Abstract
Swimming and diving are popular recreational activities, representing an effective option in maintaining and improving cardiovascular fitness in healthy people. To date, only little is known about the cardiovascular adaption to submersion in children. This study was conducted to improve an understanding thereof. We used a stepwise apnea protocol with apnea at rest, apnea with facial immersion, and at last apnea during whole body submersion. Continuous measurement of heart rate, oxygen saturation, and peripheral resistance index was done. Physiologic data and analysis of influencing factors on heart rate, oxygen saturation, and peripheral vascular tone response are reported. The current study presents the first data of physiologic diving response in children. Data showed that facial or whole body submersion leads to a major drop in heart rate, and increase of peripheral resistance, while the oxygen saturation seems to be unaffected by static apnea in most children, with apnea times of up to 75 s without change in oxygen saturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Swimming and diving are popular recreational activities, representing an effective option in maintaining and improving cardiovascular fitness in healthy people. Additionally, both skills are essential in the prevention of drowning incidents. Yet, immersion and especially submersion into the water confront the human body and cardiovascular system with several challenges. The pressure put on the body during immersion results in a rise of preload and afterload [1]. Ventricular stroke volume increases due to the Starling mechanism [1,2,3]. Simultaneously, heart rate drops [4]. Adaption to immersion and submersion has been well examined in adults [4,5,6]. In non-divers, the heart rate has been shown to drop by 10–20% from resting, while in trained divers, it may drop by 45% [7]. Peripheral blood flow is reduced in proportion with HR changes and results in an effective centralization of blood flow to the heart, lungs, and brain [8]. However, no studies have yet been conducted to investigate the diving response in children. To close this gap, this study aimed to assess the extent of the physiologic adaption to apnea and submersion in healthy children, since knowledge of a child’s normal physiologic adaption is crucial to interpret the physiologic changes in pediatric patients.
Current medical recommendations are limiting patients with chronic diseases especially heart diseases, including pediatric patients, from aquatic activities. For example, the current ESC guideline on sports and cardiovascular disease states that aquatic exercise is not recommended in patients with heart failure, and in everyday practice, there are still many doctors who prohibit patients from diving “for safety reasons” [9].
A rare yet interesting subset of cardiovascular patients are those with single ventricular physiology. These patients lack a prepulmonary heart chamber and were thought to be at high risk during diving as the volume shift and increase in pulmonary resistance were assumed to cause acute heart failure or even sudden cardiac death. As there have been no data on this topic at all, two recent studies by Paech et al. depict the first in man real-life data on this topic. Interestingly, the authors did not find any detrimental effects. Yet, the lack of data in healthy children is a major current limit for the interpretation of these study data [10, 11].
Therefore, this study aimed to generate first real-life data on the diving response in healthy children of prepubertal age. In future studies, these data might then be used to compare the physiologic reaction to the effects of the diving response in pediatric patients with severe congenital heart diseases or other chronic diseases to find reliable data predicting possible risk factors or underlining the safety of swimming and diving in these patients.
Methods
Participants
Healthy children were recruited from local swimming and sports clubs. Inclusion criteria were a prepubertal age of 6–12 years, experience with swimming or diving, and the absence of cardiovascular disease in the patient history. Exclusion criteria were signs of reduced general condition and acute illness, signs of limitations of cardiopulmonary function, mental handicap, and genetic diseases. Subjects with common pre-existing respiratory conditions were included. The subjects and their legal guardians gave their informed consent. The study received ethical approval by the ethics committee University of Leipzig and is listed under the reference 549/19-ek.
Measurements
Anthropometric data including height and weight were measured on site. Data on potential medical history and medication were obtained from personal interviews. The apnea testing took place in a local public swimming hall. Water temperature was 28° C and ambient temperature was 30° C. All tests were performed by a pediatric cardiologist and a study nurse.
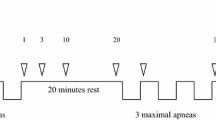
Diving protocol: Prior to the start of the testing, the subjects were instructed to the following protocol including three phases of apnea:
-
1.
2 min in a sitting position outside the pool, resting
-
2.
Static apnea for maximal duration while sitting outside the pool in normal air
-
3.
2 min in a sitting position outside the pool, resting
-
4.
Static apnea with the face immersed in a bowl of water (same temperature as pool)
-
5.
2 min rest and transition into the pool water. Beginning of step 6 after 20–30 s immersed.
-
6.
Static apnea at a depth of 0.5–1 m while holding on an underwater bar.
-
7.
1 min rest in the water after the static apnea (Fig. 1)
Transcutaneous oxygen saturation, heart rate, and perfusion index (PI), as a surrogate of peripheral vascular tone [12, 13], were recorded for the whole testing period.
PI is derived from the photoelectric plethysmography signal of the pulse oximeter and calculated as the ratio between the pulsatile component and the non-pulsatile component of the light reaching the detector. As the change in peripheral perfusion leads to a change in the pulsatile (arterial) component, the non-pulsatile part, which is determined by the tissue, does not change, and the ratio between both components changes [14]. Thus, PI reflects peripheral vasomotor tone and is a surrogate for vasoconstriction or vasodilatation, promoting a volume shift. The perfusion index subsumes vascular tone and systemic blood flow, which is influenced by stroke volume [15].
Measurements were done using a Masimo Rad-97™ patient monitor and Masimo RD SET sensors (Masimo Corporation, Irvine, USA) protected from the water by hydrophobic coating with vaseline, which were placed on an index finger.
Statistical Analysis
For the statistical analysis, IBM SPSS Statistics for Windows (V29) was used.
Apnea phases were compared regarding the percentage deviations resulting from the differences between maximum and minimum values in each apnea phase for heart rate and differences between first value and minimum value for perfusion index using the exact Wilcoxon test. Each apnea phase was compared to the other two apnea phases.
For analysis of changes in oxygen saturation, means and standard deviations of base value and minimal oxygen values were calculated.
The test population was divided into two groups to test whether differences in physiological parameters could be shown between fin swimmers, who train diving regularly, and healthy children only used to recreational swimming and diving. Analysis for differences between the two groups regarding percentage deviations of heart rate, perfusion index, and differences in oxygen saturation was performed using Mann–Whitney U test.
Spearman’s correlation analysis was used to investigate possible influencing factors on the extent of changes during apnea.
To estimate the influence of the duration of apnea on the reduction in heart rate, a Spearman’s correlation analysis was also conducted.
Female and male test persons were compared using Mann–Whitney U-test.
Results
In this study, 25 healthy children were enrolled. Table 1 shows the characteristics of the tested children. The row “subjects with pre-existing respiratory condition” includes all those who stated that they suffered from temporary respiratory problems due to asthma or allergic rhinitis (Table 1). Table 2 shows the apnea times reached in each phase.
Figure 2 shows the averaged course of heart rate, transcutaneous oxygen saturation, and perfusion index during apnea at rest and outside of the water, apnea, while face being immersed in water and apnea during full body submersion as well as resting periods in between. The primary axis shows values for heart rate and oxygen saturation, and the secondary axis shows values for perfusion index. The time axis shows intervals of 10 s in the apnea phases and intervals of 20 s in the resting periods.
Physiologic data (oxygen saturation, heart rate, and perfusion index) were first determined for the first resting phase. The absolute deviation and the percentage deviation of the heart rate and the perfusion index in the three apnea phases were then calculated. The results are seen in Figs. 3 and 4. Values of minimum oxygen saturation are shown in Fig. 5.
Figure 3 shows that heart rate was reduced by 15.7% during apnea outside the water, by 27.8% during face immersion apnea, and by 25.2% during full submersion apnea. Percentage heart rate reduction during face immersion apnea compared to percentage heart rate reduction during dry apnea showed a significantly stronger heart rate reduction for face immersion (p < 0.001). Also, a significantly stronger reduction in percentage heart rate during full body submersion apnea compared to the percentage heart rate reduction during dry apnea could be found (p = 0.007). However, the comparison of face immersion apnea to full body submersion apnea regarding percentage heart rate reduction showed no significant differences (p = 0.665).
Figure 4 shows that perfusion index was reduced by 10.6% during apnea outside the water, by 20.5% during face immersion apnea and by 43% during full submersion apnea. Only the comparison of the dry apnea phase to the full body submersion apnea phase showed a significant change in percent in the perfusion index (p = 0.018), whereby the Wilcoxon test comparing dry apnea with face immersion apnea regarding percentage perfusion index reduction (p = 0.546) and comparison of face immersion apnea and full body submersion apnea (p = 0.079) were not significant.
Figure 5 visualizes the children’s average minimal oxygen values at baseline and during apnea phases. Oxygen saturation started with a base value of 96.7% and reached mean minimum values of 95.3% (dry apnea), 95.6% (face immersion apnea), and 94.5% (full body submersion apnea).
Figure 6 presents the course of heart rate, perfusion index, and transcutaneous oxygen saturation separately for both fin swimmers and subjects who perform other sports. Analysis for differences between the two groups regarding these three parameters resulted in following p values: p (HR) = 0.331, p (PI) = 0.673, and p (SpO2) = 0.387. There were no significant differences in the diving response between trained (fin swimmers) and untrained children.
Influencing Factors
Possible factors that might influence the extent of the diving response were investigated. Spearman’s correlation analysis showed a weak to moderate correlation between the age of the test persons and the percentage deviation of the heart rate in the apnea phases (Spearman’s ρ = 0.223–0.447). Heart rate decreased more with increasing age.
The analysis showed no correlation between other factors (height, weight, hours of exercise per week) and the percentage deviation of the heart rate or the percentage deviation of the perfusion index.
Apnea length and percentage heart rate reduction correlated strongly in each apnea phase, Spearman’s ρ (first apnea) = 0.589, ρ (second apnea) = 0.568, and ρ (third apnea) = 0.824. Heart rate decreased more with increasing duration of apnea.
A comparison between female and male test persons using Mann–Whitney U test showed no significant difference between the sexes regarding changes in physiological parameters (p = 0.543).
Discussion
The current study is the first to report on the diving response in children. The following discussion will focus on the three major areas of interest when looking at physiologic data during a submersion; heart rate, perfusion index as indicator of peripheral vascular constriction, and the oxygen saturation.
First of all, there were no adverse events to report. The main finding of our study was that children show a substantial drop in heart rate with submersion. Interestingly, it seems that there is no significant difference in heart rate drop if only the face is submersed or the whole body. This phenomenon is thought to be due to the fact that the main receptors for submersion are located in the forehead [16]. The phenomenon has been reported in a similar way in adults.
The second parameter of interest is the peripheral perfusion index as a parameter for peripheral vascular constriction. It has to be stated that adaption to immersion and submersion has been examined previously only in adults. The principal mechanism is a redistribution of blood to the central oxygen-dependent organs like the heart and the brain [4, 17]. Also, blood flow in more hypoxia-tolerant tissues like the skin and resting skeletal muscles is reduced [18]. With the rise of hydrostatic pressure during immersion, in adults, a volume translocation of 500 to 1000 ml blood volume toward the thorax is initiated [5]. Cardiac output increases by around 30% and heart rate drops in average about 6% in adults [4, 6]. When looking at our cohort, a drop in peripheral perfusion index could be demonstrated in all three types of apneas (outside the water / facial immersion and submersion). Most remarkably, there was not only a rise of peripheral resistance during contact to water, but also during apnea outside the water. It can only be speculated if that rise in peripheral resistance outside the water is a sympathomimetic reaction while awaiting the first testing.
Throughout the whole testing period, values of PI decreased on average, as shown in Fig. 2. The regulation of vascular tone and volume shift are thought to be mainly a task for the autonomic nervous system [19]. Therefore, it should be expected that with increasing contact of skin to the water, there might be an increase in centralization, leading to decrease in PI which represents less peripheral volume/ more vascular tone.
It could be clearly seen that the whole body submersion induced a more pronounced rise in peripheral vascular tone than immersion of only the face. As a result, it has to be concluded that the rise in peripheral vascular tone seems not to be mainly connected to the facial receptors, at least not nearly as much as the heart rate seems to be connected to these receptors.
It was found that there were no changes in oxygen saturation during apnea maneuvers up to 75 s in our group of participants. This seems to be in accordance with hitherto reported findings; adult studies found a drop in oxygen saturation rather in longer dives of more than 40 s with a strong association to the depth of the dive [16]. Importantly, these studies report on dynamic apnea, meaning there was active muscle work leading to oxygen consumption as opposed to the participants in our study [20, 21]. So, with a median apnea time of 30 s in our study cohort, a drop in oxygen consumption could not be expected. It has to be mentioned that there were two children that were measured with an oxygen saturation below 90% (each child once in apneas of 40 s duration), despite the absence of congenital heart disease and despite repeated measurements. These low values were only seen during apnea phases or directly after. As there was no medical condition found in a following check-up, it has to be assumed that there was a certain influence of tenseness that might have led to artificially low measurements.
In addition to the reported baseline findings, the study looked at possible influencing factors of heart rate response and peripheral vascular constriction. Recognizably, the physiologic response was not affected by sex, age, and weight of the tested children in this cohort. While it might have been reasonable to suspect some training effect or adaption of the physiologic response in highly trained individuals, this coherence could not be demonstrated in the current study population, as the findings of fin swimmers, used to apnea diving, did not differ significantly from the control group of healthy children.
After all, a substantial diving response with drop in heart rate and increase in vascular tone could be demonstrated in children. Interestingly, the heart rate response was equally strong during face only immersion versus whole body submersion. Yet, further studies with a larger cohort of probands are needed to evaluate the current findings. Additionally, the conduction of the testing in colder water would be reasonable.
Conclusion
The current study presents the first data of physiologic diving response in children. Data showed that facial or whole body submersion leads to a major drop in heart rate and peripheral vasoconstriction, while the oxygen saturation seems to be unaffected by static apnea in most children, with apnea times of up to 75 s without change in oxygen saturation.
Limitations
The main limitation of this study is the small number of participants, as well as the predominance of females. Thereby, a bias due to sex and proband selection cannot be excluded. Our analyses showed no difference between males and females with regard to changes in physiologic parameters. However, as only data of 4 boys were used, this comparison is not of high information value.
Still, as sex differences were not seen in adult apnea studies, this should only be a minor limitation.
Also, one further limitation is that the study was conducted with a relatively high water temperature of 28 °C, which does not allow for concrete conclusions for much colder temperatures.
References
Epstein M (1978) Renal effects of head-out water immersion in man: implications for an understanding of volume homeostasis. Physiol Rev 58:529. https://doi.org/10.1152/physrev.1978.58.3.529
Lehmann M, Samek L (1990) Recreational swimming in CHD patients and healthy control subjects in relation to left heart function. Clin Cardiol 13:547–554. https://doi.org/10.1002/clc.4960130808
Lotshaw AM, Thompson M, Sadowsky HS et al (2007) Quality of life and physical performance in land- and water-based pulmonary rehabilitation. J Cardiopulm Rehabil Prev 27:247–251. https://doi.org/10.1097/01.HCR.0000281772.28394.30
Lin YC (1984) Circulatory functions during immersion and breath-hold dives in humans. Undersea Biomed Res 11:123–138
Arborelius M, JR, Ballidin UI, Lilja B, et al (1972) Hemodynamic changes in man during immersion with the head above water. Aerosp Med 43:592–598
Park KS, Choi JK, Park YS (1999) Cardiovascular regulation during water immersion. Appl Human Sci 18:233–241. https://doi.org/10.2114/jpa.18.233
Schagatay E, Andersson J (1998) Diving response and apneic time in humans. Undersea Hyperb Med 25:13–19
Foster GE, Sheel AW (2005) The human diving response, its function, and its control. Scand J Med Sci Sports 15:3–12. https://doi.org/10.1111/j.1600-0838.2005.00440.x
Pelliccia A, Sharma S, Gati S et al (2021) 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 42:17–96. https://doi.org/10.1093/eurheartj/ehaa605
Paech C, Gebauer RA, Weidenbach M et al (2021) The Fontan and the sea: first-in-man data on swimming and diving physiology in Fontan patients. Pediatr Cardiol 42:1614–1624. https://doi.org/10.1007/s00246-021-02649-3
Paech C, Gebauer RA, Weidenbach M et al (2023) Into the Blue: first in man data on diving physiology in Fontan patients. Pediatr Cardiol 44:179–186. https://doi.org/10.1007/s00246-022-02966-1
de Felice C, Goldstein MR, Parrini S et al (2006) Early dynamic changes in pulse oximetry signals in preterm newborns with histologic chorioamnionitis. Pediatr Crit Care Med 7:138–142. https://doi.org/10.1097/01.PCC.0000201002.50708.62
Schena F, Picciolli I, Agosti M et al (2017) Perfusion index and pulse oximetry screening for congenital heart defects. J Pediatr 183:74-79.e1. https://doi.org/10.1016/j.jpeds.2016.12.076
Lima A, Bakker J (2005) Noninvasive monitoring of peripheral perfusion. Intensive Care Med 31:1316–1326. https://doi.org/10.1007/s00134-005-2790-2
Coutrot M, Dudoignon E, Joachim J et al (2021) Perfusion index: physical principles, physiological meanings and clinical implications in anaesthesia and critical care. Anaesth Crit Care Pain Med 40:100964. https://doi.org/10.1016/j.accpm.2021.100964
Andersson JPA, Linér MH, Rünow E et al (1985) (2002) Diving response and arterial oxygen saturation during apnea and exercise in breath-hold divers. J Appl Physiol 93:882–886. https://doi.org/10.1152/japplphysiol.00863.2001
Scholander PF (1963) The master switch of life. Sci Am 209(92):106. https://doi.org/10.1038/scientificamerican1263-92
Butler PJ, Woakes AJ (1987) Heart rate in humans during underwater swimming with and without breath-hold. Respir Physiol 69:387–399. https://doi.org/10.1016/0034-5687(87)90091-0
Shattock MJ, Tipton MJ (2012) “Autonomic conflict”: a different way to die during cold water immersion? J Physiol 590:3219–3230. https://doi.org/10.1113/jphysiol.2012.229864
Mulder E, Schagatay E, Sieber A (2021) Using underwater pulse oximetry in freediving to extreme depths to study risk of hypoxic blackout and diving response phases. Front Physiol 12:651128. https://doi.org/10.3389/fphys.2021.651128
Andersson JPA, Linér MH, Fredsted A et al (1985) (2004) Cardiovascular and respiratory responses to apneas with and without face immersion in exercising humans. J Appl Physiol 96:1005–1010. https://doi.org/10.1152/japplphysiol.01057.2002
Funding
The current study was funded by the Deutsche Stiftung für Herzforschung.
Author information
Authors and Affiliations
Contributions
MR, CP, and ES wrote the main manuscript text. CP, MR, DM, and MP conducted the measurements during diving protocol. JW performed the statistical analysis. FM and AM provided Figs. 1, 2, and 6. JW, RAG, and AS prepared Table 1 and Figs. 3, 4, and 5. MW and ID supervised the study and helped in developing the study protocol. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare to have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and / or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study received ethical approval by the ethics committee University of Leipzig and is listed under the reference 549/19-ek.
Consent to participate
Informed consent was obtained from all individual participants included in the study as well as from their legal guardians.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rixen, M., Weickmann, J., Gebauer, R.A. et al. First Real-Life Data on the Diving Response in Healthy Children. Pediatr Cardiol 45, 314–322 (2024). https://doi.org/10.1007/s00246-023-03370-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03370-z