitude, and twitch tension of single motor units in human masseter muscle. J Neurophysiol 40:879–890. https://doi.org/10.1152/jn.1977.40.4.879
Henneman E (1957) Relation between size of neurons and their susceptibility to discharge. Science 126:1345–1347
Herda TJ, Miller JD, Trevino MA et al (2016) The change in motor unit firing rates at de-recruitment relative to recruitment is correlated with type I myosin heavy chain isoform content of the vastus lateralis in vivo. Acta Physiol 216:454–463. https://doi.org/10.1111/apha.12624
Herda TJ, Trevino MA, Sterczala AJ et al (2019) Muscular strength and power is correlated with motor unit action potential amplitudes, but not myosin heavy chain isoforms in sedentary males and females. J Biomech 86:215–255
Holobar A, Farina D, Gazzoni M et al (2009) Estimating motor unit discharge patterns from high-density surface electromyogram. Clin Neurophysiol 120:551–562. https://doi.org/10.1016/j.clinph.2008.10.160
Hu X, Rymer WZ, Suresh NL (2013a) Motor unit pool organization examined via spike-triggered averaging of the surface electromyogram. J Neurophysiol 110:1205–1220. https://doi.org/10.1152/jn.00301.2012
Hu X, Rymer WZ, Suresh NL (2013b) Assessment of validity of a high-yield surface electromyogram decomposition. J Neuroeng Rehabil 10:99. https://doi.org/10.1186/1743-0003-10-99
Hubal MJ, Gordish-Dressman H, Thompson P et al (2006) Variability in muscle size and strength gain after unilateral resistance training. Yearb Sports Med 2006:149–150. https://doi.org/10.1016/S0162-0908(08)70354-4
Hunter SK (2016) Sex differences in fatigability of dynamic contractions: Sex differences in skeletal muscle fatigue. Exp Physiol 101:250–255. https://doi.org/10.1113/EP085370
Johnson MA, Polgar J, Weightman D, Appleton D (1973) Data on the distribution of fibre types in thirty-six human muscles: an autopsy study. J Neurol Sci 18:111–129. https://doi.org/10.1016/0022-510X(73)90023-3
Kamen G, Sison SV, Du CC, Patten C (1995) Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol 79:1908–1913. https://doi.org/10.1152/jappl.1995.79.6.1908
Martinez-Valdes E, Negro F, Falla D et al (2018) Surface electromyographic amplitude does not identify differences in neural drive to synergistic muscles. J Appl Physiol 124:1071–1079. https://doi.org/10.1152/japplphysiol.01115.2017
Maughan RJ, Watson JS, Weir J (1983) Strength and cross-sectional area of human skeletal muscle. J Physiol (Lond) 338:37–49
McManus L, Hu X, Rymer WZ et al (2016) Muscle fatigue increases beta-band coherence between the firing times of simultaneously active motor units in the first dorsal interosseous muscle. J Neurophysiol 115:2830–2839. https://doi.org/10.1152/jn.00097.2016
Miller AEJ, MacDougall JD, Tarnopolsky MA, Sale DG (1993) Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol 66:254–262. https://doi.org/10.1007/BF00235103
Miller JD, Herda TJ, Trevino MA et al (2017a) Age-related differences in twitch properties and muscle activation of the first dorsal interosseous. Clin Neurophysiol 128:925–934. https://doi.org/10.1016/j.clinph.2017.03.032
Miller JD, Herda TJ, Trevino MA et al (2017b) Time-related changes in firing rates are influenced by recruitment threshold and twitch force potentiation in the first dorsal interosseous: recruitment threshold, potentiation and motor unit firing rates. Exp Physiol 102:950–961. https://doi.org/10.1113/EP086262
Miller JD, Sterczala AJ, Trevino MA, Herda TJ (2018) Examination of muscle composition and motor unit behavior of the first dorsal interosseous of normal and overweight children. J Neurophysiol 119:1902–1911. https://doi.org/10.1152/jn.00675.2017
Miller JD, Sterczala AJ, Trevino MA et al (2019) Motor unit action potential amplitudes and firing rates during repetitive muscle actions of the first dorsal interosseous in children and adults. Eur J Appl Physiol. https://doi.org/10.1007/s00421-019-04090-0
Milner-Brown HS, Stein RB (1975) The relation between the surface electromyogram and muscular force. J Physiol 246:549–569. https://doi.org/10.1113/jphysiol.1975.sp010904
Milner-Brown HS, Stein RB, Yemm R (1973a) The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol 230:359–370. https://doi.org/10.1113/jphysiol.1973.sp010192
Milner-Brown HS, Stein RB, Yemm R (1973b) Changes in firing rate of human motor units during linearly changing voluntary contractions. https://physoc.onlinelibrary.wiley.com/doi/abs/10.1113/jphysiol.1973.sp010193. Accessed 14 Feb 2019
Monster AW, Chan H (1977) Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol 40:1432–1443. https://doi.org/10.1152/jn.1977.40.6.1432
Nawab SH, Chang S-S, De Luca CJ (2010) High-yield decomposition of surface EMG signals. Clin Neurophysiol 121:1602–1615. https://doi.org/10.1016/j.clinph.2009.11.092
Peng Y-L, Tenan MS, Griffin L (2018) Hip position and sex differences in motor unit firing patterns of the vastus medialis and vastus medialis oblique in healthy individuals. J Appl Physiol 124:1438–1446. https://doi.org/10.1152/japplphysiol.00702.2017
Rowan SL, Rygiel K, Purves-Smith FM et al (2012) Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS ONE 7:e29082. https://doi.org/10.1371/journal.pone.0029082
Simoneau J-A, Lortie G, Boulay MR et al (1985) Human skeletal muscle fiber type alteration with high-intensity intermittent training. Eur J Appl Physiol 54:250–253. https://doi.org/10.1007/BF00426141
Staron RS, Hagerman FC, Hikida RS et al (2000) J Histochem Cytochem 48:623–629
Sterczala AJ, Herda TJ, Miller JD et al (2018) Age-related differences in the motor unit action potential size in relation to recruitment threshold. Clin Physiol Funct Imaging 38:610–616. https://doi.org/10.1111/cpf.12453
Tanji J, Kato M (1973) Recruitment of motor units in voluntary contraction of a finger muscle in man. Exp Neurol 40:759–770. https://doi.org/10.1016/0014-4886(73)90110-6
Tenan MS, Peng Y-L, Hackney AC, Griffin L (2013) Menstrual cycle mediates vastus medialis and vastus medialis oblique muscle activity. Med Sci Sports Exerc 45:2151–2157. https://doi.org/10.1249/MSS.0b013e318299a69d
Thomas CK, Ross BH, Stein RB (1986) Motor-unit recruitment in human first dorsal interosseous muscle for static contractions in three different directions. J Neurophysiol 55:1017–1029. https://doi.org/10.1152/jn.1986.55.5.1017
Trevino MA, Herda TJ, Fry AC et al (2016) Influence of the contractile properties of muscle on motor unit firing rates during a moderate-intensity contraction in vivo. J Neurophysiol 116:552–562. https://doi.org/10.1152/jn.01021.2015
Trevino MA, Sterczala AJ, Miller JD et al (2018) Sex-related differences in muscle size explained by amplitudes of higher-threshold motor unit action potentials and muscle fibre typing. Acta Physiol. https://doi.org/10.1111/apha.13151
Acknowledgements
We would like to thank the graduate and undergraduate students who aided in the collection of data, as well as each of the subjects for their selfless participation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest, financial or otherwise.
Additional information
Communicated by John C. Rothwell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Parra, M.E., Sterczala, A.J., Miller, J.D. et al. Sex-related differences in motor unit firing rates and action potential amplitudes of the first dorsal interosseous during high-, but not low-intensity contractions. Exp Brain Res 238, 1133–1144 (2020). https://doi.org/10.1007/s00221-020-05759-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-05759-1