Abstract
Purpose
The aim of the study was to investigate the effects of voluntary breath-holding on brain activity and physiological functions. We hypothesised that prolonged apnoea would trigger cerebral hypoxia, resulting in a decrease of brain performance; and the apnoea’s effects would be more pronounced in breath-hold divers.
Methods
Trained breath-hold divers and non-divers performed maximal dry breath-holdings. Lung volume, alveolar partial pressures of O2 and CO2, attention and anxiety levels were estimated. Heart rate, blood pressure, arterial blood oxygenation, brain tissue oxygenation, EEG, and DC potential were monitored continuously during breath-holding.
Results
There were a few significant changes in electrical brain activity caused by prolonged apnoea. Brain tissue oxygenation index and DC potential were relatively stable up to the end of the apnoea in breath-hold divers and non-divers. We also did not observe any decrease of attention level or speed of processing immediately after breath-holding. Interestingly, trained breath-hold divers had some peculiarities in EEG activity at resting state (before any breath-holding): non-spindled, sharpened alpha rhythm; slowed-down alpha with the frequency nearer to the theta band; and untypical spatial pattern of alpha activity.
Conclusion
Our findings contradicted the primary hypothesis. Apnoea up to 5 min does not lead to notable cerebral hypoxia or a decrease of brain performance in either breath-hold divers or non-divers. It seems to be the result of the compensatory mechanisms similar to the diving response aimed at centralising blood circulation and reducing peripheral O2 uptake. Adaptive changes during apnoea are much more prominent in trained breath-hold divers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elite breath-hold divers can hold their breath for a substantial period of time: more than 11 min in static apnoea. During maximal breath-holding, the trained divers are exposed to significant changes in arterial blood and alveolar air gas composition. Previous studies have demonstrated that immediately after breath-holding ranging 286–300 s, trained breath-hold divers exhibited the decreased alveolar oxygen partial pressure up to 26–29 mmHg and increased carbon dioxide tension up to 40–56 mmHg (Ferretti 2001; Lindholm and Lundgren 2006).
Hypoxia and hypercapnia (in general) are widely acknowledged to influence the central nervous system. Thus, acute hypoxia affects cognitive processes (Kida and Imai 1993), inducing dysfunction of psychomotor performance, attention impairment, verbal fluency deficits, language production, cognitive fluency, and short-term memory (Virués-Ortega et al. 2004). Acute hypercapnia in its turn leads to a wide range of negative consequences: lightheadedness and faintness, a mild anxiety (occasionally), sense of smothering, mild euphoria, and headache (Parsons et al. 2001).
Electrical brain activity is also affected by hypoxia and hypercapnia. The inhalation of a low-oxygen gas mixture slows down the EEG and increases the total EEG spectrum power (Papadelis et al. 2007; Schellart and Reits 2001). Higher carbon dioxide concentration in the inhaled mixture leads to a notable growth in power of delta band (Halpern et al. 2003) and robust oscillatory power loss in alpha (Hall et al. 2011; Halpern et al. 2003), beta, and low-gamma frequency bands (Hall et al. 2011).
Thus, it is most reasonable to assume that changes in blood gas composition during breath-holding may also lead to changes in brain performance, inducing changes in EEG and cognitive functions. It is well known that during breath-hold competitions, apnoea sometimes is accompanied with the loss of consciousness or loss of motor control (Fitz-Clarke 2006; Lindholm 2007). Descriptions of the EEG dynamics during breath-holding are scanty. There is some evidence of spontaneous brain activity being modified by breath-holding effects (Schellart and Reits 1999). However, only three volunteers were examined in this study, and their breath-holdings were relatively short (120–225 s).
The purpose of the study was to investigate the influence of a prolonged breath-holding on a wide range of psychophysiological functions integrating respiratory and cardiovascular variables, brain activity and cognitive functions in trained breath-hold divers, and a control group of non-divers.
We hypothesised that (1) apnoea would trigger changes in alveolar O2 and CO2 partial pressure and modify brain performance in comparison with a resting state. As the O2 partial pressure is lower and CO2 partial pressure is higher during longer breath-holding, (2) the changes in brain activity and attention deficit after the termination of apnoea are expected to be more pronounced in breath-hold divers.
Materials and methods
Participants
Two groups of participants were tested in the study. The regularly training breath-hold divers’ group (D-group) comprised 13 men (height: 177 ± 8 cm, weight: 78 ± 11 kg; lung vital capacity: 6.9 ± 0.8 l, total lung capacity: 8.5 ± 1.1 l). At the time of the experiment, the sportsmen personal best breath-holding was 309 ± 36 s. The control group (C-group) consisted of nine young men without any special breath-hold training (height: 179 ± 12 cm, weight: 77 ± 11 kg; lung vital capacity: 6.0 ± 1.2 l, total lung capacity: 7.1 ± 1.1 l). The ratios of total lung capacity to predicted values were 1.22 and 0.97 in D-group and C-group, respectively.
All subjects were non-smokers and had no previous history of cardiovascular, pulmonary, or neurological diseases. Social status and educational attainment were nearly the same among the participants. The participants were informed of the objectives and procedures of the study, and gave their written consent prior to commencement of the experiment. The experimental protocol was performed in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Institute of Biomedical Problems of the Russian Academy of Sciences.
The participants were tested in a room at a temperature of 20–22 °C. The subjects were not allowed to take food or caffeine containing beverages for at least 2 h prior to the examination. None of them was taking medications at the time of the study.
Experimental design
The examination was divided into two parts. During the first visit, the participants were instructed and trained in all experimental procedures, including several breath-holdings. During the second visit, the lung capacity of each subject was measured. Afterwards, the subjects performed three breath-holdings: the first two were considered to be pre-test and the third one to be the test breath-holding. Recreation intervals between breath-holdings were no less than 5 min each.
It is essential to note that several breath-holdings are necessary to achieve better results: the first two breath-holdings are usually shorter than the succeeding one for both trained and untrained subjects (Schagatay et al. 1999). In our study, the pre-test breath-holding of C-group subjects was 148 ± 48 s long, and the test breath-holding was 180 ± 50 s long (p > 0.05). The length of pre-test breath-holdings in the D-group was 171 ± 32 s, while the test breath-holding was 265 ± 42 s, which is significantly longer (p < 0.001). Hereunder, we will confine our attention to discussing the physiological changes during the test breath-holding only.
Apnoea was performed in a sitting position; the subject’s head was placed on a special soft cushion to thoroughly relax the subject’s neck muscles. Before holding his breath, the participant performed pulmonary ventilation in unison with a voice command “inhale-exhale”. The inhale phase lasted for 3.6 s and the exhale phase—for 3.2 s. On being subjectively ready for the breath-holding, the subject breathed out fully into the mouthpiece of a gas analyser to specify O2 and CO2 partial pressure in the final portion of the exhaled air (alveolar air). Then, the subject inhaled the ambient air from a rubber bag which contained 80 % of his vital capacity, as described previously (Wein et al. 2007). The inhale ending moment (“collapsing” of a rubber bag) was considered to be a starting point of the voluntary apnoea, and the subject put his head on the cushion. At the end of breath-holding, the subject breathed out into a mouthpiece of the gas analyser to determine O2 and CO2 partial pressure in the final portion of the exhaled air.
To estimate the apnoea effect on the attention level, a letter cancellation task was used. Each participant performed the task four times: before the experiment, and then just after every breath-holding.
Measurements and data analysis
Oxygenation of the brain tissue. Near infrared spectrometry (spectrometer NIRO-200, Hamamatsu Photonics K.K., Japan) was applied to test the oxygenation and change of total haemoglobin Δ[tHb] (blood filling) in brain tissue during apnoea. The sensor was attached to the subject’s forehead (upper part of the left frontal lobe) with adhesive tape, and was additionally fixed by an electroencephalographic cap. The source was spaced from the receiver by 4 cm, keeping the mean measurement depth of at least 2 cm. According to Kornienko and Pronin (2009), the cerebral cortex is less than 2 cm away from the skull in this region. Changes in deoxy (Δ[HHb]) and oxy haemoglobin (Δ[OHb]) concentration were estimated using the modified Lambert law. Δ[tHb] was calculated as a sum of Δ[HHb] and Δ[OHb]. Changes in concentration were expressed in μmol × l, where l means a mean pathlength (cm) passed by light in the tissue. The brain tissue oxygenation index (TOI) was calculated by the spatial resolution spectroscopy method, and was expressed as a percentage.
Arterial blood oxygenation. Arterial blood oxygenation index (arterialised oxygen saturation, SpO2) was continuously measured using pulse oximeter Nonin 8600 (Nonin, USA). A sensor was attached to a tested person’s left earlobe.
Heart rate and arterial blood pressure. ECG was continuously recorded by means of an NVX-52 amplifier (Medical Computer Systems, Russia) throughout the experiments. Disposable Ag/AgCl electrodes were positioned on the sternum and the fifth intercostal space on the left side of the body. Beat-to-beat values of heart rate were computed and analysed offline.
Arterial blood pressure was measured noninvasively using the vascular unloading technique with a Finometer monitor (FMS, Finapres Medical Systems, Netherlands). The measurement cuff was attached to the middle phalanx of the left hand long finger.
Lung volume. Vital capacity was measured by spirometry, and the residual volume was measured by the helium-rebreathing method (Sackner et al. 1975) using the differential-pressure transducer and medical mass spectrometer AMIS-2000 (Innovision, Denmark).
O 2 and CO 2 partial pressure. O2 and CO2 partial pressure was measured by the medical mass spectrometer AMIS-2000 (Innovision, Denmark) at 14 Hz in ambient air and in the final portion of exhaled (alveolar) air (PA) both before and after breath-holding.
Average pulmonary O 2 consumption and CO 2 release rates during breath-holding. Average O2 consumption and CO2 release rates during breath-holding were calculated as follows (Liner et al. 1993):
where F A o2, co2 , F b o2, co2 , and F f o2, co2 are the fraction of O2 or CO2 in alveolar air before apnoea, in the rubber bag (ambient air), and in alveolar air after apnoea, respectively; RV, V b , and V e are the residual volume, rubber bag volume, and expiratory volume after apnoea, respectively, and t is the duration of apnoea.
Chest and abdominal respiration movements were recorded using piezo-electrical sensors. The data were amplified and converted into digital signals (NVX-52, Medical Computer Systems, Russia).
Electrical brain activity. EEG activity was recorded using 19 gel-filled Ag/AgCl electrodes located in accordance with the international 10–20 system (Fp 1, Fp 2, F 7, F 3, F z , F 4, F 8, T 3, C 3, C z , C 4, T 4, T 5, P 3, P z , P 4, T 6, O 1, and O 2). A mid-forehead placement of the ground electrode and an integrated ear reference electrode (A1 + A2) were used. In addition, electrooculogram was recorded simultaneously with EEG to control eye movement’s and blinking artifacts.
All EEG data were amplified and converted into digital signals (NVX-52, Medical Computer Systems, Russia) with a sampling rate of 1000 Hz. The signals were bandpass filtered (0.5–40 Hz).
Before the first apnoea was carried out, EEG was recorded twice: during resting state with the subject’s eyes closed and in resting state with the subject’s eyes open. Then EEG was recorded continuously during the preparation for breath-holding (deep rhythmical breathing) and during apnoea.
The EEG data were processed offline and analysed by the Neocortex software (Neurobotics, Russia). The EEG recordings were visually inspected for artifacts. Only artifact-free segments were used for analysis. The EEG signals in resting state with the subject’s eyes closed and opened were analysed during 60 s each. We divided the EEG recorded before and during breath-holding into three fragments: forced ventilation before the apnoea, breath-holding up to the first respiratory muscle contraction, and breath-holding during contractions. Three intervals were chosen from each fragment: one at the beginning, one in the middle, and one at the end. The length of fragments and intervals depended upon the duration of an individual breath-holding. Therefore, six EEG intervals were analysed during breath-holding. The first one covered the short dynamic phase characterised by rapid changes in blood pressure and heart rate (Fagoni et al. 2015; Sivieri et al. 2015), the next two intervals were during the subsequent steady phase, and the last three ones—during a further dynamic phase, or the “struggle” phase (Guaraldi et al. 2009).
The Fourier spectral analysis epoch was 4 s with an overlap of 3.5 s. The following frequency bands and sub-bands were analysed: Theta (4–7 Hz), Alpha (7–14 Hz), Alpha-1 (7–9 Hz), Alpha-2 (9–11 Hz), Alpha-3 (11–14 Hz), and Beta-1 (14–20 Hz). On the basis of the spectral analysis data, the amplitude maps were constructed and averaged across subjects. EEG during involuntary diaphragmatic contractions was found full of artifacts caused by muscle activity and body movements which made a correct analysis of it impossible.
Direct current potential (DC potential) was recorded using unpolarised gel-filled Ag/AgCl electrodes: an active electrode was placed in the vertex region (C z ), while an integrated reference electrode was placed on the earlobes (A1 + A2). To control the electric skin resistance, the galvanic skin response was measured simultaneously with the DC. All the signals were amplified by means of the NVX-52 DC system (Medical Computer Systems, Russia).
The reference level of the DC potential was rated as an average value for 30 s prior to the breath-holding preparation. An averaged DC value was taken as a base line for every participant, and the following DC shift was estimated with reference to this value.
Attention test. To rate the attention level, visual search, and mental speed, the subjects performed a letter cancellation task (modified Bourdon’s test). Each participant was given a clipboard with an attached page of Russian alphabet letters organised randomly. The page comprised 554 letters in 10 rows with 69 target letters. Respondents were asked to work with these rows of letters, as if they were reading. They had to underline “H” letters and cross out “K” letters within 1 min. The task should have been performed both as quickly and as accurately as possible. After 1 min, the respondents were asked to stop and mark the letter they had reached.
The total number of letters searched provided a measure for the speed of processing. Attention level was calculated by the following formula: K = [(L1 − L2 − L3)/L] × 100 %, where L1 is a sum of correctly marked target letters, L2—a sum of missed target letters, L3—a sum of incorrectly marked letters, L—the total number of target letters up to the letter reached by the subject within 1 min. For estimating the attention level, the following scale was used: K = 0–20 %—very bad; 21–40 %—bad; 41–60 %—satisfactory; 61–80 %—good; 81–100 %—very good.
Each participant performed the letter cancellation task several times: four times during the first visit (for training, without any connection with breath-holding); and four times during the second visit (one at the very beginning of the experiment, and immediately after every breath-holding).
Anxiety level. The Spielberger State-Trait Anxiety Inventory was used to measure two types of anxiety: state anxiety, or anxiety about an event, and trait anxiety, or anxiety level as a personal characteristic. The test was performed before the breath-holdings.
Statistics
The physical data were expressed as mean and standard deviation. The sample distributions of other variables were not normal in our study. The obtained results were expressed as median and interquartile range, and non-parametric Mann–Whitney and Wilcoxon tests were applied for independent and paired samples, respectively, to discriminate variables from each other. Significance level was set at 0.05.
Results
Cardiorespiratory variables
The ability of D-group subjects to hold their breath was considerably higher (p < 0.001), than that of the C-group: 265 ± 42 vs. 180 ± 50 s, respectively.
The breath-holding induced a substantial decrease of the PA O2 and a notable increase of the PA CO2 (p < 0.01 for both). According to the duration of apnoea, breath-hold divers demonstrated more dramatic decrease of PA O2 compared with C-group (p < 0.05). At the same time, PA CO2 values were similar between breath-hold divers and non-divers at the end of the breath-holding irrespective of the apnoea duration.
Breath-hold divers showed lower rates of pulmonary oxygen consumption (\(\dot{V}\)o2) and carbon dioxide release (\(\dot{V}\)co2) during breath-holding than the subjects of the C-group (p < 0.05) (Table 1).
Arterial blood oxygenation during apnoea decreased in both groups, but at the end of the breath-holding, the SpO2 in the D-group was significantly lower (p < 0.01) compared to the C-group (Table 1; Fig. 1a).
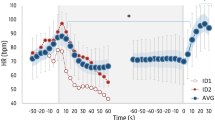
Changes in haemodynamic parameters induced by breath-holding. Arterial blood oxygenation (SpO 2 ) (a); brain tissue oxygenation index (TOI) (b); total concentration of haemoglobin in the brain tissue (Δ[tHb]) (c); heart rate (d); and mean arterial pressure (MAP) (e). Breath-holding (BH) normalized time (%) is plotted on X axis. The duration of breath-holding varied for different participants; therefore, we consider the duration of apnoea as 100 % for each participant. Data are presented as median (solid lines) and interquartile range (dashed lines). Black dots (•) indicate a significant difference between the groups (p < 0.05)
Brain tissue oxygenation index did not change during apnoea in all participants, and was quite similar in breath-hold divers and untrained subjects (Table 1; Fig. 1b).
Brain tissue haemoglobin concentration. During breath-holding, the concentration of haemoglobin in the left frontal lobe tissue increased in both groups. The increment of haemoglobin concentration was significantly higher in the D-group (p < 0.01; Table 1; Fig. 1c).
Heart rate. A considerable heart rate increase caused by the deep inhale was detected in subjects of both groups just before apnoea. Because of this, we used the mean heart rate value over the period from 60 to 30 s before breath-holding as a base level. According to Sivieri et al. (2015) and Fagoni et al. (2015), we found short dynamic phase at the beginning of breath-holding, characterised by rapid changes of heart rate. Then, the heart rate returned to the base level during the first quarter of apnoea. Consequently, there were no changes in heart rate during breath-holding in the C-group, while the heart rate of D-group subjects was decreasing. At the end of apnoea, heart rate in the D-group was significantly lower than that of C-group subjects (p < 0.05; Table 1; Fig. 1d).
Arterial blood pressure dynamic consisted of three distinct phases during apnoea (Fig. 1e), as it was previously observed by Fagoni et al. (2015) and Sivieri et al. (2015): a short dynamic phase, characterised by rapid changes in blood pressure, a steady-state phase, and a further subsequent dynamic phase, characterised by a continuous increase in blood pressure. The pronounced rise in the blood pressure was related to the so called “struggle” phase (Guaraldi et al. 2009) connected with the onset of diaphragmatic contractions.
At the end of breath-holding, arterial blood pressure was significantly higher in both groups in comparison with the base level (p < 0.01; Table 1). However, no significant difference between the groups was found before and after breath-holding.
Electrical brain activity
DC potential. Deep rhythmical breathing during the preparation phase before breath-holding did not cause any significant DC shift (Fig. 2). The beginning of apnoea was accompanied by a specific artifact (see the pointer in Fig. 2), which was seemingly caused by inhalation from a rubber bag and placing the subject’s head on the cushion. Then, the DC shifted down 1.4 (0.9–1.7) mV from the base line. Up to the end of the apnoea, DC potential was relatively stable without any significant differences between the groups. Interestingly, the interquartile range became considerably wider during breath-holding in both groups.
DC potential shifts recorded during rhythmical breathing before apnoea and during the breath-holding. RB, rhythmical breathing; BH, maximal breath-holding. Normalized time (%) for rhythmical breathing (−100 to 0) and for breath-holding (0–100) are plotted on X axis. The zero point corresponds to the starting point of apnoea. Data are presented as median (solid lines) and interquartile range (dashed lines). The arrow marks an artifact caused by inhalation from a rubber bag and placing subjects’ head on the cushion. Black dots (•) indicate a significant difference between the breath-hold divers and the control group (p < 0.05)
EEG during resting state. The EEG recorded during a resting state differed in trained breath-hold divers and untrained subjects. Some peculiarities in the EEG activity were observed in breath-hold divers: non-spindled, sharpened alpha rhythm; slowed-down alpha with the frequency nearer to the theta band; and untypical spatial pattern of alpha activity (for instance, Fig. 3a). Some of the divers demonstrated sinusoidal beta rhythm.
Electroencephalogram during resting state with eyes opened. Representative EEG records (a) of a breath-hold diver (on the left) and a subject of the control group (on the right). EEG of the majority of trained breath-hold divers is not typical for resting state with eyes opened. EEG topographic maps showing the distribution of spectrum amplitude in alpha-1 (b) and beta-1 (c) frequency bands. The displayed data are median. Plus sign (+) indicates a significant difference (increase) between the breath-hold divers and the control group (p < 0.05)
A significant discrepancy between the C- and D-groups was found during the EEG spectral analysis. It was most pronounced in alpha-1 and beta-1 frequency bands (Fig. 3b, c). During resting state with eyes opened, breath-hold divers demonstrated higher amplitude of the alpha-1 sub-band in frontal regions and higher beta-1 sub-band amplitude in posterior frontal, central, and parietal regions.
EEG during breath-holding. Due to deep rhythmical breathing, while preparing for apnoea, the total EEG spectrum amplitude increased nearly by 1.5 times in D-group subjects (Fig. 4a). At the beginning of breath-holding, the effect of deep rhythmical breathing was also evident, and then, the total EEG spectrum amplitude decreased. In the middle of breath-holding, it returned to the base level and was similar to the resting state with eyes closed. In the C-group, the changes of the total EEG spectrum amplitude were the same, but less pronounced.
Effect of rhythmical breathing and apnoea on the total EEG spectrum (0.5–30 Hz) amplitude in breath-hold divers (D-group) and non-divers (C-group). RS, resting state with eyes closed; RB, rhythmical breathing; BH, maximal breath-holding up to the first contraction; F4, T4, C4, P4, O2—frontal, temporal, central, parietal, and occipital sites of right hemisphere. The total EEG spectrum amplitudes were calculated for periods of resting state with eyes closed (RS), rhythmical breathing before breath-holding (RB), and during the breath-holding (BH). 1 and 2—intervals before breath-holding: one at the beginning and one at the end of rhythmical breathing. 3, 4, and 5—three intervals of breath-holding: one—during the dynamic phase at the beginning of apnoea, next two—during the subsequent steady phase of apnoea. Dashed lined rectangle marks represent the interval of apnoea up to the first respiratory muscle contraction. No significant changes in comparison with resting state were observed. Data are presented as median. Rectangles filled with light grey indicate significant changes from corresponding initial values during resting state with eyes closed (p < 0.05)
Comparing the spectrum amplitudes of EEG frequency sub-bands during apnoea (before the respiratory muscle contractions) and during resting state with eyes closed, we found a few significant differences: the increase in beta-1 sub-band in F8 in the D-group and the decrease in theta band in F8 and T6 in the C-group (Fig. 5).
EEG topographic maps showing the distribution of spectrum amplitude in different frequency bands and sub-bands during resting state and during breath-holding in D-group (a) and C-group (b). Resting state, resting state with eyes closed; Breath-holding, maximal breath-holding up to the first contraction (interval 5 in Fig. 4). Data are presented as median. Plus sign (+) and minus sign (−) indicate a significant difference (increase and decrease, respectively) vs. resting state with eyes closed (p < 0.05). Only three significant changes were found
The levels of attention and anxiety
Attention level. The results of the first letter cancellation task (made before any breath-holdings) were similar in D- and C-groups. No significant differences between the groups were found for the speed of processing or for the attention level. The attention level of all subjects was rated as “good” (61–80 %) or “very good” (81–100 %).
After breath-holding, the speed of processing did not change (Fig. 6), but the attention level increased significantly (p < 0.05 in the D-group; p < 0.01 in the C-group). The attention level of C-group subjects became “very good”; moreover, four participants reached the maximum rate (100 %). The majority of breath-hold divers revealed a higher attention level as well, including those who managed to hold their breath for more than 5 min.
Results of letter cancelation task in breath-hold divers (D-group) and non-divers (C-group) before and after breath-holding. Speed of processing—the total number of letters searched per minute (a). Attention level—the percentage of correctly marked target letters (b). Data are presented as median and interquartile range. *p < 0.05; **p < 0.01—significant difference vs. before breath-holding
Anxiety level. The trait anxiety level of divers was significantly lower (p < 0.05) than that of control subjects: 42 (38–44) vs. 48 (44–51), respectively. The state anxiety level was similar in both groups.
Discussion
Brain activity and cognitive functions before breath-holding
The present study showed specific EEG activity in breath-hold divers during resting state in comparison with control subjects. We could assume that this EEG pattern might be related to regular diver’s apnoeic training. Similar waking EEG activity was reported in patients with the obstructive sleep apnoea syndrome (Grenèche et al. 2008; Morisson et al. 2001). For instance, Morisson et al. (2001) observed EEG slowing in the frontal and central regions of the brain in apneic patients. The study of Grenèche et al. (2008) also showed that patients compared to healthy subjects were characterised by higher mean awake spectral power in four main EEG frequency bands and by progressive wake-dependent increase in low (0.5–7.8 Hz) and fast (12.7–29.2 Hz) frequency range activity.
We can speculate that specific EEG activity in trained breath-hold divers are neurodynamic in nature and may decrease considerably after discontinuing apnoea training. At least, Morisson et al. (2001) showed that the treatment effective in reversing respiratory impairments during the night normalized EEG patterns during wakefulness.
The EEG deviations in breath-hold divers seem not to be accompanied by cognitive impairments. For instance, the initial attention level and speed of processing (before apnoea) were similar in C- and D-groups. Ridgway and McFarland (2006) also could not find any neurocognitive impairment when testing elite breath-hold divers, who carried out standard neuropsychological tests, including speed of visuo-motor responding, speed of language comprehension, response inhibition, visual and verbal attention, and recall tasks. The results indicated that the breath-hold divers performed these tasks within the average range.
Apnoea-induced changes in EEG and cognitive functions
Breath-holding led to significant changes in alveolar O2 and CO2 partial pressure as well as in arterial blood oxygenation index. End-tidal PA CO2 increased in both groups of breath-hold divers and non-divers, and reached 51–57 mmHg (6.8–7.6 kPa) that corresponded to the breaking point of dry apnoea (Overgaard et al. 2006). PA O2 and SpO2 decreased compared with resting values, but were not the same in D-group and C-group at the end of breath-holding. In accordance with duration of the apnoea, PA O2 and SpO2 levels after the breath-holding were substantially lower in breath-hold divers than in non-divers. Nevertheless, no considerable changes in the rhythmic brain activity were recorded during and after breath-holding in comparison with the resting state. Participants of either group had their apnoeic EEG quite similar to the resting state with eyes closed. The obtained data were in agreement with the results of Schellart and Reits (1999) who noted only a minor shift of alpha and mu rhythms frequency during apnoea.
We also did not reveal any attention deficit immediately after breath-holding in either C- or D-groups. In contrast to our working hypothesis, the attention level in both groups increased after prolonged apnoea. Our finding conforms to the results of Schellart and Reits (1999). They demonstrated that untrained persons performing breath-holdings from 120 to 225 s had no notable deviations in their cognitive brain potential (P300).
Apnoea-induced changes in brain DC potential and oxygenation index
Excellent brain performance during and after breath-holding could be explained by the fact that notable cerebral hypoxia does not evolve during apnoea. This assumption is supported by the dynamics of brain DC potential and the brain tissue oxygenation index. It is well known that the DC potential is connected with the functional and metabolic state of the brain (Murik and Shapkin 2004) and is associated with P CO2 and P O2 in brain tissues (Lehmenkühler et al. 1999; Voipio et al. 2003). Our study has demonstrated that the DC potential in C- and D-groups displays similar dynamics irrespective of apnoea duration. Being reduced at the beginning of breath-holding, the DC potential stays at a relatively stable level up to the end of apnoea, even during the diaphragmatic contractions. Therefore, we can assume that the brain nerve cell functioning did not change during breath-holding. This is possible only in case of permanent rate of O2 delivery to the brain up to the end of apnoea.
Apnoea-induced cardiorespiratory changes
The constant level of brain oxygenation index and DC potential may be maintained by compensatory mechanisms aimed at centralising blood circulation similar to diving response during the “struggle” phase of apnoea (Cross et al. 2013; Guaraldi et al. 2009). It results from bradycardia, reduced cardiac output, increased arterial blood pressure, and peripheral vasoconstriction through sympathetic activation (Ferretti and Costa 2003; Gooden 1994; Lindholm and Lundgren 2009). Therefore, the vital organs, i.e., the brain and the heart, are sufficiently supplied by blood. Indeed, our study has demonstrated the substantial decrease of heart rate and increase of arterial blood pressure after the onset of diaphragmatic contractions in both groups. These changes were more prominent in breath-hold divers, as was also reported in a recent investigation by Tocco et al. (2012). All together, these results confirm that the compensatory changes were more pronounced in D-group than in C-group. Moreover, the increase of brain tissue haemoglobin level (blood filling) in D-group was three-folds higher than in C-group. It leads to the assumption that cerebral blood flow during apnoea is higher in D-group compared with C-group. Indeed, Palada et al. (2007) demonstrated that breath-hold divers showed a greater increase in the middle cerebral artery mean blood velocity contrasting with non-divers after the cessation of apnoea (105.8 vs. 52.9 %, respectively). The blood flow depends on vessels resistance and pressure difference in arterial and venous parts of the investigated region. In turn, the pressure difference depends mainly on changes in the mean arterial pressure, while regional resistance depends on the vessels’ diameters. In our study, mean arterial pressure was rose during the third phase (Fagoni et al. 2015; Sivieri et al. 2015), or the “struggle” phase (Guaraldi et al. 2009) of apnoea to similar levels in both divers and untrained persons. More pronounced augmentation of cerebral blood flow in breath-hold divers vs. non-divers, thereby, could be associated with the decreased resistance of vessels in the brain. This suggestion is consistent with the substantially higher increase of the brain tissue haemoglobin level (blood filling) at the termination of the apnoea in D-group than in untrained persons.
Ability to support constant rate of O2 delivery to the brain and brain oxygenation index during prolonged apnoea in breath-hold divers may be connected with increased oxygen stores in the organism in comparison with non-divers. Despite similar height and body weight, the total lung capacity in D-group was 1.25—higher than in C-group. The finding is in agreement with previous studies (Ferretti et al. 2012; Schagatay 2009). On the other hand, D-group showed significantly lower pulmonary \(\dot{V}\)o2 during the apnoea in comparison with the C-group. That may be connected with less pronounced redistribution of blood flow from peripheral tissues and decrease of peripheral tissue metabolic rate in divers than in non-divers. These effects could be caused by vasospasm in limb muscles (Joulia et al. 2009), as well as by specific mental techniques aimed at muscle relaxation and slowing down heart rate during breath-holding used by divers (Schagatay 2009; Kusakov 2012).
Conclusion
Our findings contradicted the primary hypothesis. Despite the fact that apnoea trigger changes in alveolar O2 and CO2 partial pressure, no considerable changes in the brain activity were found during and after breath-holding compared with resting state. The obtained data have demonstrated that apnoea up to 5 min did not lead to notable cerebral hypoxia or a decrease of brain performance in either breath-hold divers or non-divers. The apparent contradiction between the decreased PA O2 and SpO2 levels, the lack of changes in the brain oxygenation index and brain DC potential during breath-holding seems to account for specific compensatory mechanisms aimed at centralising blood circulation and reducing peripheral tissue oxygen consumption rate. These adaptive mechanisms are much more prominent in trained breath-hold divers. The adaptation of breath-hold divers to regular apnoea enables them to avoid the reduction of the oxygen supply to brain tissues and to prevent neurocognitive impairment caused by breath-holding.
Abbreviations
- C-group:
-
Control group
- D-group:
-
Breath-hold divers’ group
- DC potential:
-
Direct current potential
- ECG:
-
Electrocardiogram
- EEG:
-
Electroencephalogram
- HR:
-
Heart rate
- MAP:
-
Mean arterial pressure
- BH:
-
Breath-holding
- PA CO2 :
-
CO2 partial pressure in alveolar air
- PA O2 :
-
O2 partial pressure in alveolar air
- RB:
-
Rhythmical breathing before breath-holding
- SpO2 :
-
Arterial blood oxygenation index
- TOI:
-
Brain tissue oxygenation index
- \(\dot{V}\)CO2 :
-
Carbon dioxide release rate
- \(\dot{V}\)O2 :
-
Pulmonary oxygen consumption rate
- Δ[tHb]:
-
Change of total haemoglobin in brain tissue
References
Cross TJ, Kavanagh JJ, Breskovic T, Zubin Maslov P, Lojpur M, Johnson BD, Dujic Z (2013) The effects of involuntary respiratory contractions on cerebral blood flow during maximal apnoea in trained divers. PLoS One 8:e66950
Fagoni N, Sivieri A, Antonutto G, Moia C, Taboni A, Bringard A, Ferretti G (2015) Cardiovascular responses to dry resting apnoeas in elite divers while breathing pure oxygen. Respir Physiol Neurobiol 219:1–8
Ferretti G (2001) Extreme human breath-hold diving. Eur J Appl Physiol 84:254–271
Ferretti G, Costa M (2003) Diversity in and adaptation to breath-hold diving in humans. Comp Biochem Physiol A Mol Integr Physiol 136:205–213
Ferretti G, Costa M, Moroni R, Ranieri P, Butti F, Sponsiello N (2012) Lung volumes of extreme breath-hold divers. Sport Sci Health 7:55–59
Fitz-Clarke JR (2006) Adverse events in competitive breath-hold diving. Undersea Hyperb Med 33:55–62
Gooden BA (1994) Mechanism of the human diving response. Integr Physiol Behav Sci 29:6–16
Grenèche J, Krieger J, Erhardt C, Bonnefond A, Eschenlauer A, Muzet A, Tassi P (2008) EEG spectral power and sleepiness during 24 h of sustained wakefulness in patients with obstructive sleep apnea syndrome. Clin Neurophysiol 119:418–428
Guaraldi P, Serra M, Barletta G, Pierangeli G, Terlizzi R, Calandra-Buonaura G, Cialoni D, Cortelli P (2009) Cardiovascular changes during maximal breath-holding in elite divers. Clin Auton Res 19:363–366
Hall EL, Driver ID, Croal PL, Francis ST, Gowland PA, Morris PG, Brookes MJ (2011) The effect of hypercapnia on resting and stimulus induced MEG signals. Neuroimage 58:1034–1043
Halpern P, Neufeld MY, Sade K, Silbiger A, Szold O, Bornstein NM, Sorkine P (2003) Middle cerebral artery flow velocity decreases and electroencephalogram (EEG) changes occur as acute hypercapnia reverses. Intensive Care Med 29:1650–1655
Joulia F, Lemaitre F, Fontanari P, Mille ML, Barthelemy P (2009) Circulatory effects of apnoea in elite breath-hold divers. Acta Physiol Oxf 197:75–82
Kida M, Imai A (1993) Cognitive performance and event-related brain potentials under simulated high altitudes. J Appl Physiol 74:1735–1741
Kornienko VN, Pronin IN (2009) Diagnostic neuroradiology. Springer, Berlin
Kusakov I (2012) Deconcentration of attention: addressing the complexity of software engineering. http://deconcentration-of-attention.com. Accessed 27 Jan 2012
Lehmenkühler A, Richter F, Pöppelmann T (1999) Hypoxia- and hypercapnia-induced DC potential shifts in rat at the scalp and the skull are opposite in polarity to those at the cerebral cortex. Neurosci Lett 270:67–70
Lindholm P (2007) Loss of motor control and/or loss of consciousness during breath-hold competitions. Int J Sports Med 28:295–299
Lindholm P, Lundgren CEG (2006) Alveolar gas composition before and after maximal breath-holds in competitive divers. Undersea Hyperb Med 33:463–467
Lindholm P, Lundgren CEG (2009) The physiology and pathophysiology of human breath-hold diving. J Appl Physiol 106:284–292
Liner MH, Ferrigno M, Lundgren CE (1993) Alveolar gas exchange during simulated breath-hold diving to 20 m. Undersea Hyperb Med 20:27–38
Morisson F, Décary A, Petit D, Lavigne G, Malo J, Montplaisir J (2001) Daytime sleepiness and EEG spectral analysis in apneic patients before and after treatment with continuous positive air pressure. Chest 119:45–52
Murik SE, Shapkin AG (2004) Simultaneous recording of EEG and direct current (DC) potential makes it possible to assess functional and metabolic state of nervous tissue. Int J Neurosci 114:977–997
Overgaard K, Friis S, Pedersen RB, Lykkeboe G (2006) Influence of lung volume, glossopharyngeal inhalation and PET O2 and PET CO2 on apnea performance in trained breath-hold divers. Eur J Appl Physiol 97:158–164
Palada I, Obad A, Bakovic D, Valic Z, Ivancev V, Dujic Z (2007) Cerebral and peripheral hemodynamics and oxygenation during maximal dry breath-holds. Respir Physiol Neurobiol 157:374–381
Papadelis C, Kourtidou-Papadeli C, Bamidis PD, Maglaveras N, Pappas K (2007) The effect of hypobaric hypoxia on multichannel EEG signal complexity. Clin Neurophysiol 118:31–52
Parsons LM, Egan G, Liotti M, Brannan S, Denton D, Shade R, Robillard R, Madden L, Abplanalp B, Fox PT (2001) Neuroimaging evidence implicating cerebellum in the experience of hypercapnia and hunger for air. Proc Natl Acad Sci USA 98:2041–2046
Ridgway L, McFarland K (2006) Apnea diving: long-term neurocognitive sequelae of repeated hypoxemia. Clin Neuropsychol 20:160–176
Sackner MA, Greeneltch D, Heiman MS, Epstein S, Atkins N (1975) Diffusing capacity, membrane diffusing capacity, capillary blood volume, pulmonary tissue volume, and cardiac output measured by a rebreathing technique. Am Rev Respir Dis 111:157–165
Schagatay E (2009) Review: predicting performance in competitive apnoea diving. Part I: static apnoea. Div Hyperb Med 39:88–99
Schagatay E, van Kampen M, Andersson J (1999) Effects of repeated apneas on apneic time and diving response in non-divers. Undersea Hyperb Med 26:143–149
Schellart NA, Reits D (1999) Is magnetoencephalography applicable in clinical neurophysiology of diving? SPUMS J 29:156–158
Schellart NA, Reits D (2001) Transient and maintained changes of the spontaneous occipital EEG during acute systemic hypoxia. Aviat Space Environ Med 72:462–470
Sivieri A, Fagoni N, Bringard A, Capogrosso M, Perini R, Ferretti G (2015) A beat-by-beat analysis of cardiovascular responses to dry resting and exercise apnoeas in elite divers. Eur J Appl Physiol 115:119–128
Tocco F, Crisafulli A, Melis F, Porru C, Pittau G, Milia R, Concu A (2012) Cardiovascular adjustments in breath-hold diving: comparison between divers and non-divers in simulated dynamic apnoea. Eur J Appl Physiol 112:543–554
Virués-Ortega J, Buela-Casal G, Garrido E, Alcázar B (2004) Neuropsychological functioning associated with high-altitude exposure. Neuropsychol Rev 14:197–224
Voipio J, Tallgren P, Heinonen E, Vanhatalo S, Kaila K (2003) Millivolt-scale DC shifts in the human scalp EEG: evidence for a nonneuronal generator. J Neurophysiol 89:2208–2214
Wein J, Andersson JP, Erdeus J (2007) Cardiac and ventilatory responses to apneic exercise. Eur J Appl Physiol 100:637–644
Acknowledgments
The authors would like to thank the breath-hold divers and the control group subjects for their enthusiastic participation in the study. We also thank Anastasia Sharapkova for improving the English language of the manuscript. This work was supported by the Program of Basic Research of Department of Physiology and Basic Medicine of Russian Academy of Sciences, by the Program “Psychophysiological bases of human activity” AAAA-A16-116021660050-6 and by the grant of Russian Foundation for Basic Research 12-04-01665-a.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Massimo Pagani.
Rights and permissions
About this article
Cite this article
Ratmanova, P., Semenyuk, R., Popov, D. et al. Prolonged dry apnoea: effects on brain activity and physiological functions in breath-hold divers and non-divers. Eur J Appl Physiol 116, 1367–1377 (2016). https://doi.org/10.1007/s00421-016-3390-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3390-2