Abstract
Prior to hibernation, mammals accumulate large amounts of fat in their bodies. In temperate mammalian species, hibernation is improved by increasing the levels of poly-unsaturated fatty acids (PUFA) in the body. The saturation of fatty acids (FA) in both white adipose tissue (WAT) and membrane phospholipids of mammals often reflects their diet composition. We found that the greater mouse-tailed bat (Rhinopoma microphyllum) accumulates large amounts of fat at the end of summer by gradually shifting to a fat-rich diet (queen carpenter ants, Camponotus felah). PUFA are almost absent in this diet (<1 % of total FA), which contains a high fraction of saturated (SFA) and mono-unsaturated (MUFA) fatty acids. We found similar low levels of PUFA in mouse-tailed bat WAT, but not in their heart total lipids. The expression of two appetite-stimulating (orexigenic) hypothalamic neuropeptides, AgRP and NPY, increased in parallel to the shift in diet and with fat gain in these bats. To the best of our knowledge, this is the only documented example of specific pre-hibernation diet in bats, and one which reveals the most saturated FA composition ever documented in a mammal. We suggest that the increase in expression levels of NPY and AgRP may contribute to the observed diet shift and mass gain, and that the FA composition of the bat’s specialized diet is adaptive in the relatively high temperatures we recorded in both their winter and summer roosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long torpor bouts or hibernation are dramatic examples of phenotypic plasticity in response to periods of food shortage and/or reduced ambient temperatures (Carey et al. 2003). Most hibernating mammals, including bats, commonly exhibit robust cycles of adiposity, food intake, and energy expenditure (Rousseau et al. 2003). Preparation for hibernation involves the deposition of fat reserves, which provide the main source of energy during the prolonged seasonal fast (Young 1976). To accumulate enough energy to survive the multi-month hibernation season, energy acquisition must be optimized. This can be achieved by increasing the absorption efficiency and assimilation of food in the tissues (Bairlein and Gwinner 1994), by increasing food consumption (hyperphagia, Bairlein 1987), and by altering diet preferences to the consumption of energy-rich food sources (Hiebert et al. 2000; Levin et al. 2009; Sailer and Fietz 2009).
During hibernation, specific biochemical adaptations are needed to maintain basic physiological functions despite the fall in body temperature. Membrane fluidity and permeability are directly affected by the saturation level of their FA. In general, in hibernating species, body tissue membranes will contain higher levels of unsaturated fatty acids before winter, compared to summer (Dark 2005). PUFA constitute an important factor in the function of ion channels, the activity of membrane-attached enzymes, regulation of gene expression, endocytosis/exocytosis, survival in low ambient temperatures, and more (Pamplona 2008; Wallis et al. 2002). Mammals cannot synthesize PUFA de novo and rely on the presence of these fatty acids in their food. Therefore, the composition of FA in vertebrates’ food items is later reflected in their body fat and tissues [e.g. in mammals (Falkenstein et al. 2001), birds (Ben-Hamo et al. 2011), reptiles (Cartland-Shaw et al. 1998) and fish (Diez et al. 2007)], although tissue-specific regulation may occur. FA composition of the diet, and consequently in the body, has an effect on torpor metabolism: herbivore rodent species fed a high n-6 PUFA diet underwent longer and deeper torpor bouts compared with control animals (Florant 1998; Frank et al. 1998; Geiser et al. 1994). The way by which these FA affect torpor however, has remained unclear (Giroud et al. 2009).
Since FA composition in vertebrate bodies is often determined by their diet and affects physiological traits, they are expected to choose their diet according to their life history stage. Accordingly, some hibernators are known to alter their dietary preference prior to hibernation. For example, edible dormice (Glis glis) change their dietary preferences in the field during the pre-hibernatory period, feeding mainly on lipid-rich seeds (Sailer and Fietz 2009). An increase in the intake of oily seeds in autumn was also observed in several other hibernating rodents (Eutamias amoenus, E. speciosus, E. townsendi, E. quadrimaculatus and Citellus lateralis, Tevis 1953). Interestingly, under laboratory conditions, Djungarian hamsters (Phodopus sungorus) alter their preference for dietary unsaturated fats in a temperature-dependent fashion regardless of photoperiod (Hiebert et al. 2003).
Recently, we reported a unique pre-hibernation diet in free-ranging greater mouse-tailed bats (Levin et al. 2009). These medium-size (about 25 g) subtropical insectivore bats are abundant in northern Israel during summer. Towards the end of summer, they gradually shift from consuming mainly beetles and bugs to an extremely fat-rich prey: queen carpenter ants (Camponotus felah), which comprise up to 100 % of their diet, even though other prey items are available (Levin et al. 2009). To the best of our knowledge, this is the only known example of a specific pre-hibernation diet in bats. We suggest that this exclusive prey and its composition are important for the hibernation of the greater mouse-tailed bats. At the end of summer (October), these bats move to their winter roosts, in which ambient temperature remains at 20 °C throughout winter (until April). During winter, we observed no feces at all on the cave floor, indicating that the bats do not feed during this season (Levin et al. 2010).
Foraging behavior and energy balance in mammals are controlled by the hypothalamus, which responds to environmental and physiological inputs (Spiegelman and Flier 2001). The hormone leptin is known to play an important role as a feedback agent between adipose tissue and the central nervous system (hypothalamic arcuate nucleus), and to control energy balance (Friedman et al. 1997). Leptin influences two different groups of neuropeptides in the arcuate nucleus: the orexigenic (appetite-stimulating) neuropeptides, whose expression is inhibited by leptin; and the anorexigenic (appetite-depressing) neuropeptides, which are enhanced by leptin (Ahima and Flier 2000).
Two of the most potent known orexigenic neuropeptides are neuropeptide Y (NPY) and agouti-related protein (AgRP). These neuropeptides are released by the same set of neurons in the arcuate nucleus and affect foraging behavior and food intake in mice (Luo et al. 2011), while their expression is suppressed by leptin in hamsters (Swoap 2008). Centrally applied NPY stimulates food intake in laboratory rats (Hillebrand et al. 2006), mice (Luquet et al. 2005) and Siberian hamsters (Swoap 2008). Recent studies suggest that NPY is not merely an “orexigen”, but also stimulates behaviors that precede the intake of food, as well as stimulating the urge for food acquisition (Ammar et al. 2005; Gruninger et al. 2007). As a result, it was suggested that NPY plays a motivational role in foraging behavior and that it suppresses anxiety, fear, and responsiveness to aversive/stress stimuli (reviewed by Wu et al. 2003). Hence, NPY is a potential candidate for investigation in the context of pre-hibernation diet and fat accumulation.
Another possible candidate for studies in this context is AgRP, which is part of the melanocortin system. This system is composed of several receptors (MC-R), which have both an agonist that is derived from the POMC gene, α-MSH, and inverse agonists, agouti and an agouti-related protein—AgRP (Ahima and Flier 2000). Increased leptin levels promote POMC expression, resulting in an increase in α-MSH, and a decrease in AgRP expression. It was suggested that AgRP also affects food type choice, specifically changing the preference for fat content of the diet in mice (Barnes et al. 2010).
Based on all the above, we hypothesized that the gradual diet change and fat accumulation we observed in the greater mouse-tailed bats towards the end of the summer are adaptive, and do not merely result from a change in the availability of insects species. Since, unlike most hibernators, these bats hibernate in relatively high ambient temperature (T a), we expected that they would not consume a high n-6 PUFA diet like that of cold-climate hibernators; but rather, they would accumulate both saturated and mono-unsaturated FA. Since we did not have data on insect availability, we also monitored the diet of the generalist insectivorous noctule bat (Nyctalus noctula, Gloor et al. 1995) inhabiting the same valley during the same months. Both bat species forage in the same area. In Europe, noctule bats are known to hibernate during winter (Bat Conservation Trust 2010). In Israel, which is on this species’ southern edge of distribution and has a subtropical climate, the bats are active also during winter and we found no record of any hibernacula for this bat (bat data base of the mammalian center of the Society for the Protection of Nature in Israel). It is possible that at least part of the population migrates north for hibernation, and hibernates at low ambient temperatures. We, therefore, hypothesized that if the consumption of ant alates is indeed adaptive for hibernation in a cold climate, we would not find such a drastic change in diet composition in the noctule bats. We further hypothesized that the changes in diet preference and body mass are controlled by the orexigenic pathway in the arcuate nucleus and, therefore, expected that AgRP and NPY expression levels would increase in parallel with the observed diet shift and weight gain.
Materials and methods
Animals
Twenty-four male greater mouse-tailed bats (Rhinopoma microphyllum) were collected from a male summer roost (Gonen, 33°06′N 35°39′E, 10/6/2009: n = 5; 28/6/2009: n = 5;21/7/2009: n = 5; 30/7/2009: n = 5; 13/8/2009: n = 4). Bats were captured using hand nets, following their return from their evening foraging bout (around 10 p.m.; these bats perform only one foraging bout per night). Each bat was placed in a separate clean cotton bag for feces collection, and brought to the lab. During the following morning (10 a.m.), all feces from all individuals were collected from the bags in one pooled sample (one pool per date). From this pool, we later analyzed 50 individual feces for the identification of insect remains (Whitaker 1988). Diet of the noctule bat was monitored at a nearby colony in the same valley, ca. 6 km away (Kiryat Shemona, 33°12′N 35°34′E) during the same months. We positioned a plastic sheet below the colony, and once every 10 days (for 3 months) we collected all feces. Fifty feces from each date were analyzed as above.
We used Kuhl’s pipistrelle (Pipistrellus kuhli) as a reference species for FA composition. Unlike the subtropical mouse-tailed bat, this bat is a typical Mediterranean generalist feeder (Goiti et al. 2003), common throughout Israel. In Israel, these bats are known to feed mainly on Dipterans and Lepidopterans (Feldman et al. 2000). Four Kuhl’s pipistrelles were caught using a mist net at the end of summer 2009 (20/9) in the Hula Valley, northern Israel (33°12′N 35°34′E). Bats were trapped when emerging from their roost at dusk.
For analysis of fatty acid composition of the bats’ diet, queen carpenter ants (C. felah, five queens from five different colonies) were collected during nuptial flights in summer 2009 (20–23/8) in the grounds of the I. Meier Segals Garden for Zoological Research, Tel-Aviv University (32°06′N 34°48′E). Harvester ants (Messor sp.), a common ant species, were used as reference, with five queens collected during nuptial flights in autumn 2009 (10/10).
Body mass and composition
Bats were weighed and anesthetized with isoflurane (2 % in oxygen). Anesthetized bats (except the first group of five individuals, due to technical problems) were scanned with DXA (Dual-emission X-ray Absorptiometry, General Electric Lunar PIXImus) to evaluate the percentage of adipose tissue in their body.
Tissue collection
After scanning, the anesthetized bats were decapitated; blood was collected from the jugular vein and centrifuged (3,000 rpm, 4 min, 4 °C). Brains were surgically removed, and the hypothalami were excised and placed in Eppendorf tubes containing 500 μl trizol. White adipose tissue (WAT) from the abdominal fat and the entire heart was surgically removed into 1.5 ml screw-cap Eppendorf tubes and sealed under a N2 stream. All samples were immediately frozen in the liquid nitrogen, and stored at −70 °C for further analysis.
All procedures were carried out under permit no. 2009/31867 from the Israel Nature Reserves and Park Authority (NPA), and Ethics Committee license no. L-09-002.
Plasma leptin concentrations
We measured plasma leptin concentration using three different commercial leptin RIA kits (mouse, multispecies, and human-specific, Linco Research Inc.) and two commercial ELISA kits (mouse and human leptin, Linco Research Inc.).We use these kits routinely and successfully with rodent plasma.
Total hypothalamic RNA extraction, reverse transcription, and real-time PCR
Total RNA was extracted from the homogenized hypothalami using RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer’s instructions. Total RNA samples were treated with RQ1 DNase (Promega) according to the manufacturer’s protocol, followed by ethanol precipitation. RNA concentration was determined after DNase treatment by OD measurements. Equal amounts of RNA were used as a template for reverse transcription by the verso reverse transcription kit (Thermo) according to the manufacturer’s protocol, using the random hexamers supplied for RNA priming.
Equal amounts of cDNA were used as a template for the real-time PCR (Table 1). The real-time PCR was carried out using the absolute blue syber green mix (Thermo) in a rotorgene 6000 cycler (Corbett) using a standard cycling program. The results were analyzed by the “two standard curve” method using the β-actin as a calibrator. The relative gene expression levels of each sample were determined.
Lipid extraction and analysis
Fatty acids (both polar and non-polar) were extracted from homogenized adipose tissue and hearts and trans-esterificated (Rule 1997). The resulting methyl esters were analyzed by GC/MS using a BP5 capillary column that was temperature programmed from 100 to 280 °C at 5 °C/min. Compounds identified by their mass fragmentation were compared to authentic standards (Sigma-Aldrich, Israel). Quantification was obtained by GC analysis (VARIAN CP-3800) using a VF-5 ms capillary column (Varian, 30 m × 0.25 m, df = 0.25). The temperature was programmed from 90 to 200 °C at 3 °C/min and from 200 to 300 °C at 10 °C/min.
To determine the total fatty acid composition of the ants, fat was extracted directly from freshly excised abdomens (which were almost solely the parts found in the bat’s feces), using the same procedure as for the bat tissues. Due to their small size, fat analyses from the harvester ants were performed on pooled samples (therefore, no SD is given).
Statistical analysis
All data were tested for normal distribution (using Sigma plot 11). If data were not normally distributed, we used logit transformation or Box-Cox transformation. Normally distributed data (before or after transformation) were analyzed using one-way ANOVA or t test where appropriate. We used non-parametric statistics (Kruskal–Wallis, Spearman’s rank correlation coefficient, Mann–Whitney rank test) for data that were not normally distributed even after transformation.
Results
Diet analysis and body mass change
During the 63-day sampling period (10/6/2009 to 13/8/2009), bats almost doubled their body mass (one-way ANOVA: F = 20.5, df = 4, P < 0.001, Fig. 1). A sharp increase in body mass was observed from mid-July (21/7/2009), when more than 50 %of the bats’ diet comprised ant queens (Fig. 1). Other insect groups in the feces of the mouse-tailed bats comprised mainly Coleoptera and Heteroptera. Percentage of body fat (n = 19) ranged between 13 and 35 %, and body mass and fat were significantly correlated (Spearman’s rank correlation, r 2 = 0.89, P < 0.05). In contrast to the gradual shift to a single prey species diet that we found in the mouse-tailed bats, the diet of noctule bats from the same region was composed of many different insect groups (including Heteroptera, Lepidoptera, Ephemeroptera, Neuroptera, Diptera, Coleoptera and Orthoptera), while queen ants formed only up to 40 % of the noctule bats’ diet (Fig. 1).
Body mass increase of greater mouse-tailed bats (circles, mean values ± SD) and the percentage of alate ant in the diet of greater mouse-tailed bats (black triangles) and noctule bats (white triangles) during summer 2009. Other prey groups in the feces of the mouse-tailed bats included Coleoptera, Heteroptera, Lepidoptera; while feces of the noctule bats also contained Heteroptera, Lepidoptera, Hymenoptera, Ephemeroptera, Neuroptera, Diptera, Coleoptera and Orthoptera
Adipose tissue composition
Saturated fatty acids (SFA) comprised 50.7 ± 1.5 of all WAT fatty acids of the greater mouse-tailed bats at the end of summer, while mono-unsaturated acids (MUFA) accounted for 47.6 ± 2 (Fig. 2a; Table 2). Among the SFA, palmitic acid (C16:0) was the most common, comprising 39.5 ± 1.4 % during the second half of the summer, almost double compared with the percentage found in Kuhl’s pipistrelles (16.5 ± 3.4 %, Table 2). After the mouse-tailed bats shifted their diet to queen ants, a significant decrease in the fraction of linoleic acid (C18:2) and total PUFA was observed (Mann–Whitney rank test, T = 168, P < 0.001), while there was no significant increase in the fraction of MUFA and SFA (Mann–Whitney rank test, P = 0.055, 0.17, respectively, Fig. 2a; Table 2).
At the end of summer, the fraction of PUFA in mouse-tailed bat adipose tissue (1.3 ± 0.8 %) was extremely low compared to Kuhl’s pipistrelles (23.9 ± 9 %).
Heart total FA
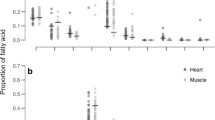
Unlike the adipose tissue, the composition of total lipids, triglycerides and phospholipids in the heart did not change significantly during summer, and was similar in the two bat species. The fraction and variation of long-chain FA (C18–C22) were higher than in WAT (see Table 2). The n-6 arachidonic acid (C20:4) and n-3 docosahexaenoic acid (C22:6) were found only in the heart tissue and were absent in WAT, and the fraction of these two FA remained constant in the mouse-tailed bat hearts during summer (Table 2).
Ant FA composition
There was no significant difference between the fatty acid composition of queen carpenter ants from the five different nests. Queen carpenter ant bodies were composed of 49.9 ± 2 oleic (C18:1) and 34.7 ± 1.5 palmitic (C16:0) acids. The percentage of PUFA in the ants’ bodies accounted for only 0.5 ± 0.2 % of the fatty acids, which is very low compared to that of PUFA of the bats’ WAT and hearts, or that of the harvester ant queens (27.2 %, Table 2).
Plasma leptin concentrations and leptin receptor mRNA levels
We failed to detect any plasma leptin in serially diluted mouse-tailed bat plasma, despite using five different commercial RIA and ELISA kits. All the positive controls were appropriate.
Relative leptin receptor mRNA (all isoforms) levels in the hypothalamus of mouse-tailed bats did not differ among the five sampling time points (Kruskal–Wallis: H = 7.812, df = 4, P = 0.1).
Hypothalamic neuropeptide mRNA levels
NPY mRNA levels in the hypothalamus of the mouse-tailed bats significantly increased during summer and peaked in mid-July, when the bats shifted their diet preferences and acquired large amounts of fat (Fig. 3a; one-way ANOVA on Logit transformed data, F = 5.009, df = 4, P = 0.0058). Although AgRP showed a similar pattern (Fig. 3b), its expression did not increase significantly between time points (Kruskal–Wallis, P = 0.182). Nevertheless, when we divided the samples into below 20 % and above 50 % ants in the bats’ feces (also parallel to the sharp increase in body mass observed in mid-July), AgRP expression levels were significantly higher in the second half of the summer (t test, t = 3.02, df = 22, P < 0.01). POMC mRNA levels did not change significantly between time points, or when comparing bats examined according to the percentage of ants in the diet (t test, t = 1 df = 21 P = 0.3).
Discussion
We found that greater mouse-tailed bats increased the percentage of queen ants in their diet from 0 to 100 % within a period of less than 2 months. These results correspond with our previous study (Levin et al. 2009), in which we found that this diet shift occurred repeatedly over the course of 6 years. In contrast, the noctule bat, which is an open-space forager like the mouse-tailed bat, and as far as we know does not hibernate in Israel, did not show such a shift but remained a generalist feeder throughout the summer (although the percentage of ant alates in its diet increased to 40 %). This finding, together with the results published in other studies regarding the diet of other insectivorous bats species from this area showing a generalist diet pattern over summer (Feldman et al. 2000; Levin et al. 2009), suggests that the gradual increase in the proportion of queen ants in the mouse-tailed bats diet during summer results from an increase in this insect’s availability, combined with a high preference for this prey item. The bats accumulate significant fat reserves towards the end of summer. In contrast to cold-climate hibernators that accumulate n-6 PUFA in their pre-hibernation body fat, we found an opposite trend in the pre-hibernating greater mouse-tailed bats WAT (Table 2; Fig. 2a). Moreover, we found that the FA composition of the mouse-tailed bat body fat reflects the composition of their main prey item-queen carpenter ants. It has been experimentally demonstrated that in relatively low ambient temperatures, high body n-6 PUFA levels constitute an important factor in determining the depth and length of hibernation (Arnold et al. 2011; Ruf and Arnold 2008). The percentage of PUFA in the WAT of such hibernators increases to 35–45 % of total FA during the pre-hibernation period (Florant 1998; Harlow and Frank 2001). Data on the FA composition of other tropical and subtropical hibernators are scarce. Low levels of body fat PUFA (2.5 %) were also measured in the nectarivore/frugivore fat-tailed dwarf lemur (Cheirogaleus medius), a tropical hibernator (Fietz and Dausmann 2003). This low level of PUFA in C. medius was related to the low level of lipids and high carbohydrate content in its diet. Nevertheless, SFA level in C. medius WAT was much lower than that found in the mouse-tailed bats. To the best of our knowledge, the level of SFA we found in the pre-hibernating greater mouse-tailed bat WAT is the highest documented in mammals to date.
It has been suggested that SFA have a negative effect on hibernation in mammals, since a high SFA percentage in the diet increases metabolic rate during torpor (Geiser et al. 1994). However, these conclusions are based primarily on observations on cold-climate zone hibernators that spend long periods of torpor at relatively low ambient temperatures. In different mammals, high levels of dietary SFA are known to reduce thermogenesis, fat oxidation, and daily energy expenditure (Takeuchi et al. 1995). Greater mouse-tailed bats hibernate for 5 months at a relatively high and constant ambient temperature of about 19–20 °C (Levin et al. 2010). We suggest that under such T a, above the melting point of the most common fatty acid (oleic acid 14 °C), these costs may be absent (allowing the bats to consume this energy-rich, abundant food source. Moreover, a high saturation level may even be beneficial to hibernators because: (a) saturated fatty acids are packed better in the adipocytes due their linear structure and contain more energy per volume; and (b) because of their double bonds, PUFA tend to be less structurally stable than SFA, and they are often attacked by oxygen-reactive species, and might form compounds (FA peroxide) with destructive potential for biological membranes (Hulbert et al. 2007; Pamplona 2008, but see Brown et al. 2011). This formation of free radicals most commonly occurs in tissues during active re-warming, when an increase in mitochondrial activity is followed by enhanced production of oxygen free radicals (Carey et al. 2000; Orr et al. 2009). We suggest that hibernators adjust the saturation levels of their WAT depending on the ambient temperature during hibernation, in order to benefit from the advantages of the different FA. Surprisingly, phospholipid composition of the heart in the studied bats remained relatively constant during summer. This provides good evidence for an active regulation of FA composition in certain tissues (in this case, the heart) despite significant dietary changes, in contrast to an earlier report by Innis and Clandinin (1981). Moreover, in contrast to WAT, heart total lipids of the mouse-tailed bats were characterized by a relatively high fraction of PUFA (Fig. 2a). It is important to note that, unlike WAT, which contains mostly stored fats (triacylglycerols) and also membrane fatty acids, the heart tissue contains almost solely polar fatty acids (phospholipids).
Unlike the dramatic difference in WAT composition between mouse-tailed bats and Kuhl’s pipistrelles, the heart FA composition of the two bat species demonstrated much greater similarity. Pamplona et al. (2000) found a negative correlation between the ratio of double bonds in the heart phospholipids and average life-span in mammals. Ruf and Arnold (2008) suggested that a high ratio between n-6/n-3 FA in the heart tissue of hibernators protects the heart from the arrhythmia that can occur during deep torpor as a consequence of massive accumulation of Ca2+ in the cytosol. A possible mechanism for explaining the benefits of a high ratio of n-6/n-3 FAs is related to the coupling between the hydrophobic core of the lipid bilayer and the hydrophobic part of a membrane-spanning protein (Phillips et al. 2009). Any conformational change of a protein involving variation in its cross-sectional area causes local bilayer deformation, with associated changes of the lateral pressure profile. Distribution of lateral stress within the hydrophobic core depends on the degree of saturation and alters the equilibrium constant for the protein conformational states. Even small changes in membrane composition, e.g., replacement of n-3 with n-6 PUFA, can lead to shifts in local pressure at a magnitude of hundreds of bars (Arnold et al. 2011). In the pre-hibernating mouse-tailed bat hearts, we found an opposite trend: the n-6/n-3 ratio in the hearts’ total lipids decreased during summer by almost 50 % (from 12.8 to 7.3, Table 2) due to a significant decrease in the n-6 linoleic acid level (Mann–Whitney rank test, T = 120, P < 0.001). This observation supports our hypothesis that hibernacula T a influences the optimal FA composition of WAT, and that reducing oxidative stress by minimizing the n-6 levels in membranes of warm temperature hibernators is advantageous.
In order to gain fat during the pre-hibernation period, bats should either be resistant to leptin (or its signaling pathway) or produce smaller amounts of leptin than usual. Chiropteran peptide hormones have been suggested to show greatest cross-reactivity with antibodies generated against human peptides (Singh et al. 2007; Widmaier et al. 1997). Unfortunately, in spite of using various kits designed for different species, including a multi-species kit, and even though leptin was successfully measured in different bat families (Vespertilionidae, Emballonuridae and Pteropodidae) (Banerjee et al. 2011; Kronfeld-Schor et al. 2000b; Singh et al. 2007; Srivastava and Krishna 2007), we failed to detect blood leptin in Rhinopoma plasma. Therefore, we could not study the possible changes in leptin levels during the pre-hibernation period. Leptin resistance was observed in the pre-hibernating little brown bat (Myotis lucifugus) (Kronfeld-Schor et al. 2000a), followed by decreased levels of leptin receptors in the CNS during pre-migration or pre-hibernation (Townsend et al. 2008). We found no significant change in leptin receptor expression levels during pre-hibernation fattening in the mouse-tailed bats.
We found a significant increase in the expression levels of both NPY and AgRP in the greater mouse-tailed bat hypothalamus during summer, which paralleled their shift in diet preference and fat gain. A similar increase in hypothalamic NPY levels was found in pregnant little brown bats (Myotis lucifigus) (Widmaier et al. 1997). NPY and AgRP are both orexigenic, stimulating increased consumption of food in mammals (White 1993). NPY activates four different receptors (Y1,2,4,5) that affect different behaviors, including foraging behavior (Day et al. 2005). In rodents, the effect on food consumption is mediated through the Y1 receptor, while foraging behavior is mediated through the Y5 receptor (Swoap 2008). We found the increase in NPY expression levels in the greater mouse-tailed bat hypothalamus during summer to be correlated with the diet shift and body mass. This change in NPY expression level may influence foraging behavior and fat gain during pre-hibernation.
High levels of AgRP increase food intake and preference for high fat diets in mice (Barnes et al. 2010). Alate ants are among the most fat-rich insects, with fat comprising up to 50 % of their body mass (Redford and Dorea 1984). It is possible that the increase in AgRP levels in the mouse-tailed bats during the pre-hibernation period increased their preference for this fat-rich diet.
The increase in NPY and AgRP expression levels in male greater mouse-tailed bats, which started immediately after the days began to get shorter, may be triggered by photoperiod, and mediated by seasonal development of leptin resistance. Effects of photoperiod on food intake were found in several mammalian species, including soay rams (Ovis aries), in which the increase in food intake is correlated with increased expression of NPY in the hypothalamus (Clarke et al. 2003), and in Siberian hamsters (Phodopus sungorus) and field voles (Microtus agrestis), in which the effect of photoperiod is mediated by changes in leptin sensitivity (Krol et al. 2006; Tups 2009). However, our results are mainly correlative, and other mechanisms and triggers could also apply, such as circannual rhythms, ambient temperature, or any combination thereof. This question deserves further studies.
In summary, we found that the greater mouse-tailed bats constitute a special case of seasonal hibernators: they hibernate in subtropical regions at relatively high T a, alter their food preferences during the pre-hibernation period, select a specific prey item with a distinctive FA profile, and accumulate large amounts of fat rich in SFA within a very short period, prior to hibernation. All these characters make these bats an extraordinary model for the study and understanding of seasonality and control of energy balance in mammals.
References
Ahima RS, Flier JS (2000) Leptin. Annu Rev Physiol 62:413–437
Ammar AA, Nergardh R, Fredholm BB, Brodin U, Sodersten P (2005) Intake inhibition by NPY and CCK-8: a challenge of the notion of NPY as an “Orexigen”. Behav Brain Res 161:82–87
Arnold W, Ruf T, Frey-Roos F, Bruns U (2011) Diet-independent remodeling of cellular membranes precedes seasonally changing body temperature in a hibernator. PLoS One 6:e18641
Bairlein F (1987) Adaptations of feeding physiology for fat deposition in garden warblers. Journal Fur Ornithologie 128:381–382
Bairlein F, Gwinner E (1994) Nutritional mechanisms and temporal control of migratory energy accumulation in birds. Annu Rev Nutr 14:187–215
Banerjee A, Udin S, Krishna A (2011) Regulation of leptin synthesis in white adipose tissue of the female fruit bat, Cynopterus sphinx: role of melatonin with or without insulin. Exp Physiol 96:216–225
Barnes MJ, Argyropoulos G, Bray GA (2010) Preference for a high fat diet, but not hyperphagia following activation of mu opioid receptors is blocked in AgRP knockout mice. Brain Res 1317:100–107
Bat Conservation Trust (2010) The noctule bat. http://www.bats.org.uk
Ben-Hamo M, McCue M, McWilliams S, Pinshow B (2011) Dietary fatty acid composition influences tissue lipid profiles and regulation of body temperature in Japanese quail. J Comp Physiol B Biochem Syst Environ Physiol 1–10
Brown JCL, Chung DJ, Belgrave KR, Staples JF (2011) Mitochondrial metabolic suppression and reactive oxygen species production in liver and skeletal muscle of hibernating thirteen-lined ground squirrels. Am J Physiol Regul Integr Comp Physiol 302:R15–R28
Carey HV, Frank CL, Seifert JP (2000) Hibernation induces oxidative stress and activation of NF-kappa B in ground squirrel intestine. J Comp Physiol B Biochem Syst Environ Physiol 170:551–559
Carey HV, Andrews MT, Martin SL (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83:1153–1181
Cartland-Shaw LK, Cree A, Skeaff CM, Grimmond NM (1998) Differences in dietary and plasma fatty acids between wild and captive populations of a rare reptile, the tuatara (Sphenodon punctatus). J Comp Physiol B 168:569–580
Clarke IJ, Rao A, Chilliard Y, Delavaud C, Lincoln GA (2003) Photoperiod effects on gene expression for hypothalamic appetite-regulating peptides and food intake in the ram. Am J Physiol Regul Integr Comp Physiol 284:R101–R115
Dark J (2005) Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr 25:469–497
Day DE, Keen-Rhinehart E, Bartness TJ (2005) Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 289:R29–R36
Diez A, Menoyo D, Perez-Benavente S, Calduch-Giner JA, de Celis SVR, Obach A, Favre-Krey L, Boukouvala E, Leaver MJ, Tocher DR, Perez-Sanchez J, Krey G, Bautista JM (2007) Conjugated linoleic acid affects lipid composition, metabolism, and gene expression in Gilthead sea bream (Sparus aurata). J Nutr 137:1363–1369
Falkenstein F, Kortner G, Watson K, Geiser F (2001) Dietary fats and body lipid composition in relation to hibernation in free-ranging echidnas. J Comp Physiol B Biochem Syst Environ Physiol 171:189–194
Feldman R, Whitaker JO, Yom-Tov Y (2000) Dietary composition and habitat use in a desert insectivorous bat community in Israel. Acta Chiropterologica 2:15–22
Fietz Tataruch, Dausmann Ganzhorn (2003) White adipose tissue composition in the free-ranging fat-tailed dwarf lemur (Cheirogaleus medius; Primates), a tropical hibernator. J Comp Physiol B 173:1–10
Florant GL (1998) Lipid metabolism in hibernators: the importance of essential fatty acids. Am Zool 38:331–340
Frank CL, Dierenfeld ES, Storey KB (1998) The relationship between lipid peroxidation, hibernation, and food selection in mammals. Am Zool 38:341–349
Friedman D, Haim A, Zisapel N (1997) Temporal segregation in coexisting spiny mice (genus Acomys): role of photoperiod and heterospecific odor. Physiol Behav 62:407–411
Geiser F, Mcallan BM, Kenagy GJ (1994) The degree of dietary fatty-acid unsaturation affects torpor patterns and lipid-composition of a hibernator. J Comp Physiol B Biochem Syst Environ Physiol 164:299–305
Giroud S, Perret M, Gilbert C, Zahariev A, Goudable J, Le Maho Y, Oudart H, Momken I, Aujard F, Blanc S (2009) Dietary palmitate and linoleate oxidations, oxidative stress, and DNA damage differ according to season in mouse lemurs exposed to a chronic food deprivation. Am J Physiol Regul Integr Comp Physiol 297:R950–R959
Gloor S, Stutz HPB, Ziswiler V (1995) Nutritional habits of the noctule bat Nycctalus noctula (Schreber, 1774) in Switzerland. Myotis 32:231–242
Goiti U, Vecin P, Garin I, Saloña M, Aihartza J (2003) Diet and prey selection in Kuhl’s pipistrelle (Pipistrellus kuhlii, Chiroptera: vespertilionidae) in south-western Europe. Acta Theriologica 48:457–468
Gruninger T, LeBoeuf B, Liu Y, Rene Garcia L (2007) Molecular signaling involved in regulating feeding and other motivated behaviors. Mol Neurobiol 35:1–19
Harlow HJ, Frank CL (2001) The role of dietary fatty acids in the evolution of spontaneous and facultative hibernation patterns in prairie dogs. J Comp Physiol B Biochem Syst Environ Physiol 171:77–84
Hiebert SM, Fulkerson EK, Lindermayer KT, McClure SD (2000) Effect of temperature on preference for dietary unsaturated fatty acids in the Djungarian hamster (Phodopus sungorus). Can J Zool 78:1361–1368
Hiebert SM, Hauser K, Ebrahim AJ (2003) Djungarian hamsters exhibit temperature-dependent dietary fat choice in long days. Physiol Biochem Zool 76:850–857
Hillebrand JJG, Kas MJH, Adan RAH (2006) To eat or not to eat; regulation by the melanocortin system. Physiol Behav 89:97–102
Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213
Innis SM, Clandinin MT (1981) Dynamic modulation of mitochondrial inner-membrane lipids in rat heart by dietary fat. Biochem J 193:155–167
Krol E, Duncan JS, Redman P, Morgan PJ, Mercer JG, Speakman JR (2006) Photoperiod regulates leptin sensitivity in field voles, Microtus agrestis. J Comp Physiol B Biochem Syst Environ Physiol 176:153–163
Kronfeld-Schor N, Richardson C, Silvia BA, Kunz TH, Widmaier EP (2000a) Dissociation of leptin secretion and adiposity during prehibernatory fattening in little brown bats. Am J Physiol Regul Integr Comp Physiol 279:R1277–R1281
Kronfeld-Schor N, Zhao J, Silvia BA, Bicer E, Mathews PT, Urban R, Zimmerman S, Kunz TH, Widmaier EP (2000b) Steroid-dependent up-regulation of adipose leptin secretion in vitro during pregnancy in mice. Biol Reprod 63:274–280
Levin E, Yom-Tov Y, Barnea A (2009) Frequent summer nuptial flights of ants provide a primary food source for bats. Naturwissenschaften 96:477–483
Levin E, Ar A, Hefetz A, Yom-Tov Y, Kronfeld-Schor N (2010) Some like it hot: hibernation patterns of the greater mouse-tailed bat (Rhinopomamicrophyllum). In: 27th Annual Meeting, Australian and New Zealand Society for Comparative Physiology and Biochemistry, Australian National University, Canberra, 3–5 December, Abstracts, p 26
Luo N, Marcelin G, Liu SM, Schwartz G, Chua S (2011) Neuropeptide Y and Agouti-related peptide mediate complementary functions of hyperphagia and reduced energy expenditure in leptin receptor deficiency. Endocrinology 152:883–889
Luquet S, Perez FA, Hnasko TS, Palmiter RD (2005) NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310:683–685
Orr AL, Lohse LA, Drew KL, Hermes-Lima M (2009) Physiological oxidative stress after arousal from hibernation in Arctic ground squirrel. Comp Biochem Physiol A Mol Integr Physiol 153:213–221
Pamplona R (2008) Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochimica Et Biophysica Acta Bioenergetics 1777:1249–1262
Pamplona R, Portero-Otin M, Ruiz C, Gredilla R, Herrero A, Barja G (2000) Double bond content of phospholipids and lipid peroxidation negatively correlate with maximum longevity in the heart of mammals. Mech Ageing Dev 112:169–183
Phillips R, Ursell T, Wiggins P, Sens P (2009) Emerging roles for lipids in shaping membrane-protein function. Nature 459:379–385
Redford KH, Dorea JG (1984) The nutritional value of invertebrates with emphasis on ants and termites as food for mammals. J Zool 203:385–395
Rousseau K, Atcha Z, Loudon ASI (2003) Leptin and seasonal mammals. J Neuroendocrinol 15:409–414
Ruf T, Arnold W (2008) Effects of polyunsaturated fatty acids on hibernation and torpor: a review and hypothesis. Am J Physiol Regul Integr Comp Physiol 294:R1044–R1052
Rule DC (1997) Direct transesterification of total fatty acids of adipose tissue, and of freeze-dried muscle and liver with boron-trifluoride in methanol. Meat Sci 46:23–32
Sailer MM, Fietz J (2009) Seasonal differences in the feeding ecology and behavior of male edible dormice (Glis glis). Mammalian Biol 74:114–124
Singh UP, Krishna A, Bhatnagar KP (2007) Year round plasma leptin and androgen concentrations in a tropical bat. Acta Theriologica 52:129–140
Spiegelman BM, Flier JS (2001) Obesity and the regulation of energy balance. Cell 104:531–543
Srivastava RK, Krishna A (2007) Adiposity associated rise in leptin impairs ovarian activity during winter dormancy in Vespertilionid bat, Scotophilus heathi. Reproduction 133:165–176
Swoap SJ (2008) Why one enters torpor: focus on “NPY Y1 receptor antagonist prevents NPY-induced torpor-like hypothermia in cold-acclimated Siberian hamsters”. Am J Physiol Regul Integr Comp Physiol 294:R234–R235
Takeuchi H, Matsuo T, Tokuyama K, Shimomura Y, Suzuki M (1995) Diet-induced thermogenesis is lower in rats fed a lard diet than in those fed a high oleic-acid safflower oil diet, a safflower oil diet or a linseed oil diet. J Nutr 125:920–925
Tevis L (1953) Stomach contents of chipmunks and mantled squirrels in northeastern California. J Mammal 34:316–324
Townsend KL, Kunz TH, Widmaier EP (2008) Changes in body mass, serum leptin, and mRNA levels of leptin receptor isoforms during the premigratory period in Myotis lucifugus. J Comp Physiol B Biochem Syst Environ Physiol 178:217–223
Tups A (2009) Physiological models of leptin resistance. J Neuroendocrinol 21:961–971
Wallis JG, Watts JL, Browse J (2002) Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem Sci 27:467–473
Whitaker JOJ (1988) Food habit and analysis of insectevorous bats. In: Kunz TH (ed) Echological and behavioral methods for the study of bats. Smithsonian Institute, Washington, DC, pp 171–189
White JD (1993) Neuropeptide Y a central regulator of energy homeostasis. Regul Pept 49:93–107
Widmaier E, Long J, Cadigan B, Gurgel S, Kunz T (1997) Leptin, corticotropin-releasing hormone (CRH), and neuropeptide Y (NPY) in free-ranging pregnant bats. Endocrine 7:145–150
Wu Y, Ma B, Zhou L, Wang H, Xu J, Kemmitt S, Brookes PC (2003) Changes in the soil microbial community structure with latitude in eastern China, based on phospholipid fatty acid analysis. Appl Soil Ecol 43:234–240
Young RA (1976) Fat, Energy and mammalian survival. Am Zool 16:699–710
Acknowledgments
We would like to thank Yotam Shenaar, Rotem Cohen-Paz, Orly Barak, Osnat Malka, and Tovit Simon for help with the laboratory work; Luzie Braulke for reading and comments on the manuscript; and Naomi Paz for editing the manuscript. This research was supported by The Israel Science Foundation (Grant No. 232/08).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Levin, E., Yom-Tov, Y., Hefetz, A. et al. Changes in diet, body mass and fatty acid composition during pre-hibernation in a subtropical bat in relation to NPY and AgRP expression. J Comp Physiol B 183, 157–166 (2013). https://doi.org/10.1007/s00360-012-0689-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-012-0689-0