Abstract
A widely held view is that torpor is avoided by mammals whenever possible because of potential costs associated with reduced body temperatures and slowed metabolic processes. We examined this hypothesis by quantifying use of torpor in relation to body condition of free-ranging northern long-eared bats (Nyctophilus bifax, approximately 10 g), a species known to hibernate, from a subtropical region during the austral summer when insects were abundant. Temperature-telemetry revealed that bats used torpor on 85% of observation days and on 38% of all nights. Torpor bouts ranged from 0.7 to 21.2 h, but the relationship between duration of torpor bouts and ambient temperature was not significant. However, skin temperature of torpid bats was positively correlated with ambient temperature. Against predictions, individuals with a high body condition index (i.e., good fat/energy reserves) expressed longer and deeper torpor bouts and also employed torpor more often during the activity phase at night than those with low body condition index. We provide the first evidence that use of torpor in a free-ranging subtropical mammal is positively related with high body condition index. This suggests that employment of torpor is maximised and foraging minimised not because of food shortages or low energy stores but likely to avoid predation when bats are not required to feed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Torpor is the most effective energy-conserving strategy available to mammals, as it substantially lowers metabolic rate (MR), which is crucial for survival on limited resources (Frank 1994; Buck and Barnes 2000; Geiser 2004). However, torpor not only reduces energy requirements, but the low body temperatures (Tb) and MR during torpor may cause potential damage to cells and organs and, therefore, the costs and benefits arising from torpor should be balanced depending on energy availability and requirements (Humphries et al. 2003). Specifically, reductions in Tb and MR can suppress the immune system, may cause neurological damage, and may impair several other important bodily functions (Prendergast et al. 2002; Arendt et al. 2003; Luis and Hudson 2006). Consequently, it is widely believed that torpor is generally employed as a last resort when energy supplies are critically low and is avoided at other times whenever possible (French 1976). Recent evidence has shown that, for example, free-ranging chipmunks (Tamias striatus) hibernate less, and torpor is shallower in years when food availability is high, in comparison to years when food is limited (Landry-Cuerrier et al. 2008). Similarly, sugar gliders (Petaurus breviceps) predominantly employ torpor when foraging and feeding is limited by cold and wet weather (Körtner and Geiser 2000; Christian and Geiser 2007).
In contrast, there are other species that appear to employ torpor under mild conditions and without apparent energetic stress. For example, small insectivorous marsupials enter spontaneous torpor (food available) under mild conditions in the laboratory (Song and Geiser 1997), likely an adaptation to deal with the low food availability in their native habitat in the Australian desert where they may express torpor on every day in winter (Warnecke et al. 2008; Körtner and Geiser 2009). Captive ground squirrels (Spermophilus spp.) hibernate even when food is freely available (Geiser and Kenagy 1990), and dormice (Glis glis) undergo a period of dormancy with multi-day torpor bouts in summer even when they are in good condition and ambient temperature (Ta) is high (Bieber and Ruf 2009). Further, there are a few observations of torpor use under mild conditions by tropical primates (Dausmann 2008; Schmid and Ganzhorn 2009) and subtropical insectivorous bats, such as Vespadelus pumilus and Mops condylurus, which enter shallow torpor even in summer (Bronner et al. 1999; Turbill et al. 2003b; Vivier and van der Merwe 2007).
As quantitative data on summer torpor use specifically by free-ranging subtropical bats are scarce, the purpose of our study was to examine whether the insectivorous northern long-eared bat, Nyctophilus bifax, employs torpor during summer and, if so, to quantify patterns of torpor. We examined N. bifax in a subtropical region of Australia, an area where this species undergoes multi-day deep torpor in winter and therefore classifies as a hibernator (Stawski et al. 2009). As this microbat roosts in trees, we predicted that weather patterns would strongly influence the use of torpor and other aspects of thermal biology. Further, because no information is currently available on torpor expression in relation to the body condition of subtropical bats, we aimed to quantify interrelations between torpor patterns and body condition of individuals to re-examine the cost-benefit hypothesis of torpor. In agreement with the cost-benefit hypothesis, we predicted that individuals in poor body condition would use deeper, longer, and more frequent torpor than those in good body condition.
Methods
The study was conducted in Iluka Nature Reserve (29°24′S, 153°22′E) in Australia, near the southern limit of the geographic distribution of N. bifax and is subtropical according to the Köppen Climate Classification. Our summer (austral) study was undertaken from 4 February to 2 March 2008. During this time, Ta, measured with temperature dataloggers (±0.5°C, iButton thermochron DS1921G, Maxim Integrated Products, Inc., Sunnyvale, CA, USA) in the shade 2 m above the ground, ranged from 15.0°C to 29.5°C. The average daily (24 h) Ta during the study period was 21.2°C and the average nightly (from sunset to sunrise) Ta was 20.1°C.
Six adult female and seven adult male bats were captured with mist nets. Bats were weighed using a pro-Fit™ electronic scale (0.1 g resolution) and their forearms measured with digital callipers (HengLiang, 0.01 mm resolution). Skin temperature (Tskin) data for N. bifax were obtained by fitting each individual with a temperature-sensitive radio transmitter (approximately 0.5 g, LB-2NT, Holohil Systems Inc., Carp, ON, Canada). After removal of a small patch of fur, transmitters were attached to the skin of bats in the mid-dorsal region using a latex adhesive (SkinBond, Smith and Nephew United, Mount Waverley, NSW, Australia). External transmitters were used to enhance transmitter range and because of the difficulties of implanting transmitters into small bats. The Tskin of resting or torpid small mammals is typically <2.0°C cooler than core Tb (Barclay et al. 1996; Körtner and Geiser 2000), and therefore, a reliable measure for quantifying torpor patterns. Transmitters were calibrated to the nearest 0.1°C at temperatures between 5.0°C and 40.0°C in a water bath against a precision thermometer before attachment to bats. Two transmitters, which were worn by bats and shed, were re-calibrated 7 and 19 days after initial calibration and had drifted by <1.0°C over the entire temperature range.
Once transmitters were attached, bats were released at the site of capture. Bats were tracked to their roost locations on all days when they carried transmitters, which ranged from 1 to 12 days per individual (mean 5.7 ± 3.2 days, n = 13) and was limited by transmitter failure or loss. The Tskin of each bat was recorded once every 10 min by a remote receiver/logger when bats were in reception range (Körtner and Geiser 2000). These receiver/loggers were moved if necessary to ensure they remained in range of the transmitter signal. Data were downloaded every 2 to 4 days from the receiver/loggers to a laptop computer.
Torpor entries and arousals were calculated from the time Tskin fell below and subsequently rose above 28.0°C. As many studies use a Tb of 30.0–31.0°C for defining the temperature threshold for torpor (Barclay et al. 2001), and with a typical Tb–Tskin differential of <2.0°C, this definition for torpor appeared appropriate.
Body condition index (BCI) was used to determine the approximate amount of fat reserves of individual bats at capture and therefore adjusted body mass for size. BCI was calculated by the following equation: \( {\text{BCI = bm}}\left( {\text{g}} \right){\text{/fa}}\left( {\text{mm}} \right) \)where bm is body mass and fa is forearm length (Speakman and Racey 1986). As the forearm length was not obtained for one individual, this bat was not included in BCI analyses. We excluded another animal from the night time torpor analysis because data were obtained for only 1 day. We assumed that BCI measured at initial capture during the current study was a reasonable approximation of BCI over the maximum period of 12 days for which we obtained torpor data, because the mean standard deviation of BCI measurements taken daily from four individuals of N. bifax kept in captivity during summer over a 2-week period changed little and was only 7% of the mean BCI of all individuals (Stawski, unpublished data). These captive individuals experienced days when they were fed and days without food, which is comparable to what this species seems to experience in the wild.
Insects were sampled at night throughout this study and also during winter (June 2009) using two insect traps with a 12-V ultraviolet light over three nights in each season (Turbill 2008). Insects were sampled for 4 h after sunset. This time period seemed appropriate as previous studies have shown that insect abundance peaks just after sunset and declines to minimal levels about 4 h later, and bats forage mostly during this time period as well (O’Shea and Vaughan 1977; Taylor and O’Neill 1988). For data analysis, the samples from two insect traps were pooled for each night.
Minitab Statistical Software (version 13.1, 2000) was used to obtain descriptive statistics and to conduct t tests and linear regressions. When physiological variables (torpor bout durations and Tskin) of males and females were statistically compared, no significant differences were observed, and as summer is a non-reproductive time of year for both males and females of N. bifax (Churchill 1998), sexes were pooled for further analyses. The MIXED procedure in SAS (version 9.01, 2001) was used to examine individuals as a random effect in a mixed model for linear regressions. The following equation was used for the model: \( {Y_{ijk}} = \mu + {T_i} + {A_j} + {e_{ijk}} \) where Y ijk is the observation value, µ is the overall mean of the tested variable, T i is the fixed effect of Ta, A j is the random effect of individual, and e ijk is the error. For linear regressions involving measures of BCI, a single mean for each individual for each of the variables (e.g., torpor bout duration) was used. We report data as means ± SD for n = the number of individuals. The mean of the values for each individual were used when calculating group means ± SD to account for repeated measures in individuals. The null hypothesis was rejected if the significance level was P < 0.05.
Results
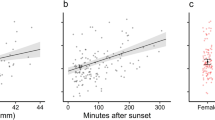
Brief bouts of torpor were frequently employed by all monitored long-eared bats, even in the subtropical summer. Bats used torpor on 85% of observation days and on 38% of all nights. Daily patterns of Tskin fluctuations varied (Fig. 1), and all bouts of torpor lasted for less than a day, ranging from 0.7 to 21.2 h. Torpor bout duration (log10) was not significantly affected by Ta when the random effect of individuals was taken into account (n = 13; P = 0.2; Fig. 2). In contrast, daily minimum Tskin of torpid bats was positively correlated with the corresponding Ta, and this relationship was significant when the random effect of individuals was taken into account (n = 13; F 1,56 = 41.9, P < 0.001, R 2 = 0.4; Fig. 3).
Tskin of free-ranging Nyctophilus bifax (upper trace, dotted line) and Ta (lower trace, smooth line) over 1 day showing: (a) typical w-pattern of torpor with one early morning torpor bout and one afternoon torpor bout, (b) a single early morning torpor bout, (c) remaining torpid throughout the day, (d) remaining normothermic throughout the day. The dashed line represents 28°C and the black and white bars represent night and day, respectively
Torpor bout duration (log10) as a function of mean Ta during each torpor bout for Nyctophilus bifax. There was no significant relationship between the two variables when the random effect of all individuals was considered (n = 13; P = 0.2). This data set was log transformed as torpor bout duration often increases exponentially with decreasing temperature
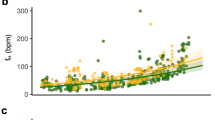
BCI was negatively correlated with the proportion of time bats spent normothermic over 24 h (n = 12; F 1,11 = 7.0, P = 0.02, R 2 = 0.4; Fig. 4a) and was positively correlated with the proportion of all nights a bat used torpor (n = 11; F 1,10 = 16.7, P = 0.003, R 2 = 0.7; Fig. 4b). Further, BCI was positively correlated with the mean duration of all torpor bouts of each individual (n = 12; F 1,11 = 5.5, P = 0.04, R 2 = 0.4; Fig. 4c). In contrast, the mean Tskin for all torpor bouts of each individual showed a negative correlation with BCI (n = 12; F 1,11 = 6.0, P = 0.04, R 2 = 0.4; Fig. 4d).
The effect of body condition index (BCI) of individual bats on the proportion of time bats spent normothermic over 24 h (a n = 12; proportion of 24 h normothermic = 2.07 − 0.144 × [BCI]; F 1,11 = 7.0, P = 0.02, R 2 = 0.4), the proportion of all nights a bat used torpor (b n = 11; proportion of all nights torpid = −3.13 + 0.377 × [BCI]; F 1,11 = 16.7, P = 0.003, R 2 = 0.7), torpor bout duration (c n = 12; torpor bout duration [h] = −5.57 + 0.929 × [BCI]; F 1,11 = 5.5, P = 0.04, R 2 = 0.4), and mean Tskin during torpor bouts (d n = 12; mean Tskin [°C] = 33.9 − 0.869 × [BCI]; F 1,11 = 6.0, P = 0.04, R 2 = 0.4)
The number of insects captured during the current summer study (482.0 ± 164.0, n = 3) was significantly higher (14-fold) than the number of insects captured during winter (33.7 ± 19.1, n = 3; P < 0.001, T = 4.7, df = 4). Therefore, insects were considered abundant during our summer study.
Discussion
Our findings demonstrate for the first time that free-ranging subtropical bats readily employ torpor during a period of food abundance in summer, especially when their BCI is high. Specifically, BCI, a measure of body condition and fat storage, was positively related to torpor bout duration and how often night torpor was used, and negatively to mean Tskin during torpor bouts. Our findings demonstrate that free-ranging bats employ torpor especially when they are in good condition and at a time when insects are abundant. These results do not support the view that, generally, mammals use torpor sparingly or not at all, unless they are in negative energy balance and/or when weather conditions are unfavourable.
The frequent use of torpor in the current study by N. bifax (85%) is only slightly less than that by the congener Nyctophilus geoffroyi in a cool temperate region, which employed torpor on every day during summer (Turbill et al. 2003a). However, unlike temperate N. geoffroyi that occasionally showed multi-day torpor bouts during summer, subtropical N. bifax only expressed torpor bouts that lasted for less than a day during summer, although they do exhibit multi-day torpor bouts during winter hibernation (Stawski et al. 2009). Torpor use during the current study was most common during the day when bats are generally inactive. During the night, when bats are typically active, N. bifax employed torpor on only 38% of all observation nights, suggesting that the weather at night was mild enough to forage throughout the entire or much of the night. In several species of bats and other mammals, the duration of torpor bouts increases with decreasing Ta (Twente and Twente 1965; Park et al. 2000; Turbill et al. 2003a; Rambaldini and Brigham 2008). However, this relationship was not significant for N. bifax during summer suggesting that the Ta range in our study was too narrow to affect torpor bout duration. In contrast, Tskin during torpor generally followed fluctuations of the environmental temperature and, as in previous studies, minimum Tskin (Tb in some studies) was positively correlated with the corresponding Ta (Arlettaz et al. 2000; Geiser and Brigham 2000; Willis et al. 2005; Rambaldini and Brigham 2008; Stawski et al. 2009). This shows that although temporal aspects of torpor were not affected by Ta, it did affect the depth of bouts, and that individuals allowed their Tskin to fall near Ta, thus maximising energy savings.
Whereas Ta had no significant effect on torpor bout duration, body condition did. Individual N. bifax with a high BCI expressed longer and deeper bouts of torpor than individuals with a low BCI, remained normothermic for shorter periods, and also entered torpor more frequently during the foraging period at night. Further, the lower mean Tskin experienced during all torpor bouts by individuals with a higher BCI (Fig. 4d) reflects that they were entering torpor more frequently at night during the activity period, when Ta and therefore Tskin will be lowest. This is the exact opposite of our prediction and that of the cost-benefit hypothesis, which proposes that individuals with high energy reserves should display less and shallower torpor than those with limited access to energy (Humphries et al. 2003). This hypothesis was primarily based on observations on mammals from temperate regions and has been supported by data from several different species (Körtner and Geiser 2000; Boyles et al. 2007; Landry-Cuerrier et al. 2008). Sugar gliders are daily heterotherms and employ torpor only under adverse conditions when they are unable to forage (Körtner and Geiser 2000). It also seems appropriate that during winter, fat-storing hibernating temperate bats select slightly colder hibernacula when they have limited energy reserves likely to maximise energy savings (Boyles et al. 2007), as food sources available to replenish their fat stores are limited. Food-caching hibernating mammals are also more likely to use torpor only when they are energetically constrained and generally prefer to remain normothermic or employ short and shallow bouts of torpor if they have enough stored food in their hibernacula (French 1976; Landry-Cuerrier et al. 2008). In contrast, our study was undertaken on a fat-storing hibernating subtropical bat during summer, when its food source was abundant and, therefore, they were able to replenish their fat stores. Clearly, this shows that bats in our study did not employ torpor as a last resort.
As many insects decrease activity at low Ta, reducing the forage that is available to bats on cool nights, the potential benefits derived from foraging will be marginal (Taylor 1963; Turbill et al. 2003a). Therefore, it seems to makes sense for the bats in our study to be flexible in their use of torpor to suit prevailing weather conditions, insect availability and, importantly, their body condition. While torpor may be most useful on nights with low insect abundance for individuals with high BCI, individuals with low BCI apparently must continue to forage to meet their energy demands. This is supported by a previous study showing that arousal times in hibernating greater horseshoe bats (Rhinolophus ferrumequinum) in winter are influenced by BCI, such that bats with low BCI were more likely to arouse at sunset than individuals with high BCI (Park et al. 2000). Therefore, similarly to our current study, it seems that greater horseshoe bats with enough energy reserves also avoid arousal from hibernation to forage if they do not need to, whereas those individuals with low energy reserves must forage. When animals forage, the risk of being eaten by a predator generally increases (Abrahams and Dill 1989; Brigham et al. 2000). As torpor decreases foraging requirements, its use likely will enhance predator avoidance (Bieber and Ruf 2009), which may in turn increase the long-term survival rate in comparison to individuals that need to forage frequently (Geiser and Turbill 2009). Hibernation does not increase mortality in some bats, but it does significantly increase the lifespan of bats (Wilkinson and South 2002; Sendor and Simon 2003). One of the possible reasons for this increased longevity is that torpid bats are well concealed from predators (Barclay and Harder 2003), which reduces the risk of predation in comparison to foraging bats. Thus, for the species investigated here, the cost-benefit hypothesis seems reversed as they maximise torpor use at night if good body condition allow them to minimise foraging, likely to avoid predation by owls.
In summary, recently emerging data suggests that the use of torpor by microbats, and other animals, is more widely employed than was previously thought and is not necessarily a response to cold temperatures or adverse conditions. Recent evidence also suggests that free-ranging microbats inhabiting subtropical regions employ torpor regularly during summer, even though conditions appear favourable for remaining normothermic. Our study supports these findings and adds new data showing that especially individual N. bifax that have high BCI enter longer and deeper torpor than those with a lower BCI. Our study demonstrates that torpor is not always used as a last resort, and it is likely that further research on other species will identify multiple functions and purposes of mammalian torpor.
Abbreviations
- MR:
-
Metabolic rate
- Ta :
-
Ambient temperature
- Tb :
-
Body temperature
- Tskin :
-
Skin temperature
- BCI:
-
Body condition index
References
Abrahams MV, Dill LM (1989) A determination of the energetic equivalence of the risk of predation. Ecology 70:999–1007
Arendt T, Stieler J, Strijkstra AM, Hut RA, Rüdiger J, Van der Zee EA, Harkany T, Holzer M, Härtig W (2003) Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci 23:6972–6981
Arlettaz R, Ruchet C, Aeschimann J, Brun E, Genoud M, Vogel P (2000) Physiological traits affecting the distribution and wintering strategy of the bat Tadarida teniotis. Ecology 81:1004–1014
Barclay RMR, Harder LD (2003) Life histories of bats: life in the slow lane. In: Kunz TH, Fenton MB (eds) Bat ecology. University of Chicago Press, Chicago, pp 209–253
Barclay RMR, Kalcounis MC, Crampton LH, Stefan C, Vonhof MJ, Wilkinson L, Brigham RM (1996) Can external radiotransmitters be used to assess body temperature and torpor in bats? J Mammal 77:1102–1106
Barclay RMR, Lausen CL, Hollis L (2001) What’s hot and what’s not: defining torpor in free-ranging birds and mammals. Can J Zool 79:1885–1890
Bieber C, Ruf T (2009) Summer dormancy in edible dormice (Glis glis) without energetic constraints. Naturwissenschaften 96:165–171
Boyles JG, Dunbar MB, Storm JJ, Brack VJ (2007) Energy availability influences microclimate selection of hibernating bats. J Exp Biol 210:4345–4350
Brigham RM, Körtner G, Maddocks TA, Geiser F (2000) Seasonal use of torpor by free-ranging Australian owlet-nightjars (Aegotheles cristatus). Physiol Biochem Zool 73:613–620
Bronner GN, Maloney SK, Buffenstein R (1999) Survival tactics within thermally-challenging roosts: heat tolerance and cold sensitivity in the Angolan free-tailed bat, Mops condylurus. S Afr J Zool 34:1–10
Buck CL, Barnes BM (2000) Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol 279:R255–R262
Christian N, Geiser F (2007) To use or not to use torpor? Activity and body temperature as predictors. Naturwissenschaften 94:483–487
Churchill S (1998) Australian bats. New Holland Publishers (Australia) Pty Ltd, Sydney
Dausmann KH (2008) Hypometabolism in primates: torpor and hibernation. In: Lovegrove BG, McKechnie AE (eds) Hypometabolism in animals: hibernation, torpor and cryobiology. University of KwaZulu-Natal, Pietermaritzburg South Africa, pp 327–336
Frank CL (1994) Polyunsaturate content and diet selection by ground squirrels (Spermophilus lateralis). Ecology 75:458–463
French AR (1976) Selection of high temperatures for hibernation by the pocket mouse, Perognathus longimembris: ecological advantages and energetic consequences. Ecology 57:185–191
Geiser F (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66:239–274
Geiser F, Brigham RM (2000) Torpor, thermal biology, and energetics in Australian long-eared bats (Nyctophilus). J Comp Physiol B 170:153–162
Geiser F, Kenagy GJ (1990) Development of thermoregulation and torpor in the golden-mantled ground squirrel Spermophilus saturatus. J Mammal 71:286–290
Geiser F, Turbill C (2009) Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften. doi:10.1007/s00114-009-0583-0
Humphries MM, Thomas DW, Kramer DL (2003) The role of energy availability in mammalian hibernation: a cost-benefit approach. Physiol Biochem Zool 76:165–179
Körtner G, Geiser F (2000) Torpor and activity patterns in free-ranging sugar gliders Petaurus breviceps (Marsupialia). Oecologia 123:350–357
Körtner G, Geiser F (2009) The key to winter survival: daily torpor in a small arid-zone marsupial. Naturwissenschaften 96:525–530
Landry-Cuerrier M, Munro D, Thomas DW, Humphries MM (2008) Climate and resource determinants of fundamental and realized metabolic niches of hibernating chipmunks. Ecology 89:3306–3316
Luis AD, Hudson PJ (2006) Hibernation patterns in mammals: a role for bacterial growth? Funct Ecol 20:471–477
O’Shea TJ, Vaughan TA (1977) Nocturnal and seasonal activities of the Pallid bat, Antrozous pallidus. J Mammal 58:269–284
Park KJ, Jones G, Ransome RD (2000) Torpor, arousal and activity of hibernating Greater Horseshoe Bats (Rhinolophus ferrumequinum). Funct Ecol 14:580–588
Prendergast BJ, Freeman DA, Zucker I, Nelson JR (2002) Periodic arousal from hibernation is necessary for initiation of immune response in ground squirrels. Am J Physiol Regul Integr Comp Physiol 282:R1054–R1062
Rambaldini DA, Brigham RM (2008) Torpor use by free-ranging pallid bats (Antrozous pallidus) at the northern extent of their range. J Mammal 89:933–941
Schmid J, Ganzhorn JU (2009) Optional strategies for reduced metabolism in gray mouse lemurs. Naturwissenschaften 96:737–741
Sendor T, Simon M (2003) Population dynamics of the pipistrelle bat: effects of sex, age and winter weather on seasonal survival. J Anim Ecol 72:308–320
Song X, Geiser F (1997) Daily torpor and energy expenditure in Sminthopsis macroura: interactions between food and water availability and temperature. Physiol Zool 70:331–337
Speakman JR, Racey PA (1986) The influence of body condition on sexual development of male brown long-eared bats (Plecotus auritus) in the wild. J Zool 210:515–525
Stawski C, Turbill C, Geiser F (2009) Hibernation by a free-ranging subtropical bat (Nyctophilus bifax). J Comp Physiol B 179:433–441
Taylor LR (1963) Analysis of the effect of temperature on insects in flight. J Anim Ecol 32:99–117
Taylor RJ, O’Neill MG (1988) Summer activity patterns of insectivorous bats and their prey in Tasmania. Aust Wildl Res 15:533–539
Turbill C (2008) Winter activity of Australian tree-roosting bats: influence of temperature and climatic patterns. J Zool 276:285–290
Turbill C, Körtner G, Geiser F (2003a) Natural use of heterothermy by a small, tree-roosting bat during summer. Physiol Biochem Zool 76:868–876
Turbill C, Law BS, Geiser F (2003b) Summer torpor in a free-ranging bat from subtropical Australia. J Therm Biol 28:223–226
Twente JW, Twente JA (1965) Regulation of hibernating periods by temperature. Proc Natl Acad Sci USA 54:1058–1061
Vivier L, Van Der Merwe M (2007) The incidence of torpor in winter and summer in the Angolan free-tailed bat, Mops condylurus (Microchiroptera: Molossidae), in a subtropical environment, Mpumulanga, South Africa. Afr Zool 42:50–58
Warnecke L, Turner JM, Geiser F (2008) Torpor and basking in a small arid zone marsupial. Naturwissenschaften 95:73–78
Wilkinson GS, South JM (2002) Life history, ecology and longevity of bats. Aging Cell 1:124–131
Willis CKR, Turbill C, Geiser F (2005) Torpor and thermal energetics in a tiny Australian vespertillonid, the little forest bat (Vespadelus vulturnus). J Comp Physiol B 175:479–486
Acknowledgments
We would like to thank Margaret Stawski for all her help with fieldwork. We are also grateful to Gerhard Körtner, Brad Law, Alexander Riek, Michal Stawski, Christopher Turbill, and Courtney Waugh for their contributions to this study. This research was undertaken in agreement with permits issued by New South Wales National Parks and Wildlife Service and the Animal Ethics Committee of the University of New England. The study was supported by grants from the University of New England and Bat Conservation International to CS and the Australian Research Council to FG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stawski, C., Geiser, F. Fat and fed: frequent use of summer torpor in a subtropical bat. Naturwissenschaften 97, 29–35 (2010). https://doi.org/10.1007/s00114-009-0606-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-009-0606-x