Abstract
Most studies on animal physiology and behaviour are conducted in captivity without verification that data are representative of free-ranging animals. We provide the first quantitative comparison of daily torpor, thermal biology and activity patterns, conducted on two groups of sugar gliders (Petaurus breviceps, Marsupialia) exposed to similar thermal conditions, one in captivity and the other in the field. Our study shows that activity in captive gliders in an outdoor aviary is restricted to the night and largely unaffected by weather, whereas free-ranging gliders omit foraging on cold/wet nights and may also forage in the afternoon. Torpor occurrence in gliders was significantly lower in captivity (8.4% after food deprivation; 1.1% for all observations) than in the field (25.9%), mean torpor bout duration was shorter in captivity (6.9 h) than in the field (13.1 h), and mean body temperatures during torpor were higher in captivity (25.3°C) than in the field (19.6°C). Moreover, normothermic body temperature as a function of air temperature differed between captive and free-ranging gliders, with a >3°C difference at low air temperatures. Our comparison shows that activity patterns, thermal physiology, use of torpor and patterns of torpor may differ substantially between the laboratory and field, and provides further evidence that functional and behavioural data on captive individuals may not necessarily be representative of those living in the wild.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most studies on animal physiology and behaviour are conducted in captivity, usually as a consequence of technological limitations and convenience. Data obtained on captive animals are considered to be representative of those of wild populations by some researchers, although few laboratory investigations have been conducted in conjunction with field studies to verify whether or not the data are comparable (Costa and Sinervo 2004).
In line with other animal studies, torpor and others aspects of thermal biology also have been investigated primarily in captivity. Torpor is characterised by a controlled reduction of body temperature (T b), metabolic rate, and other physiological processes that can substantially reduce energy expenditure (Wang 1989). Many small mammals and birds employ torpor often to overcome energy shortages during adverse environmental conditions or limited food supply (Wang 1989; Barnes and Carey 2004). Torpor can be prolonged and deep (multi-day, with T b ∼ 5°C) as in the hibernators, which generally fatten extensively before the hibernation season and often only enter torpor when fat (Wang 1989; Geiser and Ruf 1995). In contrast, daily torpor in the daily heterotherms (species entering daily torpor exclusively) lasts only for several hours, usually during the daily rest phase with T b’s falling to ∼17°C (Geiser and Ruf 1995; Lovegrove et al. 1999; Schmid 2000). Unlike hibernators, daily heterotherms mainly employ torpor at times when they are lean often in response to acute environmental stressors such as cold exposure, bad weather or lack of food. Daily heterotherms usually attempt to forage on a daily basis during their activity phase even during times when they use torpor frequently (Geiser 2004).
Captive animals may be stressed and are also usually fatter than their con-specifics in the field (Holloway and Geiser 1996; Mzilikazi and Lovegrove 2002; Costa and Sinervo 2004), therefore, use and depth of daily torpor in captivity may be adversely affected. Further, intra-specific differences in torpor physiology between captive-bred and wild-captured individuals of similar body mass have been observed in the laboratory (Geiser and Ferguson 2001), and it is likely that functional differences are even more pronounced when captive and free-ranging individuals are compared. Fortunately, the development of small temperature-sensitive radio transmitters and data loggers has permitted measurement of T b during torpor in the field (e.g. Brigham et al. 2000; Körtner et al. 2000; Chruszcz and Barclay 2002; Cooper and Withers 2004; Dausmann 2005: Willis et al. 2006) and a recent comparison has shown that indeed several birds and mammals differ between the laboratory and field with regard to torpor (Geiser et al. 2000). Nevertheless, this comparison of laboratory and field thermal biology was restricted to a qualitative treatment of torpor; quantitative analyses of functional variables in torpid and normothermic individuals were not conducted. The purpose of the present study is to provide the first quantitative comparison of daily torpor and activity patterns in a captive and a free-ranging population of sugar gliders (Petaurus breviceps; body mass ∼ 120 g, Petauridae: Marsupialia) from the same area.
Sugar gliders live along the east and north coast of Australia and in New Guinea. They are largely nocturnal and feed mainly on plant exudates, nectar, pollen and insects (Hume 1999), which can become scarce in winter or during inclement weather. Gliders use nests and huddling for social thermoregulation, but also possess the ability to enter daily torpor (Fleming 1980; Körtner and Geiser 2000a; Geiser and Körtner 2004) and therefore were chosen as our study organism.
Material and methods
Two populations of gliders from the same area were compared during our study. The first was a captive population maintained in roofed outdoor aviaries in a quiet, remote location on the campus of the University of New England, Armidale, NSW, Australia, at an altitude of ∼1,000 m. These individuals were exposed to natural photoperiod and similar temperature fluctuations as the free-ranging population (described below), but were sheltered from rain and strong wind. Of the 17 captive individuals, 14 were wild-caught from woodlands near Armidale and three were bred in captivity from the wild-caught individuals. Gliders were maintained in their family groups captured from nest boxes in the wild. In captivity, gliders in aviaries had access to nest boxes and were fed in the afternoon, well before they became active, on a mixture of high-protein cereal, honey and water supplemented with calcium and vitamins, apples, carrots and occasionally mealworms (Holloway and Geiser 2001). Food was usually available ad libitum, however, ∼once/month food was withheld for 1 day to induce torpor. Only non-reproductive adult gliders were used for our comparison. Our study was conducted between 1994 and 1998, but only data collected between May and October (late autumn to early spring), the time of year when free-ranging individuals were investigated, were used for statistical comparisons.
To quantify daily T b fluctuations, gliders were implanted intraperitoneally under Oxygen/Isoflurane anaesthesia with temperature-sensitive transmitters (Sirtrack ∼3.5 g) with a battery life of ∼6 months. Transmitters had been coated in Paraffin/Elvax (Minimitter) and calibrated to the nearest 0.1°C. Transmitters were used for measurements of T b both in the aviary and during measurements of oxygen consumption in the laboratory, but in the aviaries only T b was measured. Transmitter signals were received using a scanning receiver (Yaesu, FRG-9600) interfaced with a computer. As torpor occurrence and duration in captive animals is often quantified by open flow respirometry (e.g. Hiebert 1990; McNab and Bonaccorso 2001), we determined rates of oxygen consumption for measuring torpor variables in individual captive gliders. Air temperature (T a) was measured to the nearest 0.1°C with a calibrated thermocouple inserted 1 cm into the respirometry chamber (further details in Holloway and Geiser 2001). Animals were placed in the respirometry chamber late in the afternoon (between 1600 and 1700 hours), at T a’s that were maintained constant at values ranging between 0 and 20°C, and were measured for ∼23 h. Resting T b was averaged over at least 20 min when normothermic T b were constant and minimal during these 23-h measurements; this resting T b (1 reading/day) was used for analyses. Food and water were not available during measurements of oxygen consumption. Timing of daily activity of family groups in the aviaries was quantified using passive infrared detectors (Körtner and Geiser 1995).
The free-ranging population was studied between May and October 1998 near Armidale at Imbota Nature Reserve (30°35′S, 151°44′E) a 220-ha Eucalyptus/Acacia open woodland, ∼10 km from the UNE campus, at the same altitude of ∼1,000 m and under similar thermal conditions as the captive gliders. Ten gliders captured in autumn/winter and belonging to three different family groups were fitted with temperature-sensitive radio transmitters. Four gliders received intraperitoneal transmitters as described above, four were equipped with radio collars for measurement of skin temperature (T skin) to increase transmitter reception range in the field (Körtner and Geiser 2000a), and two individuals were fitted with both transmitter types to determine correlations between T b and T skin. In resting and torpid gliders, which assume a curled position with the transmitter near the core, T skin was within 0.6°C of core T b (T b = 1.04 × T skin − 1.06; r 2 = 0.99; Körtner and Geiser 2000a), similar to other similar-sized mammals (Dausmann 2005). Therefore these T skin values were treated as T b, however, we present means with and without correction of T skin, using the above equation, for comparison. Gliders were allowed to recover from the surgery for two days before release at the site of capture.
Gliders were radio-tracked to identify nest trees. The T b and T skin were recorded automatically in 10-min intervals with receiver/loggers, consisting of a scanner receiver (Uniden, UB60XLT) and a microprocessor-based (BASIC Stamp, Parallax) data logger (Körtner and Geiser 2000a) placed near nest trees. Absence and presence of individuals in the nest, and thus daily activity or the time at rest, were inferred from the absence and presence of transmitter signals on the logging records.
Ambient temperature (T a) was recorded in hourly intervals at the study site with temperature transducers (AD590 accuracy: 0.35°C). Daily rainfall data for Armidale were obtained from Bureau of Meteorology.
Torpor was defined by T b or T skin <30°C in resting animals, as this is the threshold commonly employed for defining torpor in marsupials (Körtner and Geiser 2000a; Cooper and Withers 2004), or during measurements of oxygen consumption when no T b measurements were available, as a reduction of oxygen consumption below 75% of resting values at the same T a (Hudson and Scott 1979). Although oxygen consumption was not measured in the field, this method was chosen for our comparison of torpor occurrence and patterns because laboratory investigations on torpor are often based on this experimental approach (e.g. Hiebert 1990; Holloway and Geiser 1996; McNab and Bonaccorso 2001). Physiological and behavioural variables of individual means of captive and free-ranging gliders were compared using two-sample t-tests. Percentage values of individual means were compared using a Mann–Whitney test. Regressions were performed by the least squares method and compared using ANCOVA. Data are presented as mean ± standard error for the number (n) of individuals measured; N is provided for additional information and denotes the number of measurements.
Results
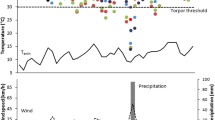
Captive and field populations displayed different activity patterns. Family groups of captive gliders were active under all environmental conditions and were almost exclusively active at night with the activity period often extending throughout the night. In contrast, individuals in the field reduced activity or were inactive on cold/wet nights. Captive individuals in the aviary began their nightly activity period (determined by passive infrared detectors) 10.1 ± 5.4 min after sunset, which was significantly (t-test, t = 3.9, df = 11, P < 0.01) earlier than in free-ranging individuals (18.1 ± 11.5 min after sunset, determined by telemetry). The activity period ended much later (t-test, t = 14.3, df = 11, P < 0.0001) in captive individuals (15.5 ± 8.9 min before sunrise) than in free-ranging individuals (138 ± 52 min before sunrise). Captive individuals were inactive during the day, whereas several free-ranging individuals occasionally foraged during the late afternoon.
Body mass and occurrence of torpor (Fig. 1) differed substantially between the captive and field populations. In the aviaries, adult gliders in autumn/early winter had body masses ranging from 115 to 170 g (mean 141 ± 5 g), whereas at the same time of the year mean body masses in captive gliders ranged from 85 to 115 g (mean 105 ± 3 g).
Torpor occurrence of captive (left bar induced torpor by food restriction; right bar all torpor bouts) and free-ranging gliders in the field. Values shown in the bar graphs are means with SE; circles represent individual gliders, the circle for 0% torpor occurrence is represented by eight captive individuals
Only one captive glider entered spontaneous torpor (food ad libitum) once with a high minimum T b of 29.5°C. Food deprivation in the aviary resulted in 13.6 ± 4.4% torpor occurrence. During oxygen consumption measurements, when T a was <20°C (the T as at which torpor was observed in the field) and no food was provided, torpor occurrence in captive gliders was 5.7 ± 2.6%, but this value was statistically indistinguishable (Mann–Whitney, P = 0.178) from the values obtained in the aviary. Overall, occurrence of induced torpor by food deprivation in captive gliders was 8.4 ± 2.5% (n = 17; N = 34 torpor bouts in n = 9 of n = 17 individuals, N = 358 animal days without food). In contrast, torpor occurrence in the free-ranging population (25.9 ± 4.8%; n = 10; N = 99 torpor bouts in n = 10 individuals, N = 556 animal days) was significantly greater (Mann–Whitney, P < 0.0001) than that observed in the captive population. When occurrence of both spontaneous torpor and induced torpor in captivity were combined, the differences between the captivity and field data were even more extreme because captive gliders displayed torpor on only 1.1 ± 0.3% (n = 17; N = 35 torpor bouts in n = 9 of n = 17 individuals, N = 2,800 animal days).
The duration of torpor bouts in the field was much longer than in captivity (Fig. 2). Mean torpor bout duration of individual captive gliders (bouts lasting >30 min) was 6.9 ± 0.8 h (n = 7, N = 21), about half (t-test, t = 9.1, df = 15, P < 0.0001) that observed in free-ranging gliders (13.1 ± 0.5 h; n = 10, N = 99). Maximum duration of torpor bouts of individual gliders also differed between captive (8.3 ± 0.9 h, n = 7) and free-ranging (19.8 ± 0.8 h, n = 10) populations (t-test, t = 11.6, df = 15, P < 0.0001). The longest individual torpor bouts observed were 13.4 h in captivity and 23 h in the field.
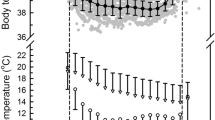
The normothermic T b of captive gliders as a function of T a (measured during respirometry) varied from that in the free-ranging gliders (Fig. 3). The T b of normothermic resting gliders in the field declined significantly (P < 0.0001) with T a, whereas the T b of captive gliders measured during respirometry showed a slight increase (P < 0.05) with declining T a over a similar T a range. The regressions of T b as a function of T a differed both in slope (ANCOVA, F 1,435 = 40.94, P < 0.0001) and intercept (ANCOVA, F 1,136 = 4.67, P < 0.05) between captive and field gliders. At low T a’s between 0 and 10°C, the average normothermic T b in the field gliders was between 3.4 and 1.5°C lower than that in captive individuals.
Body temperatures (T b) as a function of ambient temperature (T a) of captive normothermic gliders (circles, a) measured during respirometry trials when oxygen consumption was low and stable, and free-ranging gliders (b). Torpid gliders are shown as solid symbols: dots are for torpid gliders measured during respirometry, and triangles for torpid gliders measured in the aviary. The equations for the regressions for normothermic T b were: T b = 35.8 − 0.031T a; r 2 = 0.09; P < 0.05 (captive gliders); T b = 32.4 + 0.159T a; r 2 = 0.22; P < 0.001 (field gliders). The diagonal lines represent T b = T a
During torpor, the mean minimum T b from all torpor bouts was 25.2 ± 0.8°C (n = 6, N = 21) in captive gliders in comparison to 19.6 ± 0.7°C (19.4 ± 0.8°C with T skin corrected to T b, n = 10, N = 99) in free-ranging gliders (Figs. 3, 4). The mean minimum T b of captive gliders measured in the aviary (25.5 ± 0.9°C) did not differ (t-test, P = 0.33) from those measured during respirometry trials (24.2 ± 0.8°C). The means of the lowest T b minima measured for each individual were 22.2 ± 1.4°C (n = 6) in captive gliders and 12.9 ± 0.5°C (n = 10) (12.6 ± 0.4°C with T skin corrected to T b) in free-ranging gliders. All means for minimum T b’s differed significantly between captive and field gliders (t-test, t = 5.1 & 6.3, df = 14, P < 0.001) (Fig. 4). The lowest single core T b measured was 18.5°C in a captive glider and 10.4°C in a free-ranging glider. Moreover, only 8% of T b in torpid captive gliders were <20°C in comparison to 59% in the field gliders.
Discussion
Our study provides the first quantitative analysis of patterns of daily torpor and thermal biology in a mammal comparing a captive and a free-ranging population. It demonstrates that differences in thermal physiology and behaviour can be profound. Nevertheless, we do not suggest that data obtained in captivity are of little scientific value, but rather that extrapolations to the field should be made with caution.
Whereas our study demonstrates substantial quantitative differences in daily torpor between captive and free-ranging mammals, previous studies have shown some differences in hibernating species. However, in most mammals that hibernate in captivity, including pygmy-possums, bats, and rodents, torpor in the laboratory and field were qualitatively similar (i.e. prolonged torpor occurred both in the field and laboratory; Wang 1978; Geiser et al. 2000; Geiser and Körtner 2004). Quantitative differences were observed in the timing of arousals and torpor bout duration of hibernators, but the minimum T b during torpor, which differed substantially between captive and field gliders (present study), was similar in most hibernators (Wang 1978; Geiser et al. 2000; Geiser and Körtner 2004). This finding suggests that torpor patterns in hibernators are less strongly affected by captivity than in daily heterotherms, perhaps because the former spend most of the hibernation season at low T b and will be less aware of their surroundings than daily heterotherms with higher minimum T b’s. Daily heterotherms rewarm daily to normothermic T b, may even forage at low T b, and use torpor in generally a more opportunistic and less seasonal manner than hibernators (Körtner and Geiser 2000b; Geiser et al. 2002). Body mass is another likely reason for the more pronounced differences between captive and wild animals using daily torpor than for hibernation. Since daily heterotherms predominantly enter torpor when lean, whereas many hibernators only enter torpor when fat, the usually higher mass in captive individuals is likely to have a greater impact on daily heterotherms. Nevertheless, echidnas (Tachyglossus aculeatus), which hibernate in the field, are reluctant to do so in captivity (Grigg et al. 1992; Nicol and Andersen 2000), showing that even in hibernators maintenance in captivity can inhibit torpor.
Behaviour of gliders was also affected by captivity. Daily activity patterns differed substantially between captive and field populations, especially with regard to the duration of nocturnal activity, which was shorter in the field than in captivity. Moreover, as one might predict, activity patterns were strongly affected by weather in field gliders with some omitting activity during cold/wet weather, but much less so in captivity, as animals are less exposed to rain or wind. Captive gliders also remained in their nest boxes during the day, whereas free-ranging gliders foraged occasionally. These findings have implications for energy use, as well as exposure to inclement weather and predators. Our findings demonstrate that data on activity patterns, as often quantified in captivity under different experimental conditions, cannot be extrapolated to the field without verification from free-ranging individuals.
The difference in occurrence of daily torpor between captive and field gliders also has implications for energy use and foraging requirements as well as predator avoidance in the field. Although the number of known heterothermic mammals and birds is continuously growing, superficial assessments of torpor use in captive individuals may not provide reliable results for all species. The almost entire lack of spontaneous (food ad libitum) torpor in captive gliders observed here might have suggested that the species is homeothermic if the sample size was <2800 animal days. Clearly this is not the case, as in the field gliders regularly employ torpor in winter. Determination of whether or not torpor is employed by a species often is restricted to a few days of cold exposure or food restriction for one day or even less in captivity (e.g. Hiebert 1990; Holloway and Geiser 1996; McNab and Bonaccorso 2001). Positive confirmation of torpor from such captive studies is likely to provide fairly reliable information about the ability to use torpor in the wild. However, if a species refuses to enter torpor in captivity, we cannot necessarily assume it is homeothermic—this can only be unambiguously resolved by undertaking long-term studies in the field (Körtner et al. 2000). As most studies on torpor have been conducted in captivity it is likely that some species that are currently classified as homeotherms are in fact heterotherms.
As mentioned above, differences in occurrence of daily torpor between captivity and the field in sugar gliders is likely to some extent to be a consequence of body mass and fat. Captive gliders in autumn/early winter had body masses that were ∼134% of those in the field. However, these data do not indicate that our captive gliders were obese, but rather that the field gliders in the year they were studied were exceptionally lean and thus expressed frequent and deep torpor. Body mass in free-ranging gliders ranges from 100 to 170 g (Smith and Winter 1984), which is similar to our captive individuals. As it is well established that an increase in body mass is associated with a decrease in daily torpor use (Holloway and Geiser 1996; Mzilikazi and Lovegrove 2002), the low occurrence of torpor in captivity is not surprising. This result suggests that captive individuals with sufficient energy stores can remain normothermic because the energetic stress imposed during the commonly used 1-day food restriction in captivity is insufficient to induce daily torpor. Fleming (1980) had to starve captive sugar gliders for several days before daily torpor was expressed, suggesting that in the field where torpor occurs regularly, energy limitations are a normal part of a glider’s life.
Nevertheless, body mass does not appear to explain all the differences between captive and field gliders. When torpor occurrence of captive gliders was regressed against body mass, a significant regression was obtained [all torpor (%) = 7.25 – 0.044 body mass (g); P < 0.05; r 2 = 0.25]. However, when extrapolated to the mean body mass of free-ranging gliders, the predicted value for torpor occurrence is 2.6% (∼10% of that in the field). When only induced torpor (food deprivation) in captive gliders was regressed, the equation was not quite significant [induced torpor (%) = 50.8 – 0.301 body mass (g); P = 0.062; r 2 = 0.21). The predicted value for torpor occurrence at the mean mass of free-ranging gliders was 19.2% (74% of that observed in the field). These findings suggest that other captivity-based influences, in addition to larger body mass, affect daily torpor use and patterns of gliders.
The pronounced differences in torpor occurrence, duration and depth between the captive and field gliders will have consequences for energy expenditure. When the mean torpor T b, which was on average ∼6°C lower in field than in captive gliders (present study) is considered together with the longer torpor bouts (6.9 h captivity, 13.1 h field), energy expenditure (from Fleming 1980, and assuming a Q10 of 2.3, Geiser 2004) should be reduced to ∼40% in the field in comparison to the captive gliders.
Differences in physiological variables also were observed during normothermia, specifically for the resting T b. Whereas T b in captive gliders was more or less stable, in free-ranging gliders T b fell strongly with T a and, especially at low T a, this resulted in substantial differences in resting T b. Thus normothermic resting T b, which is a commonly quantified variable in captivity that is used for inter-specific comparisons (Withers 1992), can differ by several degrees from values measured in the field.
Our study suggests that captive studies may underestimate the occurrence, depth, and length of daily torpor in the wild. Our observations imply that further studies on torpor in the wild will reveal that torpor is more widespread and pronounced than is currently accepted. Thus, only further fieldwork can clarify how important torpor is for survival and fitness of mammals and birds in the wild, and, while our comparison is especially important for thermal biology and studies of activity patterns, it has implications for many other captive-based investigations.
Abbreviations
- T a :
-
Air temperature
- T b :
-
Body temperature
- T skin :
-
Skin temperature
References
Barnes BM, Carey HV (eds) (2004) Life in the cold: evolution, mechanisms, adaptation, and application. In: 12th international hibernation symposium. Biological Papers of the University of Alaska #27. Inst. Arctic Biology, University of Alaska, Fairbanks
Brigham RM, Körtner G, Maddocks TA, Geiser F (2000) Seasonal use of torpor by free-ranging Australian owlet-nightjars (Aegotheles cristatus). Physiol Biochem Zool 73:613–620
Chruszcz BJ, Barclay RMR (2002) Thermoregulatory ecology of a solitary bat, Myotis evotis, roosting in rock crevices. Func Ecol 16:18–26
Cooper CE, Withers PC (2004) Patterns of body temperature variation and torpor in the numbat, Myrmecobius fasciatus (Marsupialia: Myrmecobiidae). J Therm Biol 29:277–284
Costa DP, Sinervo B (2004) Field physiology: physiological insights from animals in nature. Annu Rev Physiol 66:209–238
Dausmann KH (2005) Measuring body temperature in the field—evaluation of external vs. implanted transmitters in a small mammal. J Therm Biol 30:195–202
Fleming MR (1980) Thermoregulation and torpor in the sugar glider, Petaurus breviceps (Marsupialia: Petauridae). Aust J Zool 28:521–534
Geiser F (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66:239–274
Geiser F, Ferguson C (2001) Intraspecific differences in behaviour and physiology: effects of captive breeding in feathertail gliders. J Comp Physiol B 171:569–576
Geiser F, Körtner G (2004) Thermal biology, energetics and torpor in the possums and gliders. In: Goldingay RL, Jackson SM (eds) The biology of Australian possums and gliders. Surrey Beatty, Sydney, pp 186–198
Geiser F, Ruf T (1995) Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool 68:935–966
Geiser F, Holloway JC, Körtner G, Maddocks TA, Turbill C, Brigham RM (2000) Do patterns of torpor differ between free-ranging and captive mammals and birds? In: Heldmaier G, Klingenspor M (eds) Life in the cold: 11th international hibernation symposium. Springer, Berlin, pp 95–102
Geiser F, Goodship N, Pavey CR (2002) Was basking important in the evolution of mammalian endothermy? Naturwissenschaften 89:412–414
Grigg GC, Augee ML, Beard LA (1992) Thermal relations of free-living echidnas during activity and in hibernation in a cold climate. In: Augee ML (ed) Platypus and echidnas. Royal Zool Soc NSW, Sydney pp 160–173
Hiebert SM (1990) Energy costs and temporal organization of torpor in the rufous hummingbird (Selasphorus rufus). Physiol Zool 63:1082–1097
Holloway JC, Geiser F (1996) Reproductive status and torpor of the marsupial Sminthopsis crassicaudata: effects of photoperiod. J Therm Biol 21:375–380
Holloway JC, Geiser F (2001) Seasonal changes in the thermoenergetics of the marsupial sugar glider, Petaurus breviceps. J Comp Physiol B 171:643–650
Hudson JW, Scott IM (1979) Daily torpor in the laboratory mouse Mus musculus var. albino. Physiol Zool 52:205–218
Hume ID (1999) Marsupial nutrition. Cambridge University Press, Cambridge
Körtner G, Geiser F (1995) Body temperature rhythms and activity in reproductive Antechinus (Marsupialia). Physiol Behav 58:31–36
Körtner G, Geiser F (2000a) Torpor and activity patterns in free-ranging sugar gliders Petaurus breviceps (Marsupialia). Oecologia 123:350–357
Körtner G, Geiser F (2000b) The temporal organization of daily torpor and hibernation: circadian and circannual rhythms. Chronobiol Int 17:103–128
Körtner G, Brigham RM, Geiser F (2000) Winter torpor in a large bird. Nature 407:318
Lovegrove BG, Körtner G, Geiser F (1999) The energetic costs of arousal from torpor in the marsupial Sminthopsis macroura: benefits of summer ambient temperature cycles. J Comp Physiol B 169:11–18
Mzilikazi N, Lovegrove BG (2002) Reproductive activity influences thermoregulation and torpor in pouched mice, Saccostomus campestris. J Comp Physiol B 172:7–16
McNab BK, Bonaccorso FJ (2001) The metabolism of New Guinean pteropodid bats. J Comp Physiol B 171:201–214
Nicol S, Andersen NA (2000) Patterns of hibernation of echidnas in Tasmania. In: Heldmaier G and Klingenspor M (eds), Life in the cold. 11th international hibernation symposium. Springer, Heidelberg pp 21–28
Schmid J (2000) Daily torpor in the grey mouse lemur (Microcebus murinus) in Madagascar: energetic consequences and biological significance. Oecologia 123:175–183
Smith AP, Winter J (1984) A key and field guide to the Australian possums, gliders and koala. In: Smith AP, Hume ID (eds) Possums and gliders. Australian mammal society and surrey beatty and sons, Chipping Norton, Australia, pp 579–594
Wang LCH (1978) Energetics and field aspects of mammalian torpor: the Richardsons’s ground squirrel. In: Wang LCH, Hudson JW (eds) Strategies in cold. Academic Press, New York, pp 109–145
Wang LCH (1989) Ecological, physiological, and biochemical aspects of torpor in mammals and birds. In: Wang LCH (ed) Animal adaptation to cold. Springer, Berlin pp 361–401
Willis CKR, Brigham RM, Geiser F (2006) Deep, prolonged torpor by pregnant, free-ranging bats. Naturwissenschaften 93:80–83
Withers PC (1992) Comparative animal physiology. Saunders, Fort Worth
Acknowledgments
We thank Chris Pavey and Craig Willis for constructive comments on the manuscript. The work was supported by a grant from the Australian Research Council to FG. The UNE Animal Ethics Committee and the National Parks and Wildlife Service of NSW approved experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Geiser, F., Holloway, J.C. & Körtner, G. Thermal biology, torpor and behaviour in sugar gliders: a laboratory-field comparison. J Comp Physiol B 177, 495–501 (2007). https://doi.org/10.1007/s00360-007-0147-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-007-0147-6