Abstract
Purpose
Acute breath-holding deprives the human body from oxygen. In an effort to protect the brain, the diving response is initiated, coupling several physiological responses. The aim of this study was to describe the physiological responses to apnea at the cardiac, peripheral and cerebral level.
Methods
31 physically active subjects (17 male, 14 female, 23.3 ± 1.8 years old) performed a maximal static breath-hold in a seated position. Heart rate (HR), muscle and cerebral oxygenation (by means of near-infrared spectroscopy, NIRS) were continuously measured. RM MANOVA’s were used to identify changes in HR, peripheral (mTOI) and cerebral (cTOI) tissue oxygenation and oxygenated (O2Hb) and deoxygenated (HHb) hemoglobin during apnea.
Results
Average apnea duration was 157 ± 41 s. HR started decreasing after 10 s (p < 0.001) and dropped on average by 27 ± 14 bpm from baseline (p < 0.001). mTOI started decreasing 10 s after apnea (p < 0.001) and fell by 8.6 ± 4.0% (p < 0.001). Following an immediate drop after 5 s (p < 0.001), cTOI increased continuously, reaching a maximal increase of 3.7 ± 2.4% followed by a steady decrease until the end of apnea. cTOI fell on average 5.4 ± 8.3% below baseline (p < 0.001).
Conclusion
During apnea, the human body elicits several protective mechanisms to protect itself against the deprivation of oxygen. HR slows down, decreasing O2 demand of the cardiac muscle. The decrease in mTOI and increase in cTOI imply a redistribution of blood flow prioritizing the brain. However, this mechanism is not sufficient to maintain cTOI until the end of apnea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygen (O2) is crucial for all living cells. The brain is especially vulnerable to a lack of O2 as it uses up to 20% of the total body O2 metabolism at rest (Madsen et al. 1993). This is illustrated by the evidence that, following sudden circulatory arrest, syncope occurs within 30 s (Shemie and Gardiner 2018) and damage is considered irreversible between 2 and 10 min following arrest (Dhanani et al. 2012). However, elite apneists can easily hold their breath for over 5 min, with the current world record set at 11 min 35 s (AIDA International 2020), which indicates that the human body is capable of ensuring O2 delivery to the brain under apneic conditions.
Indeed, in an effort to protect the brain during apnea, the body elicits a series of physiological responses called the diving response. This response is initiated by apnea and enhanced by stimulation of the facial cold receptors (Foster and Sheel 2005), reduces overall O2 demand, conserves intrinsic oxygen stores (Gooden 1994; Foster and Sheel 2005; Costalat et al. 2017) and prioritizes O2 delivery to the brain. Irving (Irving 1934, 1935) already hypothesized the existence of a mechanism composed of bradycardia combined with a redistribution of blood flow consisting of peripheral vasoconstriction to protect the brain. These responses have been well established since the 60s for both diving mammals (Scholander et al. 1962; Irving 1963) and humans (Heistad et al. 1968; Campbell et al. 1969). Bradycardia develops through increased parasympathetic nerve activity induced by reduced pulmonary stretch and removal of the inspiratory induced phasic tachycardia (Bain et al. 2018), while peripheral vasoconstriction originates from increased sympathetic activity (Heistad et al. 1968). Contraction of the spleen, which acts as a reservoir for red blood cells (Espersen et al. 2002), has also been linked to the diving response. In response to apnea and mediated by alpha-adrenoreceptors (Fredén et al. 1978), the spleen actively contracts, releasing red blood cells and thus increasing hemoglobin concentration by 2–5% (Schagatay et al. 2001; Richardson et al. 2005; Bouten et al. 2019). This increases the O2 content in the blood and therefore allows for longer breath-hold times (Schagatay et al. 2001).
The protective role of the diving response is clearly observed in studies showing increased cerebral blood flow during dry static apnea compared to rest in trained apneists (Joulia et al. 2009; Willie et al. 2015; Bain et al. 2016), although this response was less present in endurance trained athletes with no experience in breath-holding (Joulia et al. 2009). In addition, next to this preserved O2 delivery to the brain, Bain et al. (2016) also found that the brain had the capacity to decrease its metabolism, as calculated from cerebral blood flow and radial artery–jugular venous O2 content difference, in trained apneists, as such further protecting the brain from deoxygenating. Eichhorn et al. (2015, 2017) examined both cerebral and peripheral tissue oxygenation through NIRS technology during apnea. Consistent with increases in cerebral blood flow, cerebral tissue oxygenation index (cTOI) showed an increase by 10%, starting to decrease after 3 min and only reaching values below baseline at the very end of maximal static apnea, while peripheral tissue oxygenation started decreasing immediately after onset of apnea (Eichhorn et al. 2015, 2017).
The studies mentioned above all indicate the role of the diving response as a protective mechanism for the brain. However, most of these studies include elite or trained breath-hold divers while breath-holding has also been suggested (Lemaître et al. 2010) and applied (Woorons et al. 2008, 2016, 2019; Fornasier-Santos et al. 2018) as a training method to improve sports performance. From a safety perspective, the need therefore arises to obtain insight into the physiological responses to an acute bout of apnea in non-apnea trained individuals. More specifically, it is important to know if the diving response is also successful in protecting the brain against the apnea-induced hypoxia in these naïve subjects. Whereas cerebral and peripheral oxygenation has been documented (Palada et al. 2007; Eichhorn et al. 2015; Ratmanova et al. 2016), research on the factors underpinning this profile, i.e., the changes in peripheral and cerebral oxygenated and deoxygenated hemoglobin (O2Hb and HHb), especially in untrained subjects, is scarce. In addition, all studies cited above analyzed the values on respective intervals on a relative time scale (i.e., as a percentage of total apnea duration). Although this solves the issue of individual differences in apnea duration and gives valuable information on the general profile, a lot of information is lost, especially on the absolute timing of the fast onset of the mechanisms at the very beginning of breath-hold, and also the changes at the very end of the breath-hold.
The purpose of this study was therefore to quantitatively investigate the physiological responses to voluntary breath-holding at the cardiac, peripheral and cerebral level and their respective time course in naïve subjects on an absolute time scale. We hypothesize that naïve subjects will show a rapid bradycardia and peripheral tissue deoxygenation to maintain cerebral oxygenation.
Methods
Ethical approval
All procedures were conform to the Declaration of Helsinki and approved by the local ethical committee of the Ghent University Hospital (EC UZG 2016/1148). Each subject was informed about the procedure and the aim of the study and gave their written informed consent. A medical history questionnaire was completed prior to the study. All volunteers were declared to be in good health.
Subjects
Thirty-one healthy subjects (17 males and 14 females) naïve to breath-holding participated in this study. Inclusion criteria for selection were age (18- to 30-year old), being physically active and being in good general health. Smokers were excluded from the test. All participants were physically active and performed recreational physical exercise on a weekly basis and none of them were trained in breath-holding. Selected subjects were 23.3 ± 1.8 (male: 23.6 ± 1.7, female: 22.9 ± 1.9) years of age and had an average length of 175.4 ± 8.4 cm (male: 180.4 ± 6.2, female: 169.4 ± 6.8, p < 0.001) and weight of 68.6 ± 8.1 kg (male: 74.2 ± 4.8, female: 61.8 ± 5.7, p < 0.001). Subjects were instructed to refrain from caffeine, alcohol and physical activity 24 h before the test.
Experimental design
Protocol
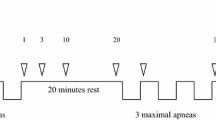
Subjects visited the Sport Science Laboratory Jacques Rogge (Ghent University, sea level) with a constant ambient air temperature of 18 °C and humidity of 45%. First, subjects filled out a medical questionnaire and the PAR-Q test (Pescatello et al. 2013). Second, an anthropometrical assessment was performed. Third, after a 3-min seated baseline, subjects performed a series of three maximal static apneas in a seated position, interspersed with 2-min recovery intervals. Subjects were notified 30 s prior to each apnea and started the attempt after a 10-s countdown. All apneas were preceded by a deep, but not maximal inspiration. During the breath-holds, participants were motivated with verbal time cues and strong verbal encouragement. No hyperventilation prior to the breath-holding was allowed to standardize the response and to avoid hypoxic syncope. Apneic times were recorded using a chronometer.
Measures
Both heart rate (HR) and muscle and cerebral oxygenation were measured continuously, starting from a 3-min baseline before the first apnea until 3 min after the last apnea. HR was registered continuously on a beat-by-beat basis (Polar H7 sensor, Polar, Kempele, Finland). Subjects were monitored for muscle and cerebral oxygenation using near-infrared spectroscopy (NIRO-200NX, Hamamatsu Phototonics, Hamamatsu, Japan). The NIRO-200NX registered at 1 Hz and detects changes [expressed in μmol. L−1 (µM)] in oxygenated (O2Hb) and deoxygenated (HHb) hemoglobin compared to baseline using the modified Beer Lambert law (Pellicer and Bravo 2011). Tissue oxygenation index (TOI) was registered based on spatially resolved spectroscopy and calculated as O2Hb/(O2Hb + HHb) × 100 (Pellicer and Bravo 2011). After shaving and disinfecting, the probe for peripheral (muscle) oxygenation was placed longitudinally on the distal area of the muscle belly of right M Vastus Lateralis. The probe for cerebral oxygenation was placed on the right prefrontal cortex located between Fp2 and F4 in compliance with the modified international EEG 10-20 system (Klem et al. 1999). For safety reasons, subjects were instructed to resume breathing if absolute cerebral tissue O2 index fell below 50% or decreased by 20% compared to baseline (Edmonds et al. 2004).
Data analysis
Because the length of apneas increases during a series of breath-holds (Schagatay et al. 1999) which allows the responses to develop more fully, only data from the third and last apnea were used for analysis. Both HR and oxygenation were analyzed: the first and last 60 s of apnea and the 30 s before and after apnea were filtered for each individual and 5-s values were selected for analysis.
Drop in HR, peripheral and cerebral oxygenation (mTOI and cTOI) and oxyhemoglobin (m[O2Hb] and c[O2Hb]) was calculated by subtracting the lowest value observed during the breath-hold and the first 30 s of recovery from the value at time 0 (= onset of breath-hold). Increase in peripheral and cerebral deoxyhemoglobin (m[HHb] and c[HHb]) was calculated by subtracting the baseline value from the maximal value achieved during the breath-hold and the first 30 s of recovery.
For [O2Hb] and [HHb], all values are set at zero at baseline. This was done by taking the mean of the last 30 s before onset of apnea and setting this as zero. This means that values express changes in (de)oxygenation expressed as ∆ µM. For HR and TOI, absolute values were used.
Statistical analysis
All data were expressed as mean ± SD. IBM SPSS statistics 24 package was used for statistical analysis. Shapiro Wilk test was used to control for normal distribution of the data and Levene’s test for the homogeneity in variances, while Mauchly’s Test of Sphericity indicated that sphericity was not violated. Statistical significance was set at p < 0.05 for all statistical tests.
A one-way Manova was used to identify differences between males and females for the depending variables age, length, weight and apnea duration.
Patterns in physiological responses were analyzed using Repeated Measures Manova for 7 parameters: HR, mTOI and cTOI, and peripheral and cerebral [O2Hb] (m[O2Hb] and c[O2Hb]) and [HHb] (m[HHb] and m[HHb]). 5-s time points for these parameters were used as repeated measures. Pairwise comparisons were made using the Least Square Difference method.
To examine the link between apnea duration and the magnitude of the responses, correlations between apnea duration on the one hand, and drop in HR, mTOI, m[O2Hb], cTOI, c[O2Hb] and the increase in m[HHb] and c[HHb] on the other, were analyzed using Pearson correlation.
Results
General
Subjects were able to hold their breath on average for 157 ± 41 s, ranging from 96 to 244 s. Although male subjects tended to hold their breath longer than females, this was not statistically significant (male: 170 ± 47, female: 142 ± 25, F = 3.611, p = 0.067). Data from 2 subjects were not included in the analysis because they showed signs of dizziness and syncope. Their patterns are illustrated with separate graphs and will be discussed in the discussion section.
Heart rate
The heart rate response is depicted in Fig. 1. Heart rate starts to increase 15 s prior to apnea (p = 0.001), reaching 87 ± 17 bpm at onset of apnea. HR starts decreasing 10 s after the start of apnea (p < 0.001) reaching values below onset values after 15 s (p = 0.003) and reaching minimal values of 60 ± 11 bpm after 83 ± 58 s. HR dropped on average by 27 ± 14 bpm (30 ± 13%) from the start (p < 0.001). HR strongly increases immediately post-apnea (p < 0.001). The difference between the value at onset and lowest HR tended to correlate (R = 0.352, p = 0.057) with apnea duration.
The heart rate response for the first and last 60 s of apnea. The grey area represents the breath-hold. The blue graph indicates the average of all subjects except two individuals who (nearly) fainted, those are depicted as dotted red graphs (ID1 and ID2). The blue beam indicates 95% confidence intervals while error bars indicate standard deviations. *Statistical differences compared to baseline (time = 0) at p < 0.05
Oxygenation
Peripheral oxygenation
Tissue oxygenation index
Figure 2a illustrates that mTOI fell on average by 8.6 ± 4% from 72.1 ± 4.5% at baseline to 63.6 ± 5.5% (Table 1, p < 0.001) within 5 s post-apnea. mTOI started decreasing 10 s after onset of apnea (p < 0.001), resulting in values significantly below baseline after 15 s (p = 0.042). mTOI continuously decreased, reaching minimal values 5 s post-apnea and returning to baseline only 30 s post-apnea (p = 0.347). The decrease in mTOI correlated significantly with apnea duration (R = 0.484, p = 0.007).
Peripheral oxygenation for the first and last 60 s of apnea. a Muscle tissue oxygenation (mTOI). b Changes in oxygenated muscle hemoglobin (m[O2Hb]). c Changes in deoxygenated muscle hemoglobin (m[HHb]). The grey area represents the breath-hold. The blue graph indicates the average of all subjects except two individuals who (nearly) fainted, those are depicted as dotted red graphs (ID1 and ID2). The blue beam indicates 95% confidence intervals while error bars indicate standard deviations. *Statistical differences compared to baseline (time = 0) at p < 0.05
∆[O2Hb] and ∆[HHb]
m[O2Hb] started to decrease 10 s (p < 0.010) after the onset of apnea, reaching values significantly below baseline (BL = − 0.52 ± 1.29 µM) after 30 s of apnea (p = 0.025) and kept decreasing to a minimum 5 s after the end of apnea (Fig. 2b, − 8.28 ± 5.15 µM). m[O2Hb] started to increase again 5 s after breathing was resumed (p < 0.001), reaching baseline 25 s post-apnea (p = 0.184, Fig. 2b). Concurrently, m[HHb] started to increase 5 s after the onset of apnea (p < 0.001), reaching values significantly above baseline after 15 s (BL = − 0.38 ± 1.09, p = 0.003, Fig. 2c). m[HHb] kept increasing until the end of the breath-hold (6.53 ± 5.64 µM) from when it slowly started to decrease again, but still remained elevated 30 s post-apnea (2.20 ± 3.00 µM, p = 0.001). Both decrease in m[O2Hb] (R = 0.362, p = 0.049), and the increase in m[HHb] (R = 0.500, p = 0.005) were significantly correlated with apnea duration.
Cerebral oxygenation
Tissue oxygenation index
Following an immediate drop 5 s (p < 0.001) after the onset of apnea, cTOI recovered and increased continuously, reaching a maximal increase of 3.7 ± 2.4% compared to baseline (p < 0.001) after 74 ± 27 s (Table 1). From then on, cTOI steadily decreased until the end of apnea significantly falling below baseline already 15 s before the end of apnea (p = 0.032, Fig. 3a). cTOI fell on average 5.4 ± 8.3% from a baseline of 67.9 ± 8.0% to minimal values of 62.6 ± 8.1% at the very end of apnea, with individual decreases up to 25%. cTOI increased immediately after apnea, already reaching baseline 10 s post-apnea (p = 0.811). The drop in cTOI correlated significantly with apnea duration (R = 0.684, p < 0.001).
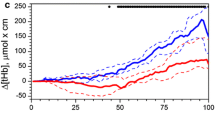
Cerebral oxygenation for the first and last 60 s of apnea. a Cerebral tissue oxygenation (cTOI). b Changes in oxygenated cerebral hemoglobin (c[O2Hb]). c Changes in deoxygenated cerebral hemoglobin (c[HHb]). The grey area represents the breath-hold. The blue graph indicates the average of all subjects except two individuals who (nearly) fainted, those are depicted as dotted red graphs (ID1 and ID2). The blue beam indicates 95% confidence intervals while error bars indicate standard deviations. *Statistical differences compared to baseline (time = 0) at p < 0.05
∆[O2Hb] and ∆[HHb]
Figure 3b shows that c[O2Hb] started to increase 10 s after the onset of apnea (p < 0.001) reaching values above baseline (BL = − 0.83 ± 1.57 µM) after 30 s (p = 0.015). c[O2Hb] increases on average by 6.42 µM which is reached after 99 ± 44 s (Table 1). After the maximal value, c[O2Hb] decreases, but never reached values below baseline on a group level. Following an initial small but significant increase from − 0.49 ± 0.90 at BL to 0.05 ± 1.28 µM, in the first 15 s (p = 0.003), c[HHb] returns to baseline and remains relatively constant (Fig. 3c). c[HHb] is increased after 60 s of breath-hold (0.135 ± 1.78 µM, p = 0.044) and continues to increase until the end of apnea (10.89 ± 8.89 µM, p < 0.001). c[HHb] starts decreasing toward baseline 5 s after cessation of apnea (p < 0.001) not reaching baseline after 30 s (p = 0.001). Both the amplitude of the increase in c[HHb] (R = 0.547, p = 0.002) and decrease in c[O2Hb] (R = 0.655, p < 0.001) correlated significantly with apnea duration.
Discussion
This study quantifies heart rate and both peripheral and cerebral oxygenation during apnea in naïve subjects. It is the first to look into the effects on changes in peripheral and cerebral TOI, [O2Hb] and [HHb] in response to acute dry static apnea on an absolute time scale. Our data show an almost immediate strong decrease in peripheral oxygenation (mTOI) combined with acute reduction in heart rate. By considering the first and last minute of breath-hold, our data revealed an initial drop in cerebral oxygenation (cTOI) in the first 10 s of apnea. This was quickly reversed and cerebral oxygenation (cTOI) was maintained and even increased above resting values and only decreased below baseline at the very end, confirming the protective role of the diving response toward the brain. During the experiments 2 of the 31 subjects experienced syncopal symptoms and abruptly stopped the apnea, which represents the first observation of cerebral and peripheral oxygenation for healthy subjects performing voluntary breath-hold until (near) syncope. These subjects showed strongly divergent patterns in peripheral and/or cerebral oxygenation indicating that the diving response insufficiently preserved O2 delivery to the brain.
Heart rate shows an initial increase prior to apnea until the first 5 s of apnea. This is most likely induced by anticipatory respiratory behavior as subjects were notified 30 and 10 s before the onset of apnea. The greater pulmonary stretch during the deeper breathing and inspiration (Sroufe 1971) before breath-hold could explain this increase as well as possible mental arousal. In addition, increased HR at onset of apnea has also been suggested to compensate for reduced stroke volume through reduced venous return caused by increased intrathoracic pressure (Ferrigno et al. 1986; Sivieri et al. 2015). Opposed to data in elite breath-hold divers who obtain minimal heart rates between 30 and 60 s after apnea onset (Ferretti 2014), our subjects reached minimal heart rates after 83 ± 58 s showing very large inter-individual variation. However, the strongest decrease is also seen in the first 30 s with seven subjects already reaching 90% or more of the maximal decrease. Decreased lung stretch and the removal of the phasic tachycardia induced during inspiration stimulate this parasympathetic nerve activity (Bain et al. 2018) and lead to bradycardia which develops during the breath-hold as a part of the diving response. In the present study, HR dropped on average by 27 bpm, varying between 9 and 53. This is in line with the 30–50 bpm decreases which are often reported for static apneas in elite breath-hold divers (Bain et al. 2018).
mTOI shows a steady, quasi-linear decrease (8.6 ± 4.0%) from the beginning of the apnea until 10 s post-apnea. m[O2Hb] shows a steady decrease from 10 s after onset until 5 s post-cessation of apnea while m[HHb] shows the opposite pattern. Physiologically, this indicates that the peripheral vasoconstriction occurs within the first 10 s and leads to limited blood flow and thus O2 delivery. This is in accordance with peripheral vasoconstriction being known as a sympathetically induced response through an increase in sympathetic muscle nerve activity from either chemostress (Heusser et al. 2009) or the elimination of ventilatory inhibition of sympathetic activity (Badrov et al. 2017). As a consequence, constant extraction of O2 on a smaller blood flow will lead to an increase in fractional O2 extraction, resulting in steadily decreasing m[O2Hb] and increasing m[HHb] until the end of the apnea. Indeed, in our study, apnea duration correlated significantly with the drop in mTOI and increase in m[HHb], indicating that the longer the breath-hold, the stronger the deoxygenation at muscular level. This is most likely due to the continued effect of peripheral vasoconstriction in deoxygenating the muscle tissue. In the present study, the drop in mTOI is less pronounced than in trained breath-hold divers (Eichhorn et al. 2015). This could easily be explained by breath-hold duration as the subjects in the study of Eichhorn et al. (2015) were trained breath-hold divers who held their breath on average for 284 s while our subjects ‘only’ reached 157 s on average. Indeed, Palada et al. (2007) also showed a stronger decrease in mTOI in breath-hold divers as compared to naïve subjects. Steady decreases in mTOI have also been observed in dynamic apneas, with attenuated O2 extraction as induced from attenuated m[HHb] and similar mTOI decreases, indicating better O2 conserving responses in trained breath-hold divers (Costalat et al. 2017).
On a cerebral level, we saw an immediate (in the first 5 s following onset of breath-hold) and significant drop in cTOI by 1.4%. It is the first time that this immediate drop in cTOI has been reported. This could be related to the fact that the relative time scales by which cTOI has been reported in previous research are not sensitive to quick changes. This decrease can be explained by the initial drop in blood pressure observed for 5–6 s after onset of apnea (Sivieri et al. 2015) caused by the acute reduction of venous return through increased intrathoracic pressure (Andersson and Schagatay 1997). Blood pressure is known to play an important role in dynamic cerebral autoregulation in which decreased blood pressure leads to decreased cerebral blood flow and oxygenation (Lucas et al. 2010). This drop in cTOI is possibly aiding in the sympathetic stimulation, as these are tightly coupled (Winklewski and Frydrychowski 2013) triggering the peripheral response. Indeed, by comparing peripheral and cerebral data in the first 15 s of apnea, we observed that the initial drop in cTOI is quickly reversed and has fully recovered almost immediately after mTOI starts to decrease. Another explanation is that the deep breaths before apnea cause a mild hypocapnia (Cross et al. 2014) which might also lead to decreased cerebral blood flow (Kety and Schmidt 1948). Following this initial small decrease and concurrent with the increase in mTOI, cTOI stabilizes and progressively increases to finally exceed baseline by 4.6 ± 3.0% at its maximal level. During this phase, c[O2Hb] slowly starts to increase while c[HHb] remains relatively constant. The combination of these data suggests a modest increase in cerebral blood flow and shows that the diving response is successful in protecting the brain, at least initially. This has also been observed in elite breath-hold divers (Eichhorn et al. 2015) although another study only found an increase in cTOI in naïve subjects but not in trained breath-hold divers (Palada et al. 2007). After reaching this maximal level, cTOI starts decreasing already more than 60 s before termination of breath-hold, with the average pattern eliciting values below baseline during the last 20 s of breath-hold. The average decrease in cTOI is 5.4 ± 8.3% below baseline with one individual going as low as 25% below baseline. c[O2Hb] not only starts to decrease later than cTOI, but also decreases until the end of apnea while the increase in c[HHb] develops at a much faster pace. This indicates that more hemoglobin is measured under the NIRS probe and thus suggests that blood flow is still increased at the end of apnea, which is supported by observations of increased cerebral blood flow in elite apneists (Bain et al. 2016). Despite increased blood flow, the delivery of O2 to the brain is ultimately insufficient to keep c[O2Hb] and cTOI at baseline levels. A possible explanation is that a decrease in SaO2 at the end of apnea, and thus a lower percentage of oxygen-bound hemoglobin, causes a decrease in O2 delivery to the brain despite increased blood flow. Indeed, Eichhorn et al. (2015) observed that SaO2 decreased long before a decrease in cTOI was seen and that cTOI started to decline when SaO2 levels fell below 93 ± 3%. The average decrease in cTOI in this study appears to be less pronounced than in elite divers (Palada et al. 2007; Eichhorn et al. 2015). The difference in apnea duration could explain for this difference in the same way as it did for mTOI: elite apneists are able to hold their breath for a longer period of time and as such, the decrease in cTOI has more time to develop. Indeed, if we look at the cTOI value from the elite apneists for the time points similar to the average apnea duration in our study, a similar amplitude of the response is seen. The positive correlation found in our data between apnea duration and drop in cTOI also supports this hypothesis. This leads us to speculate that people who are able to hold their breath for a longer period of time are not necessarily better at maintaining cerebral oxygenation, but can tolerate the urge to breathe longer and therefore seem to delay the stimulus to resume breathing. This then results in reaching a more severe state of cerebral deoxygenation. Research on cerebral oxygenation is mainly restricted to static dry breath-holds. Although we expect the same series of physiological mechanisms to occur, altering apnea modalities is likely to affect the magnitude and the speed of development of these responses. Further research is therefore needed to determine if these results also apply for other apnea modalities such as face immersion or dynamic apnea. In addition, we only measured heart rate and peripheral and cerebral oxygenation. Simultaneous measurements of cerebral blood flow, blood pressure, cardiac output and total peripheral resistance could contribute to a better understanding of the underpinning physiological mechanisms of the diving response.
During the test period, 2 subjects (ID1 and 2) showed symptoms of syncope: one subject fainted and one subject interrupted the apnea attempt prematurely because of feelings of dizziness. These subjects were therefore withdrawn from the analysis to determine the average pattern and not included in Table 1. Their data are depicted individually in Figs. 1, 2 and 3. This is the first report of peripheral and cerebral oxygenation data during voluntary apneas leading to (near) syncope. Compared to the average pattern, we see a very similar response in heart rate (Fig. 1). Moreover, both subjects appeared to demonstrate a very strong bradycardic reflex which should protect them from syncope. cTOI (Fig. 3), however, shows a very strong deviation from the average pattern for both subjects. While in the average pattern, a very modest yet significant initial drop of 1.4 ± 1.9% in cTOI is observed, the amplitude of this drop is as much as 8% in these subjects. cTOI appears to recover for both subjects, however, 30–40 s after the onset of apnea, cTOI rapidly falls again until the subjects either interrupted the apnea voluntarily because of feelings of dizziness (ID1) or fainted (ID2). Only looking at these values, the mechanisms for these two subjects appear very similar: despite a strong bradycardic response and apparent though less pronounced deoxygenation in mTOI (Fig. 2), cerebral tissue oxygenation falls short, leading to problems at the cerebral level causing dizziness and syncope. However, when looking into these mechanisms in further detail, some differences can be observed. ID1 did show a decrease in m[O2Hb] and increase in m[HHb], indicating that, although smaller than average, peripheral vasoconstriction did occur. ID2, however, shows constant m[O2Hb] combined with increased m[HHb], suggesting that peripheral vasoconstriction was impaired. The similar mTOI pattern in these subjects is thus obtained in two different ways. We hypothesize that for ID2, impaired peripheral vasoconstriction, as suggested by the m[O2Hb] and m[HHb] data, first leads to impaired redistribution of blood flow and as such hinders the possibility to increase cerebral blood flow and second, leads to a smaller decrease in muscle O2 consumption which makes SaO2 fall more quickly. Both effects should lead to a decrease in cerebral O2 delivery, leaving the brain short of O2. Indeed, the simultaneous strong decrease in c[O2Hb] and modest increase in c[HHb] at the end of the breath-hold suggest that cerebral blood flow had not increased and possibly even decreased. For ID1, peripheral oxygenation (m[O2Hb] and m[HHb]) shows a small albeit normal response. The mechanism for the fall in cTOI is therefore most likely different from ID2. Our data unfortunately cannot tell what caused the deviation in pattern, but only suggest that cerebral blood flow had, unlike the normal pattern, not increased either.
Conclusion
This study is the first to examine both peripheral and cerebral [O2Hb] and [HHb] adding insight into the underpinning profile for TOI during breath-hold on an absolute time scale. This reveals an initial decrease in cerebral oxygenation which is quickly reversed following fast peripheral vasoconstriction as suggested through decreasing mTOI. The results from this study therefore indicate that several mechanisms protecting the brain, i.e., bradycardia and peripheral vasoconstriction with concurrent increase in cTOI through elevated cerebral blood flow, are successful in maintaining cerebral oxygenation in subjects naïve to breath-holding. Our data therefore indicate that apnea training can be relatively safely applied in sports. However, these subjects also exhibit a modest decrease in cTOI at the end of breath-hold, with stronger decreases for longer breath-holds. As apnea training is effective in increasing apnea duration in naïve subjects (Bouten et al. 2019), we can expect this decrease to be more apparent and as such, caution is needed. In addition, impairment of the protective physiological mechanisms during apnea, as seen in ID1 and ID2, can lead to syncope in some individuals. It is therefore important that individuals wanting to perform apnea training have to learn the warning signs of syncope and adopt the habit of interrupting apneas when these signs occur as they might indicate that the O2 conserving mechanism protecting the brain is not functioning optimally and as such, they are at risk of hypoxic syncope. In addition, this type of training should never be performed alone.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- bpm:
-
Beats per minute
- c[HHb]:
-
Deoxygenated cerebral hemoglobin concentration
- c[O2 Hb]:
-
Oxygenated cerebral hemoglobin concentration
- cTOI:
-
Cerebral tissue oxygenation index
- HR:
-
Heart rate
- µM:
-
Micro mol. L−1
- Manova:
-
Multivariate analysis of variance
- m[HHb]:
-
Deoxygenated muscle hemoglobin concentration
- m[O2 Hb]:
-
Oxygenated muscle hemoglobin concentration
- mTOI:
-
Muscle tissue oxygenation index
- O2 :
-
Oxygen
References
AIDA International (2020) Current world record table. https://www.aidainternational.org/WorldRecords. Accessed 7 June 2020
Andersson J, Schagatay E (1997) Effects of lung volume and involuntary breathing movements on the human diving response. Eur J Appl Physiol Occup Physiol 77:19–24. https://doi.org/10.1007/s004210050294
Badrov MB, Barak OF, Mijacika T et al (2017) Ventilation inhibits sympathetic action potential recruitment even during severe chemoreflex stress. J Neurophysiol 118:2914–2924. https://doi.org/10.1152/jn.00381.2017
Bain AR, Ainslie PN, Hoiland RL et al (2016) Cerebral oxidative metabolism is decreased with extreme apnoea in humans; impact of hypercapnia. J Physiol 594:5317–5328. https://doi.org/10.1113/JP272404
Bain AR, Drvis I, Dujic Z et al (2018) Physiology of static breath holding in elite apneists. Exp Physiol 103:635–651. https://doi.org/10.1113/EP086269
Bouten J, Caen K, Stautemas J et al (2019) Eight weeks of static apnea training increases spleen volume but not acute spleen contraction. Respir Physiol Neurobiol 266:144–149. https://doi.org/10.1016/j.resp.2019.04.002
Campbell BYLB, Gooden BA, Horowitz JD (1969) Cardiovascular responses to partial and total immersion in man. J Physiol 202:239–250. https://doi.org/10.1113/jphysiol.1969.sp008807
Costalat G, Coquart J, Castres I et al (2017) The oxygen-conserving potential of the diving response: a kinetic-based analysis. J Sports Sci 35:678–687. https://doi.org/10.1080/02640414.2016.1183809
Cross TJ, Kavanagh JJ, Breskovic T et al (2014) Dynamic cerebral autoregulation is acutely impaired during maximal apnoea in trained divers. PLoS ONE. https://doi.org/10.1371/journal.pone.0087598
Dhanani S, Hornby L, Ward R, Shemie S (2012) Variability in the determination of death after cardiac arrest: a review of guidelines and statements. J Intensive Care Med 27:238–252. https://doi.org/10.1177/0885066610396993
Edmonds HL, Ganzel BL, Austin EH (2004) Cerebral oximetry for cardiac and vascular surgery. Semin Cardiothorac Vasc Anesth 8:147–166. https://doi.org/10.1177/108925320400800208
Eichhorn L, Erdfelder F, Kessler F et al (2015) Evaluation of near-infrared spectroscopy under apnea-dependent hypoxia in humans. J Clin Monit Comput 29:749–757. https://doi.org/10.1007/s10877-015-9662-2
Eichhorn L, Erdfelder F, Kessler F et al (2017) Influence of apnea-induced hypoxia on catecholamine release and cardiovascular dynamics influence of apnea-induced hypoxia on catecholamine release and cardiovascular dynamics. Int J Sports Med 38:85–91. https://doi.org/10.1055/s-0042-107351
Espersen K, Frandsen H, Lorentzen T et al (2002) The human spleen as an erythrocyte reservoir in diving-related interventions. J Appl Physiol 92:2071–2079. https://doi.org/10.1152/japplphysiol.00055.2001
Ferretti G (2014) Maximal oxygen consumption in healthy humans: theories and facts. Eur J Appl Physiol 114:2007–2036. https://doi.org/10.1007/s00421-014-2911-0
Ferrigno M, Hickey DD, Liner MH, Lundgren CEG (1986) Cardiac performance in humans during breath holding. J Appl Physiol 60:1871–1877. https://doi.org/10.1152/jappl.1986.60.6.1871
Fornasier-Santos C, Millet GP, Woorons X (2018) Repeated-sprint training in hypoxia induced by voluntary hypoventilation improves running repeated-sprint ability in rugby players. Eur J Sport Sci 18:504–512. https://doi.org/10.1080/17461391.2018.1431312
Foster GE, Sheel AW (2005) The human diving response, its function, and its control. Scand J Med Sci Sport 15:3–12. https://doi.org/10.1111/j.1600-0838.2005.00440.x
Fredén K, Lundborg P, Vilén L, Kutti J (1978) The peripheral platelet count in response to adrenergic Alpha- and Beta-1-receptor stimulation. Scand J Haematol 21:427–432. https://doi.org/10.1111/j.1600-0609.1978.tb00390.x
Gooden BA (1994) Mechanism of the human diving response. Integr Physiol Behav Sci 29:6–16. https://doi.org/10.1007/BF02691277
Heistad DD, Abbound FM, Eckstein JW (1968) Vasoconstrictor response to simulated diving in man. J Appl Physiol 25:542–549. https://doi.org/10.1152/jappl.1968.25.5.542
Heusser K, Dzamonja G, Tank J et al (2009) Cardiovascular regulation during apnea in elite divers. Hypertension 53:719–724. https://doi.org/10.1161/HYPERTENSIONAHA.108.127530
Irving L (1963) Bradycardia in human divers. J Appl Physiol 18:489–491. https://doi.org/10.1152/jappl.1963.18.3.489
Joulia F, Lemaitre F, Fontanari P et al (2009) Circulatory effects of apnoea in elite breath-hold divers. Acta Physiol 197:75–82. https://doi.org/10.1111/j.1748-1716.2009.01982.x
Kety SS, Schmidt CF (1948) The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27:484–492. https://doi.org/10.1172/JCI101995
Klem GH, Lüders H, Jasper HH, Elger C (1999) The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 52:3–6
Lemaître F, Joulia F, Chollet D (2010) Apnea: a new training method in sport? Med Hypotheses 74:413–415. https://doi.org/10.1016/j.mehy.2009.09.051
Lucas SJE, Tzeng YC, Galvin SD et al (2010) Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension 55:698–705. https://doi.org/10.1161/HYPERTENSIONAHA.109.146290
Madsen PL, Holm S, Herning M, Lassen NA (1993) Average blood flow and oxygen uptake in the human brain during resting wakefulness: a critical appraisal of the Kety-Schmidt technique. J Cereb Blood Flow Metab 13:646–655. https://doi.org/10.1038/jcbfm.1993.83
Palada I, Obad A, Bakovic D et al (2007) Cerebral and peripheral hemodynamics and oxygenation during maximal dry breath-holds. Respir Physiol Neurobiol 157:374–381. https://doi.org/10.1016/j.resp.2007.02.002
Pellicer A, del Bravo MC (2011) Near-infrared spectroscopy: a methodology-focused review. Semin Fetal Neonatal Med 16:42–49. https://doi.org/10.1016/j.siny.2010.05.003
Pescatello SL, Arena R, Riebe D, Thompson DP (eds) (2013) ACSM’s guidelines for exercise testing and prescription, ninth. American College of Sports Medicine, Baltimore
Ratmanova P, Semenyuk R, Popov D et al (2016) Prolonged dry apnoea: effects on brain activity and physiological functions in breath-hold divers and non-divers. Eur J Appl Physiol 116:1367–1377. https://doi.org/10.1007/s00421-016-3390-2
Richardson M, de Bruijn R, Holmberg H-C et al (2005) Increase of hemoglobin concentration after maximal apneas in divers, skiers, and untrained humans. Can J Appl Physiol 30:276–281. https://doi.org/10.1139/h05-120
Schagatay E, Van Kampen M, Andersson J (1999) Effects of repeated apneas on apneic time and diving response in non-divers. Undersea Hyperb Med 26:143–149
Schagatay E, Andersson JP, Hallen M, Birger P (2001) Selected contribution: role of spleen emptying in prolonging apneas in humans. J Appl Physiol 90:1623–1629
Scholander PF, Hammell HT, Lemessurier H et al (1962) Circulatory adjustment in pearl divers. J Appl Physiol 17:184–190. https://doi.org/10.1152/jappl.1962.17.2.184
Shemie SD, Gardiner D (2018) Circulatory arrest, brain arrest and death determination. Front Cardiovasc Med 5:1–6. https://doi.org/10.3389/fcvm.2018.00015
Sivieri A, Fagoni N, Bringard A et al (2015) A beat-by-beat analysis of cardiovascular responses to dry resting and exercise apnoeas in elite divers. Eur J Appl Physiol 115:119–128. https://doi.org/10.1007/s00421-014-2992-9
Sroufe LA (1971) Effects of depth and rate of breathing on heart rate and heart rate variability. Psychophysiology 8:648–655. https://doi.org/10.1111/j.1469-8986.1971.tb00500.x
Willie CK, Ainslie PN, Drvis I et al (2015) Regulation of brain blood flow and oxygen delivery in elite breath-hold divers. J Cereb Blood Flow Metab 35:66–73. https://doi.org/10.1038/jcbfm.2014.170
Winklewski PJ, Frydrychowski AF (2013) Cerebral blood flow, sympathetic nerve activity and stroke risk in obstructive sleep apnoea. Is there a direct link? Blood Press 22:27–33. https://doi.org/10.3109/08037051.2012.701407
Woorons X, Mollard P, Pichon A et al (2008) Effects of a 4-week training with voluntary hypoventilation carried out at low pulmonary volumes. Respir Physiol Neurobiol 160:123–130. https://doi.org/10.1016/j.resp.2007.09.010
Woorons X, Mucci P, Richalet JP, Pichon A (2016) Hypoventilation training at supramaximal intensity improves swimming performance. Med Sci Sports Exerc 48:1119–1128. https://doi.org/10.1249/MSS.0000000000000863
Woorons X, Millet GP, Mucci P (2019) Physiological adaptations to repeated sprint training in hypoxia induced by voluntary hypoventilation at low lung volume. Eur J Appl Physiol. https://doi.org/10.1007/s00421-019-04184-9
Acknowledgements
The authors would like to thank the subjects for their commitment and dedication to the study. The authors are also grateful to LivaNova Belgium for the use of the NIRO equipment.
Funding
JBouten is currently receiving a PhD Aspirant Scholarship (1144519N) from the Research Foundation Flanders (Fonds Wetenschappelijk Onderzoek, FWO). For the remaining authors, no grants were declared.
Author information
Authors and Affiliations
Contributions
Conception and design: JB, JGB and JB. Data collection: JB. Analysis and interpretation: JB, JGB, JB. Article drafting and revisions: JB, JGB, JB. All authors read, revised and approved of the final version.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest to disclose.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethical committee of the Ghent University Hospital (EC UZG 2016/1148) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by I. Mark Olfert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouten, J., Bourgois, J.G. & Boone, J. Hold your breath: peripheral and cerebral oxygenation during dry static apnea. Eur J Appl Physiol 120, 2213–2222 (2020). https://doi.org/10.1007/s00421-020-04445-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-020-04445-y