Abstract
Timing of winter phenotype expression determines individual chances of survival until the next reproductive season. Environmental cues triggering this seasonal phenotypic transition have rarely been investigated, although they play a central role in the compensation of climatic fluctuations via plastic phenotypic adjustments. Initiation of winter daily torpor use—a widespread energy-saving phenotype—could be primarily timed according to anticipatory seasonal cues (anticipatory cues hypothesis), or flexibly fine-tuned according to actual energy availability (food shortage hypothesis). We conducted a food supplementation experiment on wild heterothermic primates (grey mouse lemurs, Microcebus murinus) at the transition to the food-limited dry season, i.e. the austral winter. As expected under the food shortage hypothesis, food-supplemented individuals postponed the seasonal transition to normal torpor use by 1–2 month(s), spent four times less torpid, and exhibited minimal skin temperature 6 °C higher than control animals. This study provides the first in situ experimental evidence that food availability, rather than abiotic cues, times the launching of torpor use. Fine-tuning of the timing of seasonal phenotypic transitions according to actual food shortage should provide heterotherms with a flexible adaptive mechanism to survive unexpected environmental fluctuations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how individuals allocate energy between competing life-history traits (Stearns 1992) and under changing environmental conditions (Visser et al. 2010; Lane et al. 2012) are key theoretical challenges in ecology. Organisms have to adjust the timing of major biological events requiring high energy expenditure to the seasonality of their habitats (Miller-Rushing et al. 2010). Much research has focused on assessing selection for the timing of reproduction, particularly impacts of climate change at uncoupling phenologies of resources and consumers (Miller-Rushing et al. 2010; Visser et al. 2010; Sheriff et al. 2011). But to reproduce, iteroparous organisms also need to survive the unfavourable season, i.e. winter (e.g. Patil et al. 2013). Hence, the first crucial seasonal transition is the switch to a winter phenotype, defined as the suite of phenotypic changes (including gene expression, physiology, morphology, behaviour) that are expressed during the unfavourable season (Ozgul et al. 2010; Miller-Rushing et al. 2010; Helm et al. 2013).
Two main behavioural strategies have evolved to survive seasonal resource scarcity: migrate to more productive grounds or rely on energy-saving mechanisms. Timing of phenological transitions has major fitness consequences (Thomas et al. 2001; Visser et al. 2010), and flexibility of this timing is a key response to climatic variability (Canale and Henry 2010; Reed et al. 2010). Yearly migration by birds is the most studied seasonal phenotypic change among vertebrates (Gienapp et al. 2007). Non-migratory animals reduce activity and fuel metabolic needs with stored energy reserves to match energy expenditure with energy availability. Heterothermic endotherms further reduce their daily energy expenditure by entering a torpid state (Heldmaier et al. 2004; Geiser 2013), which increases survival (Turbill et al. 2011; Bieber et al. 2012). We refer to heterothermy as the faculty of some endotherms to undergo a controlled reduction of their body temperature during bouts of hibernation and daily torpor, in association with metabolism reduction (Heldmaier et al. 2004; Geiser 2013). Timing of switches to winter phenotype should be crucial for the chances of free-ranging heterotherms to survive and subsequently reproduce (Ozgul et al. 2010; Lane et al. 2012).
As heterothermy is generally expressed during harsh climatic conditions and/or lean periods (Körtner and Geiser 2000; Heldmaier et al. 2004; Vuarin and Henry 2014), it has commonly been regarded as an adaptive strategy to cope with energy shortage (Humphries et al. 2003a; Munro et al. 2005; Landry-Cuerrier et al. 2008; see Geiser 2013; Vuarin and Henry 2014, for other functions). But heterothermy comes with costs, such as oxidative stress, reduced immunocompetence or cognitive impairments (Humphries et al. 2003a; Angilletta et al. 2010). Hence, heterothermy use should be optimized according to current temperature and food constraints (Humphries et al. 2003a). For heterothermy to remain beneficial, organisms need to rely on environmental cues that allow them to accurately assess and anticipate forthcoming energetic constraints (Visser et al. 2010). A major risk when compensating environmental changes by phenotypic flexibility is to express a suboptimal phenotype that does not maximize fitness in the new environment (Auld et al. 2010; Reed et al. 2010). When studying phenotypically flexible traits, it is therefore crucial to identify the primary environmental cues that allow animals to accurately fine-tune seasonal adjustments of their phenotype (Piersma and Drent 2003; Canale and Henry 2010; Reed et al. 2010; Helm et al. 2013).
To date, it remains controversial whether heterothermy is mainly expressed in anticipation of upcoming seasonal unfavourable conditions, and triggered by fixed seasonal cues, or mainly a flexible thermal strategy that fine-tunes energy expenditure according to short-term fluctuations in energy availability (Vuarin and Henry 2014; Williams et al. 2014). Photoperiod is an ubiquitous, proximal environmental cue for seasonal acclimation (Helm et al. 2013). But proximate energetic constraints also influence the regulation of heterothermy. Decreasing air temperatures enhance heterothermy expression (e.g. Heldmaier et al. 2004; Landry-Cuerrier et al. 2008; Vuarin et al. 2013; Helm et al. 2013), and food shortage triggers its expression in captive mammals (see Vuarin and Henry 2014 for a review). Whether free-ranging heterotherms adjust their seasonal transition to regular hibernation/daily torpor use primarily according to seasonal food shortage (food shortage hypothesis) versus to other seasonal cues, such as photoperiod and weather conditions (anticipatory cues hypothesis), has never been tested experimentally.
In the wild, food supplementation during periods of natural food shortage is the most direct experimental approach to test for the dependence of heterothermy on energy availability (Humphries et al. 2003b; Vuarin and Henry 2014). The aim of the present study was to provide the first test of a direct dependence of the launching of torpor use on seasonal food availability under natural conditions. We experimentally manipulated food availability and quantified torpor use of grey mouse lemurs (Microcebus murinus; SEM Fig. S1) at the transition between the wet season and the dry season. This primate inhabits dry deciduous forests of western and southern Madagascar, where it experiences severe water and food shortage during the dry season, i.e. austral winter (Dammhahn and Kappeler 2008a). Grey mouse lemurs prepare in advance to this unfavourable season by a suite of physiological modifications (e.g. Génin and Perret 2003; Giroud et al. 2008, 2009; Canale et al. 2011), including fat deposition (Perret and Aujard 2001; Schmid 2001). Internal energy storage is complemented with torpor-based energy saving to maintain a favourable energy balance. Grey mouse lemurs enter torpor mostly on a daily basis (Schmid 2001; Fig. S3) with large inter-individual variation in its expression (Vuarin et al. 2013). Because they evolved in a highly seasonal environment, but with poorly predictable intra- and inter-annual fluctuations in resource availability (Dewar and Richard 2007), we predict that they directly cue in on local resource availability to time the launching of their winter phenotype (Dawson 2008; Canale and Henry 2010; Helm et al. 2013), i.e. the use of daily torpor during the dry season. Animals living in unpredictable environments are indeed expected to be more phenotypically flexible, and to rely more on proximate cues of resource constraints, than those from more predictable environments (Canale and Henry 2010; Florant and Healy 2012; Geiser 2013). Therefore, if food shortage is a major seasonal environmental constraint, we predict food-supplemented individuals to postpone their transition to winter phenotype, i.e. to minimize torpor use relative to control individuals exposed to the seasonal, gradual reduction in food availability.
Materials and methods
Study site and animals
The study was conducted in the dry deciduous forest of Kirindy/CNFEREF (44°39′E, 20°03′S, 30−60 m above sea level) in western Madagascar between March and October 2010. The climate is highly seasonal, with a hot wet season from December to March and a cool dry season with little or no rain from April to November (Dammhahn and Kappeler 2008a). Throughout the experiment from April to June, mean T a was 23.9 ± 7.4 °C, and it rained only twice, before the start of the experiment (13.7 mm on 12 March and 2.2 mm on 13 March).
Grey mouse lemurs forage in overlapping home ranges and do not cache food (Dammhahn and Kappeler 2008b). Hence, our experimental design had to be based on the comparison of daily torpor patterns between a control plot (named N5) and a plot with food supplementation (named CS5). The two plots are ca. 2 km apart within a continuous forest and defined by boundaries of grid systems (500 × 500 m) of small foot trails at 25-m intervals (Dammhahn and Kappeler 2008a). We compared the two plots in terms of population density, vegetation structure, food availability, air temperature, and grey mouse lemurs’ condition and torpor use (ESM1, Table S1). The only notable difference was that, before the experiment, body condition of individuals from the control plot was 22 % higher than in individuals from the food-supplemented plot. Unfortunately, we do not have the necessary data to document the temporal covariation between body condition and torpor use throughout the experiment. Because food supplementation is expected to induce a rapid modification of body condition, body condition at capture is likely to be a poor predictor of body condition days and weeks later during the experiment. We still assessed the robustness of our results to initial differences in body condition between plots (ESM2). Because effects related to body condition were non-significant (Table S2), and because the sign, strength and significance of effects of the other variables remained similar after the addition of a body condition effect (Table S3), we are confident that the effect of food supplementation in our experimental design was not confounded by ecological or physiological differences between the two plots.
Study animals were captured between 27 March and 8 April (1 April ± 4 days), using 120 Sherman live traps baited with banana, opened at dusk and collected at dawn. Captured individuals were kept in their trap for the day. Upon first capture, individuals were briefly anesthetized (10 μl Ketanest 100), marked individually with subdermally implanted microtransponders (Trovan, Germany), and measured (including body mass and head width; Dammhahn and Kappeler 2008a; Vuarin et al. 2013). Forty-three adult (i.e. >50 g) individuals, 19 from the control plot and 24 from the food-supplemented plot, were equipped with collar-mounted temperature loggers (~3 g Weetag Lite; AlphaMach, Sainte-Julie, Canada; Fig. S1), which recorded skin temperature (T sk) every 10 min. T sk is a reliable indicator of core body temperature, at least for the characterization of daily torpor use (Dausmann 2005). All captured individuals were released at their site of capture shortly before dusk on the same day. At the end of the food supplementation experiment, study animals were recaptured between 9 June and 3 November (12 August ± 60 days) to retrieve the temperature loggers. Twenty-one individuals still equipped with loggers were recaptured [8 loggers out of 21 were retrieved 13 ± 4 days (range 9–19 June) before the end of the food supplementation to maximize recovery rates as some individuals had already lost their collars at the beginning of June]. Twelve other individuals were recaptured but had lost their collars, and 10 individuals disappeared. T sk data could be recovered from all loggers but one. Statistical analyses are based on 9 individuals from the control plot (5 females, 4 males) and 11 from the food-supplemented plot (4 females, 7 males), for which T sk was recorded over 68 ± 14 days (range 15–76 days; daily sample sizes per plot are presented in Table S4).
Food supplementation design
Food supplementation started on 8 April and lasted until 23 June (77 days) (Table S4). In the food-supplemented plot, food was provided daily at 12 feeding platforms (Fig. S2) that were distributed across the plot according to locations of mouse lemurs aggregates. Grey mouse lemurs mainly forage on fruits and invertebrates (Dammhahn and Kappeler 2008a). Food supplementation consisted of a mix of smashed banana, raisins and cat pellets (ca. 395 kJ per feeder) provided every second day, and only smashed banana (ca. 144 kJ per feeder) provided on the following day. Mean daily energetic provisioning was ca. 270 kJ day−1 per feeder, which corresponds to daily energetic requirements of 3–4 individuals of 50 g body mass (Schmid and Ganzhorn 2009; Canale et al. 2011). Feeding platform design demonstrated that they were highly attractive, and safe, for grey mouse lemurs, while preventing access by larger animals (Fig S2). Similar-sized species were unlikely to feed at the platforms either because the type of food provided did not match their diet (Microgale and Geogale tenrecs, tufted-tailed rats Eliurus myoxinus), or because they were inactive at that time of the year (fat-tailed dwarf lemurs, Cheirogaleus medius). For logistical reasons, food provisioning was interrupted for three consecutive nights every second week (17–19 April, 1–3 May, 15–17 May, 29–31 May and 12–14 June; Table S4).
Characterization of torpor use
Continuous profiles of T sk (see Tsk_rawdata; ESM) were used to compute three variables that quantify the daily use of torpor (see Dataset; ESM). First, we determined whether an individual entered torpor or not on a given day (i.e. individual probability to enter torpor: P torp, binomial variable; Table S4). Animals were considered torpid when their T sk dropped below 30 °C (following Schmid 2001). When T sk oscillated around 30 °C (184 measures of 1357), we applied the rules presented in Fig. S3 to diagnose torpor bouts. Second, for each identified torpor event, we computed the duration of torpor bout (D torp, in hours ±0.1), which corresponds to the time spent below 30 °C during a day. These definitions of P torp and D torp allowed testing for the separate effect of food supplementation on the probability to enter torpor (P torp) and the amount of time allocated to torpor by a torpid individual. Third, we also analysed minimal skin temperature (T sk min, °C), which objectively quantifies hypothermia without relying on a threshold temperature. During torpor bouts, T sk min quantifies torpor depth (Vuarin et al. 2013).
Daily torpor use strongly depends on air temperature: the lower T a during the resting phase, the longer and the deeper torpor bouts (Heldmaier et al. 2004; Geiser 2013; Vuarin et al. 2013). To adjust fluctuations in daily torpor use for temporal fluctuations in T a, we recorded T a at resting sites in both plots. As grey mouse lemurs generally rest in tree holes (Schmid 1998), we placed a temperature logger (iButtons; Maxim Integrated, USA) in four tree holes per plot (as in Vuarin et al. 2013). T a was recorded every 20 min throughout the study period. We considered daily minimum T a (T a min) in tree holes as a reliable index of the thermal constraint faced by resting animals, since T sk min is achieved during the coldest hours of the day (i.e., when T a is the lowest; Schmid 2001; Vuarin et al. 2013). Daily T a min values for each plot were averaged across the four corresponding temperature loggers. Thermal conditions were similar and highly correlated between the two plots (Fig. 1; Table S1; Pearson’s R = 0.92, p < 0.01).
Statistical analyses
As we recorded temporally repeated measures of P torp, D torp and T sk min for the same individuals, we applied mixed effects models with individual identity entered as a random intercept to account for the within-individual non-independence among data points.
First, we tested whether torpor use (P torp, T sk min) differed between the control and food-supplemented plots over the 9 days preceding the experiment (9 and 7 individuals, respectively). Difference in D torp could not be tested for since only a single individual used torpor once in the food-supplemented plot. Second, we tested our predictions about the negative effect of food supplementation on torpor use by analysing P torp, D torp and T sk min data collected after the start of the experiment, from 9 April to 23 June. P torp data were analysed with generalized linear mixed effects models fitted with a binomial distribution, using the ‘lmer’ function of ‘lme4’ package (v.0.999999-2) for R, whereas both D torp and T sk min data were analysed using linear mixed effects models using the ‘lme’ function of ‘nlme’ package (v.3.1-109) for R. We adjusted torpor variables for the additive fixed effects of time (assuming a linear effect of the number of days since the start of the experiment with 9 April set to 1; temporal patterns other than linearity did not receive a better statistical support), sex and daily T a min, and their interactions (time and sex, time and T a min, T a min and sex). These variables influence daily torpor use: (1) low T a is associated with longer and deeper torpor bouts, (2) female grey mouse lemurs are less active in the dry season and, thus, might express more torpor than males (Schmid and Kappeler 1998; Kraus et al. 2008; Dammhahn and Kappeler 2008b; Terrien et al. 2010), and (3) torpor is maximal during the height of the dry season (Schmid 2001).
To test our predictions, we added the fixed effect of experimental treatment (natural food availability, named ‘control’ versus food supplementation, named ‘food-supplemented’), and its interactions with other fixed effects: (1) between treatment and time to account for a potential change of the effect of treatment throughout the study period, (2) between treatment and sex because females are more prone to use daily torpor, and therefore might show a stronger response to the treatment than males, and (3) between treatment and T a min as the effect of treatment could be countered by low T a. Finally, we also tested for an interaction (1) between treatment, time and sex effects because the sex-dependence of the response to the treatment could change over time, and (2) between time, sex and T a min effects because the sex-dependence of the sensitivity to T a could change over time. To account for remaining non-independence between data points due to temporal autocorrelation, we also parameterised an auto-regressive structure of order 1 (‘corAR1’ function) for temporal dependence between residuals in the analyses of D torp and T sk min (see Vuarin et al. 2013 for details). For the treatment effect, the control plot was defined as intercept.
Final models were obtained after removal of all non-significant interactions and additive effects based on likelihood ratio tests between nested models. We proceeded in three steps: assessment of third order interactions, then of second order interactions and, eventually, of additive effects in presence of significant, non-nested interactions. At each step, the most parameterised model included all terms, so that each effect was assessed with a model adjusted for all others. The significance level was set to p < 0.05. We checked whether residuals of each final model followed a Gaussian distribution. Slopes of effects (β), and their standard errors (SE), are presented for full models and final models to allow assessment of the effects to the potential weaknesses of downward, stepwise model selection. Torpor parameter estimates are presented as mean ± standard deviation (SD).
Results
Over the 9 days before the start of the experiment, torpor parameters did not differ between the two plots (respectively for P torp, and T sk min: \(\chi^{2}_{ 1}\) = 0.74, p = 0.39, β = 1.75 ± 2.55 and \(\chi^{2}_{ 1}\) = 0.78, p = 0.38, β = −0.50 ± 0.62 °C). The percentage of individuals entering torpor on a given day (P torp) was 12.5 % in the control plot and 3.2 % in the food-supplemented one (respectively, 3 of 9, and 1 of 7 individuals entered torpor at least once). T sk min was 30.6 ± 1.6 °C in the control plot and 31.1 ± 1.0 °C in the food-supplemented one. D torp was 1.5 ± 1.3 h in the control plot and 0.5 h in the food-supplemented plot for the single individual that used torpor once.
After the start of food supplementation, torpor use rapidly differed between the two plots (Fig. 2). P torp was much lower in the food-supplemented plot than in the control one (Table 1). On average throughout the experiment, control individuals expressed torpor on 93.4 % of the days sampled (n = 672 individual days), whereas food-supplemented individuals expressed torpor on only 27.5 % of the days sampled (n = 685 individual days). These results are adjusted for the effects of time and T a min, with a general trend for increasing torpor use throughout the experiment, which was reinforced by low T a min (time × T a min interaction; Table 1), and for time and sex, with males increasing their use of torpor later than females (time × sex interaction; Table 1) (Fig. 2). However, the response to the food treatment did not vary through time (treatment × time interaction, Table 1), and did not differ according to sex (treatment × sex interaction, Table 1). The response was not reinforced by low T a either (treatment × T a min interaction, Table 1).
Observed (histogram) and predicted (dots) percentages of a, b control and c, d food-supplemented male (♂) and female (♀) grey mouse lemurs (Microcebus murinus) entering torpor according to the date. White corresponds to normothermia and grey to torpor. Predicted values were estimated with the final model’s parameters. Control refers to the control plot with natural food availability (4 males, 6 females) and Food suppl. to the food-supplemented plot (7 males, 4 females; daily sample sizes are reported in Table S4)
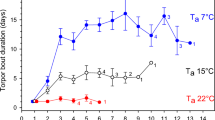
Among individuals using torpor, food supplementation reduced D torp: control individuals spent more time torpid (12.7 ± 7.2 h) than food-supplemented ones (3.3 ± 6.4 h; Table 1; Fig. 3). Torpor duration increased through time and increased with decreasing T a min (Table 1), regardless the food treatment (treatment × time and treatment × T a min interactions; Table 1). This sensitivity of torpor duration to T a min decreased throughout the season (time × T a min interaction, Table 1). Males exhibited shorter torpor bouts than females (1.8 ± 5.0 versus 6.3 ± 7.7 h for the food-supplemented plot and 11.6 ± 7.3 versus 13.6 ± 7.1 h for the control plot; Table 1), regardless the food treatment (treatment × sex interaction; Table 1).
Observed (±SD; dots) and predicted (lines) torpor bout duration for a, b control and c, d food-supplemented male (♂) and female (♀) grey mouse lemurs according to the date (each dot corresponds to the mean ± SD of the duration for the day ±2 days); and observed (±SD; dots) and predicted (lines) minimal skin temperature for e, f control and g, h food-supplemented male (♂) and female (♀) grey mouse lemurs according to the date (each dot corresponds to the mean ± SD of the temperature for the day ±2 days). Predicted durations and temperatures were estimated from the final model’s parameters. Control refers to the control plot with natural food availability (4 males, 6 females) and Food suppl. refers to the food-supplemented plot (7 males, 4 females; Table S4)
Grey mouse lemurs from the food-supplemented plot maintained higher T sk min during rest than those from the control plot (Table 1). Control individuals exhibited an average T sk min of 20.7 ± 4.9 °C whereas T sk min of supplemented ones was 26.7 ± 5.8 °C. This was no longer the case at the end of the experiment when food-supplemented females reached values similar to control females, while food-supplemented males still exhibited higher T sk min than control males (treatment × time × sex interaction; Table 1; Fig. 3). There was a general trend for decreasing T sk min with time (i.e. increasing torpor depth; Table 1). Furthermore, T sk min decreased with decreasing T a min (Table 1), and this sensitivity of T sk min to T a min increased throughout the season (time × T a min interaction, Table 1). Finally, males were less responsive to T a min than females (T a min × sex interaction, Table 1).
Discussion
This food supplementation experiment demonstrated that the increasing use of torpor at the transition between the wet season and the dry season is flexible, and adjusted to actual food availability in a free-ranging heterotherm. Individuals with access to supplementary food reduced torpor use, duration and depth. These results demonstrate that the timing of winter phenotype expression is fine-tuned according to energy availability, superseding the influence of abiotic cues of seasonal transition, such as photoperiod and climatic conditions. Nonetheless, daily torpor flexibility proved to be limited to the first months of the transition to the dry season. One–two months later than control individuals, food-supplemented ones also switched to a winter phenotype, with regular daily torpor, despite continuous provisioning of supplementary food.
As predicted, grey mouse lemurs flexibly adjusted their use of torpor according to food availability, supporting the torpor optimization hypothesis (Humphries et al. 2003a), i.e. individuals use available energy to minimize heterothermy expression, which in turn reduces associated costs (Humphries et al. 2003a; Angilletta et al. 2010). The two other experiments of food supplementation with free-ranging heterotherms also support a proximate role of food availability in heterothermy regulation (Humphries et al. 2003b; Munro et al. 2005). They were both conducted with a temperate hibernator, the eastern chipmunk (Tamias striatus) during winter. Chipmunks rely on external energy storage (hoard of seeds) and on prolonged bouts of hypometabolism to survive the 4–7 months cold, boreal winter. Individuals with experimentally enlarged food stores spent more time euthermic and exhibited shallower torpor bouts (Humphries et al. 2003b; Munro et al. 2005). Six other studies support a proximate role of food availability in heterothermy regulation in free-ranging animals, despite major differences in ecological and energetic constraints operating in the different systems (Vuarin and Henry 2014). They either highlighted correlative evidence of the dependence of heterothermy use on food availability under natural conditions, experimentally manipulated food availability under semi-natural conditions, or indirectly induced food shortage through an experimental increase of intraspecific competition for food. One compelling correlative study showed that the probability to use torpor in Australian owlet-nightjars (Aegotheles cristatus) depended primarily on insect availability, and to a lesser extent on rainfall, with no influence of low air temperature (Doucette et al. 2012).
A key assumption of our experimental design was that natural food availability decreases during the transition to the dry season. Monitoring of insect biomass and plant phenology supported the assumption that fleshy fruit availability and flying insect abundance decrease to a minimal level during the heart of the dry season (Dammhahn and Kappeler 2008b). During our experiment, meteorological conditions corresponded to a typical start of the dry season (Schmid and Kappeler 1998; Dammhahn and Kappeler 2008b), with an abrupt stop of rainfall and decreasing night temperatures (Fig. 1), simultaneously with a typical, major increase of the proportion of animals using torpor in the control plot (Fig. 2; e.g. Schmid 2001). Overall, meteorological conditions and heterothermic responses of control individuals demonstrate that our experiment actually took place at the ecological transition between wet and dry seasons.
If food availability was the only determinant of torpor use, food-supplemented individuals should have maintained their summer phenotype (i.e., normothermia) throughout the experiment. However, they started to regularly enter torpor after 1 (for females) to 2 (for males) months of food supplementation, and most individuals entered torpor by the end of the experiment (end of June; Fig. 2). Three non-mutually exclusive hypotheses could explain these temporal dynamics of the effect of food supplementation on torpor use. First, the influence of anticipatory seasonal cues (photoperiod) on torpor regulation may increase through time, as the predictable risk of resource shortage increases. At the onset of the dry season, daily torpor would be flexibly adjusted to proximal environmental cues reflecting actual ecological conditions. Later on, the influence of photoperiod would take over that of proximal cues of resource constraints to guarantee that individuals enter into the adaptive physiological and behavioural states that maximize survival chances to the predictable, harshest part of the dry season. The existence of such a time window of reduced influence of photoperiod relative to proximal cues during the transition between the favourable and the unfavourable seasons, i.e. when day length is a poor predictor of actual energetic constraints, has already been suggested in other heterotherms (Geiser 2004; Munro et al. 2005; Sheriff et al. 2012). It also allows birds to fine-tune the timing of spring migration (Cornelius et al. 2013) and reproduction initiation according to local conditions (Lambrechts and Perret 2000; Dawson 2008). Moreover, Paul et al. (2009) experimentally demonstrated in captive Siberian hamsters (Phodopus sungorus) that non-photic cues (food availability, social structure) inhibit the reproductive axis under autumn photoperiod, but not under summer photoperiod. Overall, these observations suggest that, during seasonal transitions, phenotypic change is facultative and largely influenced by proximal cues of environmental constraints, whereas it becomes obligate and mainly driven by photoperiod when the animals enter the height of the harsh season (winter/dry season). Second, torpor use may be regulated according to the size of internal fat stores, rather than directly by food availability. Anticipatory fattening is a prerequisite for sustained regular daily torpor and hibernation (Körtner and Geiser 2000). And individuals with high body condition are more prone to use daily torpor, and use it more flexibly, than lean individuals (Vuarin et al. 2013). Grey mouse lemurs would have used supplementary food to constitute larger fat reserves than usual, postponing their physiological shift to winter phenotype. According to the asset protection principle (Dammhahn 2012), food-supplemented animals would have initiated regular daily torpor use when the prospect of supplementary fat storage was offset by the costs of additional food acquisition (e.g. predation; Humphries et al. 2003a; Sheriff et al. 2011; Turbill et al. 2011; Bieber et al. 2014). The first bouts of daily torpor may have even increased the efficiency of conversion of supplemented food into fat stores (Sheriff et al. 2013a; Giroud et al. 2014). Third, natural food availability (Dammhahn and Kappeler 2008a) is expected to decrease through time. Grey mouse lemurs may have remained normothermic as long as natural food availability, supplemented by artificial food provisioning, was sufficient to fulfil their daily energetic requirements. But when natural food supplies were exhausted, individuals would have started to use daily torpor to counterbalance growing energetic deficits associated with insufficient supply of artificial food.
In grey mouse lemurs, as in other heterotherms (like eastern chipmunks or arctic ground squirrels Urocitellus parryii; Munro et al. 2005; Sheriff et al. 2011, 2013b), males use less daily torpor than females. They remain more active and can be captured throughout the dry season (Schmid and Kappeler 1998; Kraus et al. 2008; Dammhahn and Kappeler 2008b, 2013). Our results clarify this sex difference in daily torpor use: at the heart of the dry season (June), males expressed daily torpor as much as females in the control plot, although less than females in the food-supplemented plot (Figs. 2, 3). The main difference is that males begun regular daily torpor use one month later than females (Fig. 2), and exhibited higher minimal skin temperatures throughout the experiment than supplemented females, who ended up exhibiting similar patterns than control individuals (Fig. 3). These sex-specific differences in seasonal timing of daily torpor use might result from reduced body condition of males relative to females during the dry season (Schmid and Kappeler 1998), and associated reduced propensity to use daily torpor (Kobbe et al. 2011; Vuarin et al. 2013), or from sex-differences in energetic requirements to engage in reproduction just after the dry season (Schmid and Kappeler 1998; Canale et al. 2012; see also Munro et al. 2005; Sheriff et al. 2013b). Our study is the first to document, in free-ranging grey mouse lemurs, that sex differences in activity patterns (Rasoazanabary 2006) result from the fact that males and females do not start using daily torpor at the same period of the dry season, males entering daily torpor later than females.
To conclude, the transition to winter phenotype is plastic and not primarily timed according to abiotic cues that allow the anticipation of the dry season. During the transition between wet and dry seasons, the seasonal transition to winter phenotype is fine-tuned according to food availability. The cues used to time the final food-independent shift to the full winter phenotype remain to be identified. It could be photoperiod, which would override the influence of current environmental conditions. In our changing world, identification of the cues that time heterothermy is an obligate preliminary step to determine which, and how, heterothermic animals could be resilient to human-induced disturbances of environmental cycles, like habitat destruction or climate change (Canale and Henry 2010; Lane et al. 2012; Geiser 2013; Williams et al. 2014).
Author contribution statement
MD, PMK and PYH conceived and designed the experiments. MD and PYH conducted fieldwork. PV, MD and PYH performed statistical analyses. PV, MD and PYH wrote the manuscript; PMK provided editorial advice. PMK created, supervised and gave access to unique field facilities.
References
Angilletta MJ, Cooper BS, Schuler MS, Boyles JG (2010) The evolution of thermal physiology in endotherms. Front Biosci E2:861–881
Auld JR, Agrawal AA, Relyea RA (2010) Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc R Soc B 277:503–511
Bieber C, Juškaitis R, Turbill C, Ruf T (2012) High survival during hibernation affects onset and timing of reproduction. Oecologia 169:155–166
Bieber C, Lebl K, Stalder G, Geiser F, Ruf T (2014) Body mass dependent use of hibernation: why not prolong the active season, if they can? Funct Ecol 28:167–177
Canale CI, Henry P-Y (2010) Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic unpredictability. Clim Res 43:135–147
Canale CI, Perret M, Théry M, Henry P-Y (2011) Physiological flexibility and acclimation to food shortage in a heterothermic primate. J Exp Biol 214:551–560
Canale CI, Perret M, Henry P-Y (2012) Torpor use during gestation and lactation in a primate. Naturwissenschaften 99:159–163
Cornelius JM, Boswell T, Jenni-Eiermann S, Breuner CW, Ramenofsky M (2013) Contributions of endocrinology to the migration life history of birds. Gen Comp Endocrinol 190:47–60
Dammhahn M (2012) Are personality differences in a small iteroparous mammal maintained by a life-history trade-off? Proc R Soc Lond B 279:2645–2651
Dammhahn M, Kappeler PM (2008a) Small-scale coexistence of two mouse lemur species (Microcebus berthae and M. murinus) within a homogeneous competitive environment. Oecologia 157:473–483
Dammhahn M, Kappeler PM (2008b) Comparative feeding ecology of sympatric Microcebus berthae and M. murinus. Int J Primatol 29:1567–1589
Dammhahn M, Kappeler PM (2013) Seasonality and behavioral energy strategies in Microcebus berthae and M. murinus. In: Masters J, Gamba M, Génin F (eds) Leaping ahead advances in prosimian biology. Springer, New York, pp 215–223
Dausmann KH (2005) Measuring body temperature in the field-evaluation of external vs. implanted transmitters in a small mammal. J Therm Biol 30:195–202
Dawson A (2008) Control of the annual cycle in birds: endocrine constraints and plasticity in response to ecological variability. Philos Trans R Soc Lond B 363:1621–1633
Dewar RE, Richard AF (2007) Evolution in the hypervariable environment of Madagascar. Proc Natl Acad Sci USA 104:13723–13727
Doucette LI, Brigham RM, Pavey CR, Geiser F (2012) Prey availability affects daily torpor by free-ranging Australian owlet-nightjars (Aegotheles cristatus). Oecologia 169:361–372
Florant GL, Healy JE (2012) The regulation of food intake in mammalian hibernators: a review. J Comp Physiol B 182:451–467
Geiser F (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66:239–274
Geiser F (2013) Hibernation. Curr Biol 23:R188–R193
Génin F, Perret M (2003) Daily hypothermia in captive grey mouse lemurs (Microcebus murinus): effects of photoperiod and food restriction. Comp Biochem Physiol B 136:71–81
Gienapp P, Leimu R, Merilä J (2007) Responses to climate change in avian migration time-microevolution versus phenotypic plasticity. Clim Res 35:25–35
Giroud S, Blanc S, Aujard F, Bertrand F, Gilbert C, Perret M (2008) Chronic food shortage and seasonal modulations of daily torpor and locomotor activity in the grey mouse lemur (Microcebus murinus). Am J Physiol Regul Integr Comp Physiol 294:R1958–R1967
Giroud S, Perret M, Gilbert C, Zahariev A, Goudable J, Le Maho Y, Oudart H, Momken I, Aujard F, Blanc S (2009) Dietary palmitate and linoleate oxidations, oxidative stress, and DNA damage differ according to season in mouse lemurs exposed to a chronic food deprivation. Am J Physiol Regul Integr Comp Physiol 297:R950–R959
Giroud S, Zahn S, Criscuolo F, Chery I, Blanc S, Turbill C, Ruf T (2014) Late-born intermittently fasted juvenile garden dormice use torpor to grow and fatten prior to hibernation: consequences for ageing processes. Proc R Soc Lond B 281:20143111
Heldmaier G, Ortmann S, Elvert R (2004) Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol 141:317–329
Helm B, Ben-Shlomo R, Sheriff MJ, Hut RA, Foster R, Barnes BM, Dominoni D (2013) Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc R Soc Lond B 280:20130016
Humphries MM, Thomas DW, Kramer DL (2003a) The role of energy availability in mammalian hibernation: a cost-benefit approach. Physiol Biochem Zool 76:165–179
Humphries MM, Kramer DL, Thomas DW (2003b) The role of energy availability in mammalian hibernation: an experimental test in free-ranging eastern chipmunks. Physiol Biochem Zool 76:180–186
Kobbe S, Ganzhorn JU, Dausmann KH (2011) Extreme individual flexibility of heterothermy in free-ranging Malagasy mouse lemurs (Microcebus griseorufus). J Comp Physiol B 181:165–173
Körtner G, Geiser F (2000) The temporal organization of daily torpor and hibernation: circadian and circannual rhythms. Chronobiol Int 17:103–128
Kraus C, Eberle M, Kappeler PM (2008) The costs of risky male behaviour: sex differences in seasonal survival in a small sexually monomorphic primate. Proc R Soc Lond B 275:1635–1644
Lambrechts MM, Perret P (2000) A long photoperiod overrides non-photoperiodic factors in blue tits’ timing of reproduction. Proc R Soc Lond B 267:585–588
Landry-Cuerrier M, Munro D, Thomas DW, Humphries MM (2008) Climate and resource determinants of fundamental and realized metabolic niches of hibernating chipmunks. Ecology 89:3306–3316
Lane JE, Kruuk LEB, Charmantier A, Murie JO, Dobson FS (2012) Delayed phenology and reduced fitness associated with climate change in a wild hibernator. Nature 489:554–557
Miller-Rushing AJ, Høye TT, Inouye DW, Post E (2010) The effects of phenological mismatches on demography. Philos Trans R Soc Lond B 365:3177–3186
Munro D, Thomas DW, Humphries MM (2005) Torpor patterns of hibernating eastern chipmunk Tamias striatus vary in response to the size and fatty acid composition of food hoards. J Anim Ecol 74:692–700
Ozgul A, Childs DZ, Oli MK, Armitage KB, Blumstein DT, Olson LE, Tuljapurkar S, Coulson T (2010) Coupled dynamics of body mass and population growth in response to environmental change. Nature 466:482–485
Patil VP, Morrison SF, Karels TJ, Hik DS (2013) Winter weather versus group thermoregulation: what determines survival in hibernating mammals? Oecologia 173:139–149
Paul MJ, Galang J, Schwartz WJ, Prendergast BJ (2009) Intermediate-duration day lengths unmask reproductive responses to nonphotic environmental cues. Am J Physiol Regul Integr Comp Physiol 296:R1613–R1619
Perret M, Aujard F (2001) Regulation by photoperiod of seasonal changes in body mass and reproductive function in gray mouse lemurs (Microcebus murinus): differential responses by sex. Int J Primatol 22:5–24
Piersma T, Drent J (2003) Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol 18:228–233
Rasoazanabary E (2006) Male and female activity patterns in Microcebus murinus during the dry season at Kirindy forest, western Madagascar. Int J Primatol 27:437–464
Reed TE, Waples RS, Schindler DE, Hard JJ, Kinnison MT (2010) Phenotypic plasticity and population viability: the importance of environmental predictability. Proc R Soc Lond B 277:3391–3400
Schmid J (1998) Tree holes used for resting by gray mouse lemurs (Microcebus murinus) in Madagascar: insulation capacities and energetic consequences. Int J Primatol 19:797–809
Schmid J (2001) Daily torpor in free-ranging gray mouse lemurs (Microcebus murinus) in Madagascar. Int J Primatol 22:1021–1031
Schmid J, Ganzhorn JU (2009) Optional strategies for reduced metabolism in gray mouse lemurs. Naturwissenschaften 96:737–741
Schmid J, Kappeler PM (1998) Fluctuating sexual dimorphism and differential hibernation by sex in a primate, the gray mouse lemur (Microcebus murinus). Behav Ecol Sociobiol 43:125–132
Sheriff MJ, Kenagy GJ, Richter M, Lee T, Tøien O, Kohl F, Buck CL, Barnes BM (2011) Phenological variation in annual timing of hibernation and breeding in nearby populations of arctic ground squirrels. Proc R Soc Lond B 278:2369–2375
Sheriff MJ, Williams CT, Kenagy GJ, Buck CL, Barnes BM (2012) Thermoregulatory changes anticipate hibernation onset by 45 days: data from free-living arctic ground squirrels. J Comp Physiol B 182:841–847
Sheriff MJ, Fridinger RW, Tøien Ø, Barnes BM, Buck CL (2013a) Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol Biochem Zool 86:515–527
Sheriff MJ, Richter MM, Buck CL, Barnes BM (2013b) Changing seasonality and phenological responses of free-living male arctic ground squirrels: the importance of sex. Philos Trans R Soc Lond B 368:20120480
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Terrien J, Perret M, Aujard F (2010) Gender markedly modulates behavioral thermoregulation in a non-human primate species, the mouse lemur (Microcebus murinus). Physiol Behav 101:469–473
Thomas DW, Blondel J, Perret P, Lambrechts MM, Speakman JR (2001) Energetic and fitness costs of mismatching resource supply and demand in seasonally breeding birds. Science 291:2598–2600
Turbill C, Bieber C, Ruf T (2011) Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc R Soc Lond B 278:3355–3363
Visser ME, Caro SP, van Oers K, Schaper SV, Helm B (2010) Phenology, seasonal timing and circannual rhythms: towards a unified framework. Philos Trans R Soc Lond B 365:3113–3127
Vuarin P, Henry P-Y (2014) Field evidence for a proximate role of food shortage in the regulation of hibernation and daily torpor: a review. J Comp Physiol B 184:683–697
Vuarin P, Dammhahn M, Henry P-Y (2013) Individual flexibility in energy saving: body size and condition constrain torpor use. Funct Ecol 27:793–799
Williams CT, Barnes BM, Kenagy GJ, Buck CL (2014) Phenology of hibernation and reproduction in ground squirrels: integration of environmental cues with endogenous programming. J Zool 292:112–124
Acknowledgments
We acknowledge the support of this study by Prof. Daniel Rakotondravony and the other members of the Département de Biologie Animale, Université d’Antananarivo, the Commission Tripartite, and the CAFF of the Direction des Eaux et Forêts, and the CNFEREF Morondava. This study was partly funded by CNRS and MNHN (UMR 7179), the German Primate Centre and the Ethologische Gesellschaft. The authors declare that they have no conflict of interest. We thank Martine Perret, Rodin Rasoloarison and Léonard Razafimanantsoa for their support, as well as Susanne Schliehe-Diecks, Bruno Tsiverimana, Jean-Pierre Tolojanahary and the Kirindy team for assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Joanna E. Lambert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vuarin, P., Dammhahn, M., Kappeler, P.M. et al. When to initiate torpor use? Food availability times the transition to winter phenotype in a tropical heterotherm. Oecologia 179, 43–53 (2015). https://doi.org/10.1007/s00442-015-3328-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3328-0