Abstract
One of the most obvious physiological changes accompanying seasonal heterothermy in mammals is a fattening stage preceding periods of resource scarcity. This phenomenon reflects the interplay of both diet and physiology. Though the accrual of fat stores is known to be essential for overwintering in some species, the influence of diet on the physiology of torpor is not fully understood. Results from captive studies in heterothermic rodents and marsupials have indicated that when autumn diets are enriched with polyunsaturated fatty acids (PUFAs), animals receiving these diets experience deeper and more frequent torpor bouts than their counterparts receiving a control diet. Our study investigates this potential effect of dietary composition in animals that use daily torpor rather than prolonged torpor (i.e., hibernation). In so doing, we investigate the degree to which dietary effects on torpor are restricted to cold-adapted rodents and marsupials, or are a more general feature of mammalian heterothermy. We examined the effects of a PUFA diet and a control diet on the thermoregulation of one of the few species of primates known to use daily torpor: the grey mouse lemur (Microcebus murinus). Though the results of this study are largely inconclusive regarding the impact of dietary manipulations on torpor frequency and duration, we nonetheless find that the propensity of animals to enter torpor is directly influenced by age and seasonal changes in body mass, and thus reflect important physiological aspects of flexible thermoregulatory responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hibernation and daily torpor are among the most extreme adaptations of endothermic mammals to energetic demands posed by the environment (Boyer and Barnes 1999; Carey et al. 2003; Heldmaier et al. 2004). The hibernation season is defined by multiple torpor bouts punctuated by brief periods of rewarming known as interbout arousals. If a torpor bout lasts longer than 24 h, we classify it as prolonged torpor, or hibernation; in contrast, torpor bouts that last less than 24 h are known as daily torpor or daily heterothermy. Torpor bouts are characterized by regulated metabolic depression and body temperatures that approximate ambient. During torpor, body temperature differentials between euthermic and torpid states can range from 25 to 40° C for animals that use hibernation. For daily heterotherms, however, this differential is usually much smaller (Geiser and Ruf 1995, 2015). These temperature fluctuations impact whole animal physiology in numerous ways, especially regarding metabolic changes impacting energy budgets.
For animals that use prolonged torpor or hibernation, it is well known that large amounts of stored fat reserves are important for successful hibernation to occur. Accumulating evidence suggests that not only the direct effects of quantity, but also the indirect effects of quality of fat accrued which has energetic advantages (Florant 1998; Dark 2005). Studies involving multiple hibernating rodent species and hibernating marsupial species have concluded that a diet enriched with high levels of polyunsaturated fatty acids (PUFAs) has positive effects on both the frequency of torpor bouts, as well as the duration of torpor bouts (Geiser and Kenagy 1987; Geiser 1990; Frank 1991, 1992; Geiser et al. 1992, 1994; Florant et al. 1993; Thorp et al. 1994; Harlow and Frank 2001; Corn 1997, Frank and Storey 1996; but see Bartness et al. 1991; Contreras et al. 2014). Or, in other words, these studies indicate that animals fed a diet rich in PUFAs had longer bouts of torpor and maintained lower body temperatures than animals fed a control diet. PUFAs, which cannot be synthesized by mammals, and must be ingested via diet, are incorporated into fat reserves and cellular membranes. Due to a higher unsaturation index, fat reserves containing greater amounts of PUFAs melt at correspondingly lower temperatures relative to typical mammalian fat (~30° C; Florant 1998; Gunstone et al. 1986), making them more readily available for energy conversion. For hibernating species, this is especially important, because body temperatures are lowered significantly during torpor bouts, and fat reserves, as the sole fuel source available during hibernation, are thought to remain fluid to be accessible for fueling metabolism (Florant 1998). In addition, cellular and subcellular membranes of adipocytes and other cells are also hypothesized to remain fluid during torpor to allow for normal activity of transmembrane proteins (Munro and Thomas 2004).
Hibernating species can extend torpor bouts for up to 2 weeks at a time, making the necessity of increasing lipid fluidity through PUFAs apparent in these species. For animals that express daily torpor, however, the temporal pattern of torpor bouts can be varied. In contrast to hibernators, daily heterotherms experience euthermia for at least part of the day (Geiser and Ruf 1995) and, therefore, require optimal physiological functioning at both high and low body temperatures, rapidly transitioning between the two states. To determine whether the same relationship holds true to those observed in hibernators, several studies have attempted to clarify the relationship of dietary fatty acids to thermoregulation in daily heterotherms. For example, studies done on rodent and marsupial species [deer mouse (Peromyscus maniculatus; Geiser 1991), Djungarian hamster (Phodopus sungorus; Geiser and Heldmaier 1995; Hiebert et al. 2000; Diedrich et al. 2014), and stripe-faced dunnart (Sminthopsis macroura; Withers et al. 1996)] also suggest that PUFA-rich diets have a positive influence on torpor patterns in daily heterotherms.
Another factor that remains less well studied is cholesterol’s impact thermoregulation in heterotherms. Cholesterol has been shown to impact both lipid concentration and steroid hormone concentration (Geiser et al. 1997) which can, in turn, contribute to higher cellular membrane fluidity (Munro and Thomas 2004). Geiser et al. (1997) conducted a study in which they manipulated the dietary cholesterol in chipmunks (T. amoenus) preceding seasonal heterothermy. The study reveals that study animals on high cholesterol diets had significantly lower Tb, metabolic rates, and longer torpor bouts than the control animals fed lower portions of dietary cholesterol. How dietary composition relates to torpor patterns in daily heterotherms remains poorly understood, but is an area rich for investigation.
The grey mouse lemur (Microcebus murinus) endemic to Madagascar, another heterothermic mammal, belongs to one of the few groups of primates that employ torpor as an energy saving strategy (Ortmann et al. 1997; Schmid 2001). These small (50–95 g), nocturnal tropical primates display torpor behavior during the austral winter during intense periods of resource scarcity (Ortmann et al. 1997). In response, mouse lemurs in natural populations enter periods of inactivity that can range from a few hours to multi-day torpor bouts (i.e. hibernation or prolonged torpor; Ortmann et al. 1997; Schülke and Ostner 2007; Schmid and Ganzhorn 2009). In captivity, however, mouse lemurs have a tendency to restrict torpor bouts to less than 24 h (Genin and Perret 2003).
Although there is convincing evidence that dietary PUFAs and cholesterol may affect thermoregulation, more research on phylogenetically diverse species is warranted to ascertain whether there are general effects or considerable species-dependent differences. To our knowledge, studies concerning the impacts of diet manipulation on the physiology of torpor in mouse lemurs and other tropical heterotherms are rare. Using a captive colony of mouse lemurs housed at the Duke Lemur Center (DLC), USA, this study aims to answer the following questions: Do dietary fatty acids influence the frequency and duration of torpor bouts? What is cholesterol’s role in thermoregulation? And if not dietary effects, what factor or factors are most likely to contribute to the variation seen in thermoregulatory strategies among captive mouse lemurs?
Materials and methods
Study animals
Ten adult (1–5-year-old) male grey mouse lemurs (Microcebus murinus), born to the breeding colony at DLC, were used in this study. The study was conducted from September to December in the years 2013 and 2014 (hereafter referred to as year one and year two, respectively). To minimize social influences and allow accurate determination of amount of diet consumed during the study, animals were caged separately in a climate-controlled “hibernaculum,” but allowed visual and auditory access to other individuals. Cage location within the hibernaculum and relative humidity were kept constant for the duration of the study, and animals were maintained under North Carolina photoperiods that were reduced every 2 weeks to mimic the transition from fall to winter (Fig. 1). We acknowledge that this differs from the animals’ native habitat in Madagascar (see Table S1). To aid in animal care, which occurs during the morning hours, light cycles are reversed, so that lights go off at 1200 h daily, with the time of lights on modified every 2 weeks to get desired light–dark (LD) cycles (between 2130 and 0230).
Fattening phase
Mouse lemurs in natural populations exhibit seasonal fattening in the tail and hind legs in preparation for periods of resource scarcity (Ortmann et al. 1997; Atsalis 1999; Schmid 1999; Kobbe et al. 2011), and use those fat reserves when food resources become scarce. This cyclic weight gain and loss are governed by an endogenous rhythm that is, in turn, entrained by photoperiod (Perret et al. 1998; Aujard et al. 1998; Seguy and Perret 2004). Consequently, we can replicate body mass changes in captivity using a regulated photoperiodic regimen.
During the 2-month period before torpor behavior typically begins (mid-September to mid-November), animals were divided into two groups that were balanced for age and body mass at the start of year one and fed an “autumn” diet (see Table S2 for animal ages and diet treatments between the two years); in year one, group one was fed an experimental diet containing chow enriched with 10% linoleic acid (safflower oil, rich in PUFAs, and lower in cholesterol) and group 2 received a diet with an isocaloric control chow (higher in saturated fatty acids and cholesterol). These two types of chow varied in fatty acid and cholesterol composition and were custom-formulated by the TestDiets Division of Purina Mills (Table 1). Diets were supplemented with a fruit and vegetable mix that remained constant across individuals and across years. Diet amounts were determined by DLC veterinarians at the start of year one, adjusted accordingly based upon body mass of each individual animal, but equal across matched pairs of animals. Animals were fed their assigned diets for 8 weeks to allow fatty acids to be incorporated into depot fats, ensuring any effect on torpor would be seen. Eight weeks was chosen as the standard for how the previous studies concerning dietary fatty acids and torpor have been conducted (e.g. Geiser and Kenagy 1987; Geiser 1990; reviewed in Munro and Thomas 2004). Furthermore, a study done by Florant et al. (1993) demonstrates that after 8 weeks of diet treatment, fatty acid composition of WAT mirrors the composition of the food that the animals had ingested during that time frame, and Frank (2002) shows these changes can be seen in as little as 19 days.
During year two, each group received the opposite diet from the previous year, so that each animal served as its own control. Quantity of diet items fed was based on each individual’s body weight at the beginning of the study and water was provided ad libitum throughout the study period. In year two, the same diet amount was fed to each individual to reduce variability from year to year. Throughout the entire fattening phase, ambient temperature (T a) was kept at 25 °C.
Torpor phase
In mid-November, we reduced caloric intake by 20% to simulate a natural reduction in food resources (“winter” diet). Day length was reduced to LD10:14, and ambient temperatures were lowered to 20 °C to induce torpor behavior. It has been well documented in the literature that this is an appropriate photoperiod and ambient temperature in which torpor can occur in captive M. murinus (Aujard et al. 1998; Genin and Perret 2000; Giroud et al. 2008). Indeed, in their native environments, wild mouse lemurs experience winter temperatures ranging from 6 to 20 °C, and enter torpor throughout this range (Ortmann et al. 1997; K. Dausmann, pers. comm.). Historically, mouse lemurs at DLC have displayed torpor behavior after experiencing reduced day length, even in the absence of temperature reduction. However, lowered T a and reduced caloric intake have been shown to amplify torpor behavior in mouse lemurs (Giroud et al. 2008; DLC staff; pers. comm.).
Animals were fitted with temperature-sensitive collars to monitor skin temperature (T sk) in hourly intervals throughout the duration of the torpor period. T sk is routinely used as a proxy for core body temperature and using external temperature-sensitive collars is a non-invasive alternative to implanted transmitters (Dausmann 2005; Geiser et al. 2007). Temperature-sensitive transmitters placed in contact with the skin have been shown to provide a reliable index of core body temperature with accuracy usually around 2 °C (Munro et al. 2005). Our collars were modified iButton data loggers (model: DS1922L) attached to wire collars coated with plastic tubing and weighed less than 1 g. The range of body mass for our study animals for both years combined was 62–119 g; thus, collar weight is well under the upper limits for safety. This is typically determined by the upper threshold of 5% of an animals’ body weight (Dausmann 2005). A torpor bout was defined as T sk <33 °C for at least 6 h with a depth of at least 30 °C for at least 2 consecutive hours. The threshold of 33 °C was based on the bimodal distribution of minimum body temperature values recorded for mouse lemurs (Ortmann et al. 1997; Genin and Perret 2003) and the observation that in the active state, mouse lemurs never show T sk lower than 33 °C at DLC (unpub. data). While testing for collar safety in June of 2012, we determined average euthermic T sk for an active mouse lemur during a 2-week trial. At a T a of 25.5 ± 0.76 °C, average T sk is 35.6 ± 0.81 °C, which is comparable with euthermic T sk recorded from free-ranging mouse lemurs in western Madagascar (36.3 °C; Schmid 2001). These data were not included in our study, as we tested the collar on only one individual during the active season.
T sk profiles during the torpor phase
All T sk data were analyzed using a program written in-house that extracted torpor bout information from the logged time and T sk recordings. Figure 2 shows a representative body temperature recording logged during study period in both year one while receiving a PUFA diet (panel a) and year two while on a control diet (panel b) and in a second representative animal in year one on a PUFA diet (panel c) and year two while on a control diet (panel d). Two collars failed to record during year two leaving us with a total of 18 body temperature recordings from years one (n = 10) and two (n = 8) for data analysis.
T sk recording traces from two representative animals on a PUFA diet in year one; b control diet in year two; c PUFA diet in year one; and d control diet in year two. These T sk traces demonstrate the remarkable variability in torpor patterns that we find. In b, Arrow on the left indicates a decrease in T sk that would not be counted as a torpor bout, as T sk does not reach below 30 °C for 2 consecutive hours. Arrow on the right marks a clear torpor bout, as defined by our study parameters
To merge the data collected in year one and year two, the dates were transformed from the calendar date to the number of days after T a was lowered to 20 °C to initiate torpor, which was defined as day zero. We extracted the total number of torpor bouts (i.e., frequency) and duration of each torpor bout during the study period. Mean frequency and duration of torpor bouts for each individual were calculated from all recorded torpor bouts, and the percentage of time that animals spent in torpor was calculated by dividing the total time spent in torpor by the entire length of T sk recordings. These metrics were used as measures of the degree of expression of torpor and allowed for comparisons between diet groups, as well as between years when appropriate. In addition, we also report the shortest and longest torpor bouts and the minimum T sk recorded in any individual during our study. Our program also allowed us to extract information regarding the timing of daily entrance into torpor to test whether entrance into torpor showed a clear daily rhythm.
Body weight changes and food intake measurements
Animals were weighed weekly during the entire study period for both years and food intake was recorded daily to determine the total daily consumption of each food type and to test whether or not animals showed a preference for diet type.
Data analysis
All statistical analyses were done in R (version 3.2.3; R Core Team 2015). Differences in the occurrence frequency and duration of torpor bouts between experimental groups were assessed using paired t tests after a manual trimming of data to normalize length of recording. The recording period for year one and year two differed by 3 days. The longer recording period (year one) was trimmed to match the same number of days as year two, relative to the beginning of the period of lowered T a. We also used a binomial family generalized linear mixed model which modeled the data at the level of individual days, where days were used as repeated measurements within an observation period. To determine how covariates impacted frequency of torpor bouts, data were coded, so that a torpor bout occurring on a given day was a binary event (0 = no and 1 = yes). We also used a linear mixed model to assess study factors’ impacts on torpor bout duration. Rhythmicity of torpor bouts was evaluated using the circular statistical package in R (CircStats, version 0.2-4). Specifically, the Rayleigh test was used to assess the uniformity of time at the first entrance of torpor for each torpor bout occurrence, as well as to determine the uniformity of time regarding emergence from torpor bouts. A significant p value (<0.05) indicates that the entrance and emergence times were not uniformly distributed throughout the 24-h day (i.e., animals tended not to enter torpor at specific times). Food intake values were normalized using percentage of food consumed daily; percentage values were arcsine-transformed before testing for differences in consumption using a paired t test. Numerical values are expressed as means ± standard deviation (SD).
Results
T sk profiles during the torpor phase
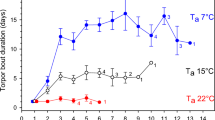
All individuals across both diet groups and both years experienced at least one torpor bout during the torpor phase when T a was lowered to 20°C, photoperiod was reduced to LD10:14, and calories were restricted. The mean frequency of torpor bouts for all individuals across both years was 10.3 ± 6.5 occurrences (n = 18). The highest number of torpor bouts recorded was 27 in a single animal extracted from both years of combined data, while the lowest was observed in one individual that entered torpor only once in year one. The distribution of torpor frequencies was bimodal. In other words, while some individuals are prone to readily and frequently enter torpor, others are torpor-averse (Fig. 3). The mean torpor bout duration was 8 ± 3 h for all individual recordings combined. The longest bout was 22 h and the shortest was 6 h. In year one of the study, our hibernaculum experienced a malfunctioning heating system that resulted in T a dropping to as low as 12 °C for 36 h. This incidence allowed us to record the lowest T sk observed. While the majority of the individuals continued to defend T sk at near euthermic levels, one animal had a recorded T sk of 14.5 °C during that time frame.
Diet does not influence mean torpor bout frequency or mean torpor bout duration
There were no significant differences in either mean duration or mean frequency of torpor bouts when comparing animals fed PUFA diets and animals receiving a control diet. This is due to extreme variation and lack of consistency in torpor patterns between diet groups, as well as between years (Fig. 4; Fig. S1). We also observed large variations in torpor depth and duration within the same animal throughout the study period, with neither metric correlating to diet or year effects (an example can be seen in Fig. 2; panels b, c). After removing the two individuals that had collar failures in year two and conducting paired t tests on the remaining eight animals, we found that the mean frequency of torpor bouts was not significantly different between animals on PUFA diets (n = 8) or the control diet (n = 8; p = 0.39; t test; Fig. 4, panel a). Similarly, the mean duration of torpor bouts observed was not significantly different between the two diet groups (p = 0.79; t test; Fig. 4, panel b). There was no significant difference between groups in total hours spent in torpor (p = 0.37; t test), or total percentage of time spent in torpor (p = 0.38; t test). Finally, there was no significant difference in mean frequency of torpor bouts or mean torpor bout duration observed between years (Fig. 4; panels c (frequency; p = 0.18; t test) and d (duration; p = 0.09; t test)).
Torpor bout frequency and duration of bouts in captive grey mouse lemurs by treatment and year. a Frequency of torpor bouts between diets; b duration of torpor bouts between diets; c frequency of torpor bouts between years; and d duration of torpor bouts between years. Medians are indicated by black lines, and data outliers are indicated by open circles. All p values = n.s
Torpor patterns are likely influenced by differences in body mass
Because of the extreme variation in torpor bout frequency and duration that cannot be explained by diet or by year, binomial family generalized linear mixed models (for frequency of torpor bouts) and linear mixed models (for duration of torpor bouts) were used to fit the data with added study factors allowing us to consider the impact of variables, such as body mass, rate of weight loss, average daily ambient temperature (T a), hibernaculum location, breeding pre-study and post-study, offspring sired, and age. In addition, many traces show a gradual increase in torpor usage after the beginning of the torpor phase. In other words, they tend to enter torpor more frequently towards the end of the recording period (again, see Fig. 2, panels b, c). This is characteristic of the torpor patterns documented in many heterothermic mammals (Geiser et al. 1990; Heldmaier et al. 2004). Therefore, we used R package lme4 (Bates et al. 2015) to investigate the relationship between torpor bout frequency and study factors, with day as a random effect (i.e., each day was included as a repeated effect). An additional intercept was included for animal ID. Fixed effects were entered for diet, year, T a, hibernaculum location, weight, and daily weight fluctuation (i.e., rate of weight loss, in most cases, although two animals did gain a slight amount of weight during 1 week of the torpor phase). Daily weight was extrapolated from rate of weekly change, so that this variable matched the other variables in terms of time units measured. Our extrapolation assumed that weight loss occurred at a steady rate over the 7-day period in between weighing. Breeding and offspring were also included as binary events (i.e., breeding, no = 0 and yes = 1; offspring, no = 0 and yes = 1). We ran our models with interaction terms for diet and weight (weight*diet), and without the interaction term, and compared the models using a likelihood ratio test. There was no interaction between diet and weight (\(x^{2}\)(2) = 4.31, p = 0.12), so these were included as distinct effects. We also tested age as a factor, first confirming that age and year were not significantly interdependent variables (\(x^{2}\)(1) = 0.03, p = 0.8), and including these as distinct effects in our model. Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. Overall, these statistical models confirmed that diet does not influence torpor bout frequency or torpor bout duration in captive mouse lemurs, as results from our paired t tests indicated. However, we do find a significant interaction of weight on torpor usage. We also find that age significantly impacts torpor bout frequency, but not duration. P values were obtained using likelihood ratio tests of the full model containing the effect in question (body mass or rate of weight loss), against the model without the effect in question.
Body mass significantly affected both torpor bout frequency (\(x^{2}\)(1) = 7.98, p = 0.005) and torpor bout duration (\(x^{2}\)(1) = 7.03, p = 0.008) with greater body mass positively associated with frequency and length of torpor bout. We also investigated the effect of rate of weight loss on torpor bout frequency and duration. Rate of weight loss was significantly impacted by torpor bout frequency (\(x^{2}\)(1) = 10.63, p = 0.001) and torpor bout duration (\(x^{2}\)(1) = 6.01, p = 0.01). Animals that had less frequent torpor bouts, and for a shorter amount of time also lost more weight.
Age also significantly impacted torpor bout frequency (\(x^{2}\)(1) = 4.23, p = 0.04), with older animals experiencing more torpor bouts. However, age did not affect torpor bout duration (\(x^{2}\)(1) = 3.28, p = 0.07).
Comparing body weights between diet groups and between years shows that body weight was not significantly different between diet groups at the beginning of the fattening phase (p = 0.73; t test), or at the beginning of the torpor phase (p = 0.74; t test; Fig. 5). Body weight was not significantly different between year one and year two at the beginning of the fattening phase (p = 0.11; t test), but it was significantly different between years at the beginning of the torpor phase (p < 0.001; t test), with animals in year one on average weighing more at the start of the torpor phase than average body mass of animals in year two.
We did not observe significant differences in food preferences between PUFA and control diets (p = 0.35; t test). However, we did find that animals ate significantly more in year two than they did in year one (p = 0.001; t test), which is surprising given the fact that, overall, animals gained less weight in year two. This suggests that the animals were eating more to recover the weight that they had lost in year one of the study, especially those being subjected to decreased environmental temperatures, but using torpor less frequently.
In addition, our animals do not show uniform daily rhythmicity in entrance of torpor bouts (Rayleigh uniformity p < 0.001) or uniform daily rhythmicity in emergence from torpor bouts (Rayleigh uniformity p < 0.001). The timing of reductions in core body temperatures as determined from T sk recordings suggests that animals start a bout of torpor preferentially during the simulated night, with the majority of torpor bouts initiated at 1400 h, shortly after the lights in the hibernaculum are turned off, corresponding to the animals’ active period (Fig. 6). However, we also find that our study animals can enter torpor during 17 h in a 24-h day cycle, with 8 h representing the most likely times for an animal to enter torpor (defined as ≥15 instances). Animals tend to emerge from torpor shortly after the lights in the hibernacula are turned off around 1200, which correspond to simulated night and their natural active period, a result we expected to find. However, there is much variability in timing as animals emerged primarily between 1300 and 2400 h, with few instances of animals emerging during the “lights on” period (Fig. 6).
Discussion
This is the one of the few detailed studies investigating the effects of diet manipulation on torpor patterns in a heterothermic primate species. Taken as a whole, our results suggest that in captive mouse lemurs, the dietary manipulations we used in this study did not have a significant effect on torpor patterns. This can be explained by four factors, which our study was unable to test: (1) the possibility that dietary fatty acids did not assimilate into fat reserves; (2) if they did assimilate into fat depots, this did not have any noticeable impact on torpor patterns under the environmental regime used in our study design; (3) the trace amounts of PUFA in the control diet were enough to allow membranes to remain fluid at lowered ambient and body temperatures, thereby further supporting the homeoviscosity hypothesis, or (4) the cholesterol content of our experimental diets is a contributing factor to the variability in torpor patterns we have described in this study. The previous studies on the impact of dietary cholesterol on torpor patterns in chipmunks suggest that, just as with high-PUFA diets, high cholesterol diets also have the capacity to increase torpor bout depth and duration. However, our study might be indicating interaction effects of dietary cholesterol and fatty acids, as our control diets were five times higher in cholesterol content (i.e., our PUFA diets were high PUFA and low cholesterol, while our control diets were low PUFA and high cholesterol). Therefore, any positive effect from cholesterol might be potentially masked by the higher saturated fatty acid content of our control diet. Furthermore, it has been suggested that dietary fatty acids and dietary cholesterol may be using similar mechanisms on membrane properties of adipose stores (Geiser et al. 1997), although this is still not well understood.
Fietz et al. (2003) conducted autumnal feeding observations and investigated biochemical changes in white adipose tissue stores before and during hibernation in fat-tailed dwarf lemurs, (Cheirogaleus medius)—the hibernating sister lineage to mouse lemurs. They found that fat-tailed dwarf lemurs show extremely low PUFA concentrations in fat stores preceding hibernation and further suggest that monounsaturated fatty acids (MUFAs)—synthesized from high-sugar fruits consumed during autumn—were the main fuel source in this species during hibernation (Fietz et al. 2003). The authors conclude that in C. medius, consuming PUFAs and incorporating them into fat reserves as a means to maintain lipid fluidity during hibernation does not appear to be critical for hibernation. This is likely due to the relatively high body temperatures in these animals during hibernation wherein near-freezing levels are never reached (minimum averages for C. medius are approximately 18 °C, with lowest recorded T b of 9.3 °C (Dausmann et al. 2005).
Furthermore, a recent study conducted in a captive colony of M. murinus in Brunoy, France found a strikingly similar fatty acid profile in the WAT of their study animals (Vuarin et al. 2014). Although the investigators were not subjecting their animals to diet manipulations, results from that study also suggest a positive correlation between depth and duration of shallow torpor bouts and a greater proportion of unsaturated fatty acids in WAT. However, they find that the majority of the unsaturation index was due to MUFAs, with PUFAs to a lesser degree. In a recent study by members of the same research team, the authors did, in fact, subject mouse lemurs to dietary manipulation studies to test how the two major classes of PUFAs (n-6 and n-3) influence preparation for and expression of heterothermy. They find that higher dietary content of n-3 can suppress torpor usage in their animals (Vuarin et al. 2016), although their study was conducted under thermoneutrality and, as the authors note, should be repeated at lower T a.
Frank and Dierenfeld (1998) argue that there may be a physiological constraint associated with high-PUFA diets, that is, increased peroxidation of lipids in fat reserves. Under normal physiological conditions, PUFAs undergo lipid peroxidation more readily than saturated fatty acids or MUFAs (Gunstone et al. 1986). High levels of lipid peroxidation can lead to hemolysis, muscle degeneration, or nervous system damage (Halliwell and Gutteridge 1986; Seppanen et al. 2013; Frankel 1995). Heterotherms that increase PUFAs in their fat reserves may, therefore, be particularly susceptible to tissue damage by lipid peroxidation. In addition, as with PUFAs, MUFAs can play a role in keeping fat stores fluid at low body temperatures as the presence of even one double bond can lead to an increase in fluidity. Further addition of double bonds (e.g., as in PUFAs) can have smaller and sometime negative effect on the resulting fluidity (Hazel and Williams 1980; Vuarin et al. 2014). Mouse and dwarf lemurs, therefore, may have developed means to avoid toxic fatty acids peroxides by consuming relatively lower PUFA diets preceding torpor, and instead relying on MUFAs in their fat stores to keep fat reserves fluid at body temperatures below euthermic, although this correlation needs to be further tested. This relationship has also been documented for P. sungorus (Diedrich et al. 2014) and for greater mouse-tailed bats (Rhinopoma microphyllum; Levin et al. 2013). In greater mouse-tailed bats, which hibernates at relatively high T a as mouse lemurs do, their adipose stores directly preceding hibernation contain a high fraction of saturated fatty acids and MUFAs, with PUFAs being nearly absent (<1% of total fatty acid composition; Levin et al. 2013).
The response of the interaction between dietary factors and how they impact torpor is set by the ecological context in which species evolve. It is, perhaps, not surprising that species without ready access to PUFAs in their habitats may not show identical responses to species who have high accessibility to PUFAs in their natural food resources. While herbivorous species that thrive on high-PUFA plants, nuts, and seeds have evolved a physiological dependence on high-PUFA intakes, highly insectivorous (e.g., mouse lemurs) or carnivorous (e.g., bears) heterotherms could be expected to have developed alternative approaches, such as a reliance on MUFAs or lowered need for PUFAs. Furthermore, cholesterol is rare in plants but can be quite abundant in invertebrates, such as insects (Munro and Thomas 2004). This might suggest that mouse lemurs in the wild are relying on a combination of MUFAs and cholesterol, or solely on one factor, as a means to achieve lowered body temperatures during torpor bouts.
Even despite the fact that mouse lemurs in their natural settings are likely not relying on PUFAs for thermoregulatory strategies, this study nonetheless holds promise for new avenues of research. More conclusive studies should be done with, for example, room temperatures that are lowered below the threshold of 20°C imposed by this study, or follow-up studies conducted with wild animals under semi-captive conditions to more accurately mimic environmental conditions. Furthermore, diets should be modified, such that the control diet is completely absent of PUFAs to rule out the fact that even trace amounts may play a role, as our study was unable to address. Finally, although the current IACUC standards precluded us from acquiring adipose tissue samples to investigate how dietary fatty acids are assimilated into body composition in these animals, this avenue of inquiry still remains open.
Why is there so much variation in torpor patterns?
It is well established, based on the previous studies on wild mouse lemurs, that individuals display remarkable flexibility in their torpor usage. Considerable variation in patterns of heterothermy was observed with some individuals using daily torpor exclusively, while others used prolonged heterothermy and still others remained euthermic (Schmid and Ganzhorn 2009; Kobbe et al. 2011). The previous field studies document that torpor episodes vary in intensity, duration, and frequency between populations and even among individuals of the same population (Ortmann et al. 1997; Schülke and Ostner 2007; Schmid and Ganzhorn 2009; Kobbe et al. 2011). Presumably, this flexibility allows mouse lemurs to (1) adapt quickly to the extremely unpredictable environments of Madagascar; (2) cope with large variations in food availability and subsequent variations in body conditions, and (3) incorporate breeding status, or lack thereof, into thermoregulatory strategies for over-winter survival.
Extreme variation in torpor patterns and study covariates allowed us to tease apart the factors that might influence thermoregulatory strategies in captive mouse lemurs. Our main finding is that the amount of fat stored is an important predictor for increased torpor use, as animals that gained more weight relative to starting body mass used torpor more frequently and for longer duration than animals that did not gain as much weight. This same effect is seen in wild populations of grey mouse lemurs (Schmid 2000, 2001; Kobbe et al. 2011) and has also been shown to be the case in free-ranging subtropical bats (Nyctophilus bifax; Stawski and Geiser 2010). The authors find that in this population of bats, individuals with more fat reserves expressed longer and deeper torpor bouts. They hypothesize that torpor is not due to food shortages or low energy stores, as previously suggested to be the main trigger, but instead most likely used to avoid predation. This idea in wild mouse lemurs remains unknown, but is an intriguing possibility.
A second finding concerning the relationship between body mass and torpor use in our study is that animals that lose weight at a faster rate during the torpor phase were also the ones that spent less time in torpor. This relationship is seen in the animals that did not gain sufficient fat stores leading up to the torpor phase and is presumably due to the higher costs associated with maintaining euthermic body temperatures at lowered ambient temperatures, although it remains unclear why an animal would remain euthermic—and more quickly burn through their fat stores—if torpor was an option. Many studies have investigated the effects of abiotic factors and torpor use (e.g., ambient temperature, water availability, illumination, and food availability, but less is understood regarding how ecological interactions play a role (e.g., predator avoidance, inter-and intra-specific competition; reviewed in Kronfeld-Schor and Dayan 2013).
A similar situation has been documented by Nowack et al. (2010, 2013). Their studies on the African lesser bushbaby (Galago moholi) have shown that torpor is physiologically possible during the cold, dry season in this species, though this behavior is employed only under life-threatening circumstances (Nowack et al. 2010). The authors suggest that these animals have evolved alternative strategies, such as behavioral thermoregulation (i.e., huddling, opting for better insulated sleeping sites, and/or reducing locomotion), to survive periods of energetic constraints (Nowack et al. 2013). In our study, huddling behavior can be ruled out as a means of behavioral thermoregulation, because animals were caged separately, though individuals were provided with blankets and multiple types of nesting material (e.g., PVC piping, Kleenex boxes, and pockets cut from jeans) in keeping with IACUC protocols. It is, therefore, possible that those animals that remained euthermic had chosen better insulated sleeping/resting sites. Alternatively, as we did not track locomotion throughout the study, we cannot rule out the possibility that certain euthermic animals had instead reduced their locomotion to save energy stores rather than employ torpor.
Timing of entrance into torpor is also variable
In general, torpor on a daily basis is restricted to an animal’s period of inactivity, which in the DLC colony, and is between the hours of 0200 h and 1200 (the simulated day on a reverse light cycle). The majority of torpor bout entrances occur at the transition between lights on and lights off, with about 35 instances that take place in the hour before the lights turn on. It is an interesting result, but it might not be surprising. Ortmann et al. (1997) found that torpor onset varies from day to day in a wild population of mouse lemurs. In those animals, torpor is, instead, terminated on a strict daily rhythm; however, torpor bout duration—which varied greatly in their study animals—did so due to timing of entrance into torpor. The differences observed may be due to certain ecological interactions, like predator avoidance; however, this has not been well studied (Kronfeld-Schor and Dayan 2013). We also find much variability in timing of torpor bout emergences, but these are primarily restricted to the animals’ natural active period, and do not appear to be terminated on a daily rhythm as Ortmann et al. (1997) find in the population of wild M. murinus they studied.
Conclusion
Our study demonstrates that our dietary manipulations in captive mouse lemurs do not have positive effects on torpor bout frequency and duration. Furthermore, we have shown that body mass has a significant positive correlation to both number of torpor bouts and to torpor bout duration, and older animals have more frequent torpor bouts, but not necessarily longer torpor bouts. This study is the first attempt to investigate how nutritional ecology may influence thermoregulatory strategies that mouse lemurs use to survive the unpredictable climates in Madagascar. Numerous avenues for future research include exploring how diets high in sugar or MUFAs influence fat storage and torpor use, or determining how choice of sleeping sites, or locomotion during the animals’ activity period is correlated with time spent in torpor. The maintenance of energy budgets throughout the duration of the hibernation season is crucial and is a delicate balance of energy intake and expenditure. If energy balance isn’t maintained, the animal faces a depletion of fat stores precisely at the time when food resources are gone with potentially devastating fitness consequences.
References
Atsalis S (1999) Seasonal fluctuations in body fat and activity levels in a rain-forest species of mouse lemur, Microcebus rufus. Int J Primatol 20:883–910
Aujard F, Perret M, Vannier G (1998) Thermoregulatory responses to variations of photoperiod and ambient temperature in the male lesser mouse lemur: a primitive or an advanced adaptive character? J Comp Physiol B 168:540–548
Bartness TJR, Milner R, Geloen A, Trayhurn P (1991) Effects of high fat diets on hibernation and adipose tissue in Turkish hamsters. J Comp Pysiol B 161:451–459
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Soft. 67:5. doi:10.18637/jss.v067.i01
Boyer B, Barnes B (1999) Molecular and metabolic aspects of mammalian hibernation. Bioscience 49:713–724
Carey H, Andrews M, Martin S (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83:1153–1181. doi:10.1152/physrev.00008.2003
Contreras C, Franco M, Place NJ, Nespolo RF (2014) The effects of poly-unsaturated fatty acids on the physiology of hibernation in a South American marsupial, Dromiciops gliroides. Comp Biochem Physiol 177:62–69. doi:10.1016/j.cbpa.2014.07.004
Corn JG (1997) The role of polyunsaturated fats in hibernation and daily torpor. Ph.D. disseration. Colorado State University
Dark J (2005) Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr 25:469–497. doi:10.1146/annurev.nutr.25.050304.092514
Dausmann K (2005) Measuring body temperature in the field—evaluation of external vs. implanted transmitters in a small mammal. J Therm Biol 30:195–202. doi:10.1016/j.jtherbio.2004.11.003
Dausmann K, Glos J, Ganzhorn J, Heldmaier G (2005) Hibernation in the tropics: lessons from a primate. J Comp Physiol B 175:147–155. doi:10.1007/s00360-004-0470-0
Diedrich V, Steinlechner S, Scherbarth F (2014) Effects of unsaturated fatty acids on torpor frequency and diet selection in Djungarian hamsters (Phodopus sungorus). J Exp Biol 217:4313–4319. doi:10.1242/jeb.113217
Fietz J, Tataruch F, Dausmann K, Ganzhorn J (2003) White adipose tissue composition in the free-ranging fat-tailed dwarf lemur (Cheirogaleus medius; Primates), a tropical hibernator. J Comp Physiol B 173:1–10. doi:10.1007/s00360-002-0300-1
Florant G (1998) Lipid metabolism in hibernators: the importance of essential fatty acids. Am Zoologist 38:331–340
Florant GL, Hester L, Ameenuddin S, Rintoul DA (1993) The effect of a low essential fatty acid diet on hibernation in marmots. Am J Physiol 264:R747–R753
Frank CL (1991) Adaptations for hibernation in the depot fats of a ground squirrel (Spermophilus beldingi). Can J Zool 69:2707–2711
Frank CL (1992) The influence of dietary fatty-acids on hibernation by golden-mantled ground squirrels (Spermophilus lateralis). Physiol Zool 65:906–920
Frank CL (2002) Short-term variations in diet fatty acid composition and torpor by ground squirrels. J Mammal 83(4):1013–1019
Frank C, Dierenfeld E (1998) The relationship between lipid peroxidation, hibernation, and food selection in mammals. Am Zool 38:341–349
Frank CL, Storey KB (1996) The effect of total unsaturate content on hibernation. In: Geiser F, Hulbert AJ, Nicol SC (eds) Adaptations to the cold: 10th international hibernation symposium, University of New England Press, Armidale, pp 211–216
Frankel EN (1995) Oxidation of polyunsaturated lipids and its nutritional consequences. In: Castenmiller WAM (ed) Oils-fats-lipids: proceedings of the 21st World Congress of the International Society for Fat Research (ISF)
Geiser F (1990) Influence of polyunsaturated and saturated dietary lipids on adipose tissue, brain and mitochondrial membrane fatty acid composition of a mammalian hibernator. Biochim Biophys Acta 1046:159–166
Geiser F (1991) The effect of unsaturated and saturated dietary lipids on the pattern of daily torpor and the fatty acid composition of tissues and membranes of the deer mouse Peromyscus maniculatus. J Comp Physiol B 161:590–597
Geiser F, Heldmaier G (1995) The impact of dietary fats, photoperiod, temperature and season on morphological variables, torpor patterns, and brown adipose tissue fatty acid composition of hamsters, Phodopus sungorus. J Comp Physiol B
Geiser F, Kenagy GJ (1987) Polyunsaturated lipid diet lengthens torpor and reduces body temperature in a hibernator. Am J Physiol 252:R897–R901
Geiser F, Ruf T (1995) Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool 68:935–966
Geiser F, Ruf T (2015) Daily torpor and hibernation in birds and mammals. Biol Rev 90:891–926. doi:10.1111/brv.12137
Geiser F, Hiebert S, Kenagy G (1990) Torpor bout duration during the hibernation season of two sciurid rodents: interrelations with temperature and metabolism. Physiol Zool 63:489–503
Geiser F, Stahl B, Learmonth R (1992) The dffect of dietary fatty-acids on the pattern of torpor in a marsupial. Physiol Zool 65:1236–1245
Geiser F, Mcallan BM, Kenagy GJ (1994) The degree of dietary fatty acid unsaturation affects torpor patterns and lipid composition of a hibernator. J Comp Physiol B 164:299–305
Geiser F, Kenagy GJ, Wingfield JC (1997) Dietary cholesterol enhances torpor in a rodent hibernator. J Comp Physiol B 167:416–422
Geiser F, Holloway JC, Körtner G (2007) Thermal biology, torpor and behaviour in sugar gliders: a laboratory-field comparison. J Comp Physiol B 177:495–501. doi:10.1007/s00360-007-0147-6
Genin F, Perret M (2000) Photoperiod-induced changes in energy balance in gray mouse lemurs. Physiol Behav 71:315–321
Genin F, Perret M (2003) Daily hypothermia in captive grey mouse lemurs (Microcebus murinus): effects of photoperiod and food restriction. Comp Biochem Physiol B 136:71–81. doi:10.1016/S1096-4959(03)00172-6
Giroud S, Blanc S, Aujard F et al (2008) Chronic food shortage and seasonal modulations of daily torpor and locomotor activity in the grey mouse lemur (Microcebus murinus). AJP Reg Integ Comp Physiol 294:R1958–R1967. doi:10.1152/ajpregu.00794.2007
Gunstone ED, Harwood JL, Padley FB (1986) The lipid handbook. Chapman and Hall, New York, pp 265–270
Halliwell B, Gutteridge J (1986) Oxygen free-radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys 246:501–514
Harlow HJ, Frank CL (2001) The role of dietary fatty acids in the evolution of spontaneous and facultative hibernation patterns in prairie dogs. J Comp Physiol B 171:77–84. doi:10.1007/s003600000148
Hazel JR, Williams EE (1980) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Re 29(3):167–227
Heldmaier G, Ortmann S, Elvert R (2004) Natural hypometabolism during hibernation and daily torpor in mammals. Resp Physiol Neurobi 141(3):317–329. doi:10.1016/j.resp.2004.03.014
Hiebert SM, Fulkerson EK, Lindermayer KT, McClure SD (2000) Effect of temperature on preference for dietary unsaturated fatty acids in the Djungarian hamster (Phodopus sungorus). Can J Zool 78:1361–1368
Kobbe S, Ganzhorn JU, Dausmann KH (2011) Extreme individual flexibility of heterothermy in free-ranging Malagasy mouse lemurs (Microcebus griseorufus). J Comp Physiol B 181:165–173. doi:10.1007/s00360-010-0507-5
Kronfeld-Schor N, Dayan T (2013) Thermal ecology, environments, communities, and global change: energy intake and expenditure in endotherms. Annu Rev Ecol Evol Syst 44:461–480. doi:10.1146/annurev-ecolsys-110512-135917
Levin E, Yom-Tov Y, Hefez A, Kronfeld-Schor N (2013) Changes in diet, body mass and fatty acid composition during pre-hibernation in a subtropical bat in relation to NPY and AgRP expression. J Comp Physiol B 183:157–166. doi:10.1007/s00360-012-0689-0
Munro D, Thomas DW (2004) The role of polyunsaturated fatty acids in the expression of torpor by mammals: a review. Zoology 107:29–48. doi:10.1016/j.zool.2003.12.001
Munro D, Thomas D, Humphries M (2005) Torpor patterns of hibernating eastern chipmunks (Tamias striatus) vary in response to the size and fatty acid composition of food hoards. J Anim Ecology 74:692–700. doi:10.1111/j.1365-2656.2005.00968.x
Nowack J, Mzilikazi N, Dausmann KH (2010) Torpor on demand: heterothermy in the non-lemur primate, Galago moholi. PLoS One 5:e10797. doi:10.1371/journal.pone.0010797
Nowack J, Whippich M, Mzilikazi N, Dausmann KH (2013) Surviving the cold, dry period in Africa: behavioral adjustments as an alternative to teterothermy in the African Lesser Bushbaby (Galago moholi). Int J Primatol 34:49–64. doi:10.1007/s10764-012-9646-8
Ortmann S, Heldmaier G, Schmid J, Ganzhorn JU (1997) Spontaneous daily torpor in Malagasy mouse lemurs. Naturwissenschaften 84:28–32
Perret M, Aujard F, Vannier G (1998) Influence of daylength on metabolic rate and daily water loss in the male prosimian primate Microcebus murinus. Comp Biochem Physiol A 119:981–989
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Schmid J (1999) Sex-specific differences in activity patterns and fattening in the gray mouse lemur (Microcebus murinus) in Madagascar
Schmid J (2000) Torpor in the tropics: the case of the gray mouse lemur. Basic Appl Ecol 1:133–139
Schmid J (2001) Daily torpor in free-ranging gray mouse lemurs (Microcebus murinus) in Madagascar. Int J Primatol 22:1021–1031
Schmid J, Ganzhorn JU (2009) Optional strategies for reduced metabolism in gray mouse lemurs. Naturwissenschaften 96:737–741. doi:10.1007/s00114-009-0523-z
Schülke O, Ostner J (2007) Physiological ecology of cheirogaleid primates: variation in hibernation and torpor. Acta Ethol 10:13–21. doi:10.1007/s10211-006-0023-5
Seguy M, Perret M (2004) Factors affecting the daily rhythm of body temperature of captive mouse lemurs (Microcebus murinus). J Comp Physiol B 175:107–115. doi:10.1007/s00360-004-0467-8
Seppanen CM, Cho H, Csallany AS (2013) Comparison between high-PUFA and low-PUFA fats on lipid peroxidation and LDL oxidation. FNS 04:572–579. doi:10.4236/fns.2013.45074
Stawski C, Geiser F (2010) Fat and fed: frequent use of summer torpor in a subtropical bat. Naturwissenschaften 97:29–35. doi:10.1007/s00114-009-0606-x
Thorp CR, Ram PK, Florant GL (1994) Diet alters metabolic rate in the yellow-bellied marmot (Marmota flaviventris) during hibernation. Physiol Zool 67:1213–1229
Vuarin P, Henry PY, Guesnet P, Alessandri JM, Aujard F, Perret M, Pifferi F (2014) Shallow hypothermia depends on the level of fatty acid unsaturation in adipose and liver tissues in a tropical heterothermic primate. J Therm Biol 43:81–88. doi:10.1016/j.jtherbio.2014.05.002
Vuarin P, Henry PY, Perret M, Pifferi F (2016) Dietary supplementation with n-3 polyunsaturated fatty acids reduces torpor use in a tropical daily heterotherm. Physiol Biochem Zool. doi:10.1086/688659
Withers K, Billingsley J, Hirning D, Young A, McConnell P, Carlin S (1996) Torpor in Sminthopsis macroura: effects of dietary fatty acids. In: Geiser F, Hulbert, AJ, Nicol SJ ( eds) Adaptations to the cold. University of New England Press, Armidale, Australia, pp 217–222
Acknowledgements
We thank Sarah Zehr and Kathrin Dausmann for careful review of the manuscript and appreciate the assistance of Duke Lemur Center staff, especially Erin Ehmke, David Brewer, and Bobby Schopler for making this project possible. We would especially like to thank Edwin Iversen and the Duke Statistical Computing Center for guidance on statistical analysis. This is Duke Lemur Center publication number 1334. This work was supported by grants from the Mazuri Exotic Animal Nutrition Grant to S.L.F., and a National Science Foundation Doctoral Research Improvement Grant [NSF-BCS 1455809 to A.D.Y and S.L.F].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted. Animal handling was carried out with the approval of Duke University’s Institutional Animal Care and Use Committee (IACUC Protocol #A146-12-05). The authors declare no competing interests.
Additional information
Communicated by G. Heldmaier.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faherty, S.L., Campbell, C.R., Hilbig, S.A. et al. The effect of body mass and diet composition on torpor patterns in a Malagasy primate (Microcebus murinus). J Comp Physiol B 187, 677–688 (2017). https://doi.org/10.1007/s00360-016-1045-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1045-6