Abstract
Salt stress reduces plant growth by negatively interfering with the division rate and cellular expansion, limiting the growth and development of the roots, stems, and leaves. 24-Epibrassinolide (EBR) is a molecule extracted from plant tissues and is a plant growth regulator with a high capacity to modulate tolerance to abiotic stresses. The objective of this study was to verify the possible improvements promoted by pretreatment with EBR in salt-stressed tomato plants, evaluating the variables related to root anatomy, photosynthetic pigments, antioxidant system, and biomass accumulation. The experiment comprised four treatments: two salt conditions (0 and 150 mM NaCl, described as Na+ (−) and Na+ ( +), respectively) and two concentrations of 24-epibrassinolide (0 and 100 nM EBR, described as EBR (−) and EBR ( +), respectively). EBR modulated the protection and vascularization of root structures, as demonstrated by the increases in epidermis thickness (12%) and metaxilem diameter (119%), respectively. This steroid relieved oxidative damage, which was clearly linked to elevated activities of superoxide ascorbate peroxidase (24%) and guaiacol peroxidase (31%). EBR also benefited photosynthetic pigments, reducing the degradation of chlorophylls. In addition, pretreatment with EBR favoured a higher biomass, which was due to positive effects on leaf and root tissues, including better performance of photosynthetic machinery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tomato is considered a model plant in research conducted in the agricultural sector (Suresh et al. 2014) because it has interesting characteristics, such as a short cycle, rapid growth, easy pollination, wide adaptability to climatic conditions, and high rates of seed production (Bai and Lindhout 2007; Gerszberg et al. 2015; Gerszberg and Hnatuszko-Konka 2017). In this context, this species plays an important role in the generation of knowledge, more specifically on the deleterious effects caused by salt stress (Zheng et al. 2016) and ways to mitigate these interferences on metabolism (Shu et al. 2016) and growth in higher plants (Maia et al. 2018).
Soil salinity is a problem in several regions of the world, including agricultural areas (Qureshi et al. 2008; Qadir et al. 2014), and the incorrect use of irrigation and inadequate soil management are the main causes of soil salinization due to anthropogenic action (Bouksila et al. 2013). In plants, salt stress generated by sodium (Na+) causes osmotic and ionic imbalances (Dalio et al. 2011), reducing the plant water potential and impairing plant metabolism (Rengasamy 2010). Na+ ions also negatively impact nutrient uptake, often causing nutritional deficiency (Hafsi et al. 2017; Cao et al. 2019). Additionally, Na+ accumulation in the cytoplasm and/or vacuoles causes oxidative stress in the cells (Chaves et al. 2009), resulting in anatomical disturbances and interferences with the photosynthetic machinery, growth rate, and biomass (Ashraf and Harris 2004; Munns and Tester 2008b).
Salt stress reduces plant growth, negatively impacting the division rate and cellular expansion and limiting the growth and development of roots, stems, and leaves (Yu et al. 2015; Forieri et al. 2016a; Khoshbakht et al. 2018). Specifically, in root tissue, this stress induces a decrease in the metaxylem as a response to minimize cavitation and loss of function of vessel elements, which are essential structures for the conduction of water and assimilation of nutrients (Risopatron et al. 2010; Oliveira et al. 2018). Additionally, mild or moderate Na+ stress can provoke an increase in the thickness of the cell wall in an attempt to reduce the accumulation of this potentially toxic ion; however, at high concentrations, cell plasmolysis often ensues, triggering damage to protective tissues, including the epidermis and endoderm (Silva et al. 2020).
Chlorophyll (Chl) is an essential component of the photosynthetic machinery (Johnson 2016) and is responsible for the absorption of light in specific bands of the spectrum (Chen 2014) and the biosynthesis of carbohydrates indispensable for plant metabolism (Kalaji et al. 2018). Structurally, Chl has a hydrophobic tail that inserts into the thylakoid membrane, a head formed by a porphyrin ring, and a circular group of Mg2+ atoms (Hohmann-Marriott and Blankenship 2011). Typically, exposure to Na+ ions induces intense formation of O2− ions, which are generated by the fusion of excess electrons with O2 molecules in the chloroplasts (Shelke et al. 2017). They cause damage to membranes and disturbances in the centres of PSII reactions (Nishiyama et al. 2004; Kalaji et al. 2011), resulting in photoinhibition (Tavakkoli et al. 2011; Ruban 2015).
Plants under salt stress often overproduce reactive oxygen species (ROS), which superoxide (O2−) and hydrogen peroxide (H2O2) are the major components (Gill and Tuteja 2010). ROS are highly toxic substances that cause oxidative damage to membranes, proteins and nucleic acids in plant cells (Shahzad et al. 2018). Antioxidant metabolism plays a promising role in mitigating the impacts of ROS accumulation (Kang and Nam 2016). Under oxidative stress, the enzymes superoxide dismutase (SOD), guaiacol peroxidase (POX), catalase (CAT) and ascorbate peroxidase (APX) (He et al. 2016) are responsible for ROS elimination, reducing damage to the photosynthetic apparatus and cell membranes (Guerrero et al. 2015).
24-Epibrassinolide (EBR) is a molecule extracted from plant tissues and is natural and biodegradable (Tanveer et al. 2018). Chemically, it is one of the brassinosteroids (BRs) with plant growth regulatory activities (Müssig 2005). EBR is involved in multiple metabolic processes, including cell division (Tong et al. 2014), cell elongation (Tang et al. 2011), vascular differentiation (Ibanes et al. 2009), reproductive development (Huang et al. 2013; Vogler et al. 2014; Maita and Sotomayor 2015), germination (Wang et al. 2011), formation of the root system (González-García et al. 2011; Cai et al. 2018), senescence, abscission and maturation (Hansen et al. 2009; Mazorra et al. 2013), gene regulation (Mao et al. 2017), and modulation of tolerance to biotic and abiotic stresses (Sasse 2003; Choudhary et al. 2012; Pereira et al. 2019).
The hypothesis of this research was based on the negative effects induced by Na+ stress in plants, such as disturbances in the anatomy of stem and root tissues (Akhtar et al., 2017; Silva et al., 2020), oxidative damage (Sheikh-Mohamadi et al. 2017), and interference with photosynthetic performance (Hasanuzzaman et al. 2018). On the other hand, studies available in the literature demonstrate that EBR can mitigate the problems associated with this stress (Ahammed et al. 2020), increasing ROS scavenging (El-Mashad and Mohamed 2012; Rattan et al. 2020), and modulating anatomical adaptations (Oliveira et al. 2018). Therefore, the objective of this study is to verify the possible improvements by pretreatment with EBR in salt-stressed tomato plants, evaluating the variables linked to root anatomy, photosynthetic pigments, antioxidant system, and biomass accumulation.
Materials and Methods
Location and Growth Conditions
The experiment was performed at the Campus of Paragominas of the Universidade Federal Rural da Amazônia, Paragominas, Brazil (2°55′ S, 47°34′ W). The study was conducted in a greenhouse with controlled temperature and humidity. The minimum, maximum, and median temperatures were 27, 33 and 26.2 °C, respectively. The relative humidity during the experimental period varied between 60 and 80%.
Plants, Containers and Acclimation
Seeds of Solanum lycopersicum L. cv. Caline IPA-7 Hortivale™ were germinated using Plantmax™ substrate. Fifteen-day-old seedlings with similar features and sizes were selected and placed in 1.2 l containers (0.15 m in height and 0.10 m in diameter) filled with a mixed substrate of sand and vermiculite in a 3:1 ratio. A solution was used for nutrients (Lima and Lobato 2017), and the ionic force was started at 50% and was modified to 100% after two days. After two days, the nutritive solution remained at total ionic force.
Experimental Design
The experiment was randomized with four treatments, including two salt conditions (0 and 150 mM NaCl, described as Na+ (−) and Na+ ( +), respectively) and two concentrations of brassinosteroids (0 and 100 nM EBR, described as EBR (-) and EBR ( +), respectively). Six replicates for each of the four treatments were conducted, yielding a total of 24 experimental units used in the experiment, with one plant in each unit. Na+ concentration was defined based in study conducted by Stevens et al. (2006) with tomato plants, while EBR treatment was chosen in agreement with Maia et al. (2018).
24-Epibrassinolide (EBR) Preparation and Application
Twenty-day-old young plants were sprayed with 24-epibrassinolide (EBR) or Milli-Q water (containing a proportion of ethanol that was equal to that used to prepare the EBR solution) at 5-d intervals until day 35. The 0 and 100 nM EBR (Sigma-Aldrich, USA) solutions were prepared by dissolving the solute in ethanol followed by dilution with Milli-Q water [ethanol:water (v/v) = 1:10,000] (Ahammed et al. 2013).
Plant Conduction and Water Deficit Treatment
One plant per pot was used to examine the plant parameters. The plants received the following macro- and micronutrients contained in the nutrient solution in agreement with Lima and Lobato (2017). To simulate Na+ exposure, NaCl was used at concentrations of 0 and 150 mM Na+, which was applied over 15 days (days 25–40 after the start of the experiment). During the study, the nutrient solutions were changed at 07:00 h at 3-day intervals, with the pH adjusted to 5.5 using HCl or NaOH. On day 40 of the experiment, physiological and morphological parameters were measured for all plants, and leaf tissues were harvested for anatomical and biochemical analyses.
Measurement of Chlorophyll Fluorescence and Gas Exchange
Chlorophyll fluorescence was measured in fully expanded leaves under light using a modulated chlorophyll fluorometer (model OS5p; Opti-Sciences). Preliminary tests determined the location of the leaf, the part of the leaf, and the time required to obtain the greatest Fv/Fm ratio; therefore, the acropetal third of the leaves, which was the middle third of the plant and adapted to the dark for 30 min, was used in the evaluation. The intensity and duration of the saturation light pulse were 7500 µmol m−2 s−1 and 0.7 s, respectively. Gas exchange was evaluated in all plants and measured in the expanded leaves in the middle region of the plant using an infrared gas analyser (model LCPro+; ADC BioScientific) in a chamber under constant CO2, photosynthetically active radiation, air-flow rate, and temperature conditions at 360 μmol mol−1 CO2, 800 μmol photons m−2 s−1, 300 µmol s−1, and 28 °C, respectively, between 10:00 and 12:00 h. The water-use efficiency (WUE) was estimated according to Ma et al. (2004), and the instantaneous carboxylation efficiency (PN/Ci) was calculated using the formula that was described by Aragão et al. (2012).
Measurements of Anatomical Parameters
Samples were collected from the middle region of the leaf limb of fully expanded leaves, and roots were collected 5 cm from the root apex. Botanical material was fixed in FAA 70 for 24 h and then dehydrated in a series of ethanol and butanol before being embedded in histological paraffin (Johansen 1940). Transverse sections were prepared according to the procedures described by Maia et al. (2018). For stomatal characterization, the epidermal impression method was used as described by Segatto et al. (2004). The slides were observed and photographed under an optical microscope (Motic BA 310; Motic Group Co. LTD.) equipped with a digital camera (Motic 2500; Motic Group Co., LTD.). The images were analysed using Moticplus 2.0 software. In both leaf faces, the stomatal density (SD) was calculated as the number of stomata per unit area, and the stomatal functionality (SF) was calculated as the ratio PDS/EDS according to Castro et al. (2009). The stomatal index (SI %) was calculated as the percentage of stomata in relation to the total number of epidermal cells in a given area.
Determination of the Antioxidant Enzymes, Superoxide and Soluble Proteins
Antioxidant enzymes (SOD, CAT, APX, and POX), superoxide, and soluble proteins were extracted from leaf tissues according to the method of Badawi et al. (2004). The total soluble proteins were quantified using the methodology described by Bradford (1976). The SOD assay was measured at 560 nm (Giannopolitis and Ries 1977), and the SOD activity was expressed in mg−1 protein. The CAT assay was detected at 240 nm (Havir and McHale 1987), and the CAT activity was expressed in μmol H2O2 mg−1 protein min−1. The APX assay was measured at 290 nm (Nakano and Asada 1981), and the APX activity was expressed in μmol AsA mg−1 protein min−1. The POX assay was detected at 470 nm (Cakmak and Marschner 1992), and the activity was expressed in μmol tetraguaiacol mg−1 protein min−1. O2− was measured at 530 nm (Elstner and Heupel 1976).
Quantification of Hydrogen Peroxide, Malondialdehyde and Electrolyte Leakage
Stress indicators (H2O2 and MDA) were extracted using the methodology described by Wu et al. (2006). H2O2 was measured using the procedures described by Velikova et al. (2000). MDA was determined by the method of Cakmak and Horst (1991) using an extinction coefficient of 155 mM−1 cm−1. EL was measured according to Gong et al. (1998) and calculated by the formula EL (%) = (EC1/EC2) × 100.
Determination of Na+ Content, Photosynthetic Pigments and Biomass
Na+ contents were determined using flame photometry (model 910; Analyser) based on procedures described by Carmo et al. (2000). Chlorophyll and carotenoid determinations were performed using a spectrophotometer (model UV-M51; Bel Photonics) according to the methodology of Lichtenthaler and Buschmann (2001). The biomass of roots, stems, and leaves was measured based on constant dry weights (g) after drying in a forced-air ventilation oven at 65 °C.
Data Analysis
Data were subjected to normality of residuals using Shapiro–Wilk test and data applied to one-way ANOVA. Significant differences between the means were determined using the Scott-Knott test at a probability level of 5% (Steel et al. 2006). Standard deviations were calculated for each treatment.
Results
EBR Reduced Na+ Contents and Protected Tissue Structures in Salt-Stressed Plants
Tomato plants under salinity (150 mM NaCl) presented significant increases in Na+ contents (Table 1). However, EBR application induced reductions in roots (39%) and leaves (60%) compared to the treatment without EBR. For root structures, plants subjected to NaCl toxicity suffered reductions (Table 2 and Fig. 1). However, the application of 100 nM EBR in plants submitted to 150 mM NaCl promoted increases of 12%, 10%, 9%, 66%, and 119% in RET, RDT, RCD, VCD, and RMD, respectively, compared to comparable treatment without EBR. On leaf structures, salinity provoked negative interferences (Table 2 and Fig. 2). However, plants under the combined actions of NaCl and EBR had ETAd, ETAb, PPT, and SPT values that increased by 6%, 11%, 10%, and 14%, respectively, and the PPT/SPT ratio was reduced by 3% compared to similar treatments without EBR. Plants without NaCl and sprayed with EBR also exhibited increases in ETAd, ETAb, PPT, and SPT of 5%, 10%, 6%, and 13% and a reduction of 6% in PPT/SPT compared to treatment without NaCl and without EBR.
Steroid Alleviated the Damage Provoked by Salinity on the Photosynthetic Machinery
Na+ toxicity induced decreases in chloroplastic pigments (Table 3). Samples exposed to Na+ and EBR exhibited increases of 39%, 45%, 65%, and 40% in the variables Chl a, Chl b, Car, and Total Chl, respectively, but reductions of 3% and 12% in the ratios Chl a/Chl b and Chl total/Car, respectively, when compared to equal treatment (150 mM NaCl) without EBR. Regarding chlorophyll fluorescence, plants exposed to NaCl toxicity were negatively affected (Table 3 and Fig. 3). Plants under NaCl and EBR exhibited decreases of 10% and 1% in F0 and Fm, respectively. However, these plants presented increases of 2% and 4% in Fv and Fv/Fm, respectively, compared to the same parameters in plants exposed to a similar treatment without EBR. Plants under the combined effects of Na+ and EBR exhibited increases in ΦPSII (17%) and ETR (16%) and qp (2%) and reductions in NPQ (19%), EXC (8%), and ETR/PN (6%) compared with the same parameters in plants that received an equivalent treatment without EBR. Regarding gas exchange, Na+-induced toxicity provoked negative repercussions (Table 3). The spray with EBR in plants under salt stress promoted increases in PN, E, gs, WUE, and PN/Ci values by 15%, 6%, 22%, 11%, and 28%, respectively. However, a decrease of 1% in Ci occurred compared to plants exposed to Na+ without EBR.
Minimal fluorescence yield of the dark-adapted state (F0), maximal fluorescence yield of the dark-adapted state (Fm), variable fluorescence (Fv), and maximal quantum yield of PSII photochemistry (Fv/Fm) in tomato plants sprayed with EBR and exposed to salt stress. Columns with different letters indicate significant differences from the Scott-Knott test (P < 0.05). Columns corresponding to means from six repetitions and standard deviations
EBR Spray-induced Beneficial Effects on Stomatal Characteristics in Plants Under Na+ Stress
Salinity promoted negative effects on stomatal characteristics (Table 4). On the adaxial surface, plants exposed to NaCl and EBR had increases of 7%, 4%, and 14% in SD, SF, and SI, respectively, showing decreases of 3% in PDS and 7% for EDS. On the abaxial face, EBR promoted 7%, 6%, and 12% increases in SD, SF, and SI variables, as well as reductions of 2% and 7% in PDS and EDS variables, respectively, when compared to the respective parameters in plants that received similar treatments without EBR.
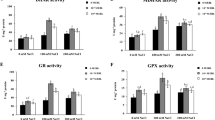
Redox Metabolism was Upregulated in Plants Sprayed with EBR and Subjected to Salt Toxicity
The high Na+ in the solution applied to plants caused increases in antioxidant enzyme activities (Fig. 4). Plants under the combined effects of Na+ and EBR exhibited increases of 5%, 22%, 24%, and 31% in SOD, CAT, APX, and POX, respectively, compared to the same parameters in plants that received the same treatment without EBR. Regarding stress indicators, salinity promoted increases (Fig. 5) but plants treated with Na+ and EBR presented reductions in O2−, H2O2, EL, and MDA values by 16%, 26%, 19%, and 24%, respectively, compared to the same parameters in plants that received similar treatments without EBR.
Activities of superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and peroxidase (POX) in tomato plants sprayed with EBR and exposed to salt stress. Columns with different letters indicate significant differences from the Scott-Knott test (P < 0.05). Columns corresponding to means from six repetitions and standard deviations
Superoxide (O2−), hydrogen peroxide (H2O2), malondialdehyde (MDA), and electrolyte leakage (EL) in tomato plants sprayed with EBR and exposed to salt stress. Columns with different letters indicate significant differences from the Scott-Knott test (P < 0.05). Columns corresponding to means from six repetitions and standard deviations
Pretreatment with EBR Mitigated the Negative Impacts Associated with Na+ on Biomass
Plants treated with Na+ exhibited significant decreases in biomass (Figs. 6, 7). EBR spray on plants exposed to NaCl induced increases in LDM, RDM, SDM, and TDM of 24%, 159%, 20%, and 61%, respectively, compared with the same parameters in plants that received equal treatment without EBR.
Leaf dry matter (LDM), root dry matter (RDM), stem dry matter (SDM), and total dry matter (TDM) in tomato plants sprayed with EBR and exposed to salt stress. Columns with different letters indicate significant differences from the Scott-Knott test (P < 0.05). Columns corresponding to means from six repetitions and standard deviations
Discussion
Treatment with 100 nM EBR reduced the Na+ contents in leaf and root tissues of tomato plants exposed to 150 mM NaCl for 15 days. EBR minimized Na+ contents, probably due to its beneficial action on cation transporters because under salt stress, K+ transporters are often blocked, resulting in Na+ uptake and causing reductions in K+/Na+ ratios in plants (Sarabi et al. 2017; Alam et al. 2019; Debnath et al. 2019). Na+ can accumulate in all plant tissues (leaves, stems, and roots); in other words, these ions can accumulate in the cytoplasm and/or vacuole (Hasegawa 2013) and consequently cause damage to root anatomical structures and photosynthetic machinery (Farooq et al. 2015). Dong et al. (2017) studied the effect of EBR spray on Triticum aestivum plants under salinity (120 mM NaCl) and reported that EBR (10 nM) significantly reduced Na+ contents in the roots.
EBR promoted increases in structures related to root anatomy (RET, RDT, RCD, VCD, and RMD). This steroid acts on several genes, such as the ERF2 and ERF5 genes, which are involved in ethylene biosynthesis and often have effects on the growth and division of meristematic tissues of the root, such as RET, RDT, and RCD (Mussig et al. 2003; Hacham et al. 2011). On the other hand, the plants subjected to 150 mM NaCl presented a reduction in all the anatomical parameters analysed, demonstrating the susceptibility of Lycopersicon esculentum roots to salt stress (Hameed et al. 2009; Céccoli et al. 2011). EBR spray induced increases in the RET, RDT, and RCD tissues, mitigating the adverse effects of NaCl because these structures are intrinsically involved in protection under abiotic stress conditions, such as saline stress (Cui 2016). Ribeiro et al. (2019) analysed Glycine max seedlings under conditions of water deficiency and reported that EBR had positive effects on RMD, which is an intensely vascularized tissue that contributes to the absorption of water and nutrients; they also verified the positive effects of EBR on RET, RDT, and VCD, indicating that EBR stimulated cell division and root growth.
Plants treated with NaCl and sprayed with EBR had positive outcomes in regard to leaf anatomy variables, such as ETAd, ETAb, PPT, SPT, and PPT/SPT ratio. Increases in PPT and SPT clearly contributed to increases verified in PN/Ci and PN, reflecting ETAd and ETAb (Abbruzzese et al. 2009). PPT and SPT are tissues with large amounts of chloroplasts and intracellular spaces and are both efficient channels for CO2 capture (Pereira et al. 2016; Maia et al. 2018). Salinity promotes negative interferences on gas exchange, as well as it lowers rates of expansion and cell division (Hu and Schmidhalter 2005). However, this steroid probably improved Ca+ 2 uptake, affecting cell expansion and division (Hayat et al. 2012), with a subsequent increase in the ETAd and ETAb values observed in our study. Oliveira et al. (2018) studied the deleterious effects of saline stress (0 and 250 mM NaCl) and the consequences of EBR administration (0 and 50 nM EBR) on young Eucalyptus urophylla plants and found increases in ETAd (7%), ETAb (22%), PPT (14%), and SPT (25%) after the application of EBR.
Plants subjected to Na+ and EBR presented increases in photosynthetic pigments, and this result was related to the role of EBR in mitigating the degradation of chlorophylls occasioned by photoinhibition. Photoinhibition is a phenomenon that occurs when photosynthetic pigments absorb light excessively, which leads to damage at the reaction centres of PSII, suggesting that EBR improves heat dissipation and electron transfer (Munns and Tester 2008b; Pereira et al. 2019). Alzahrani et al. (2019) evaluated the physiological, biochemical, and antioxidant responses of two Vicia faba genotypes subjected to three concentrations of NaCl (50, 100, and 150 mM) and found significant reductions of 54%, 51%, and 51% for Chl a, Chl b, and Car, respectively, when compared to the control in the Hassawi-3 genotype.
Steroids increased Fm, Fv, and Fv/Fm values and decreased F0, alleviating the damage provoked by salinity on PSII reaction centres. The low Fv/Fm values in plants under salinity are clear indicators of injury to thylakoid membranes due to negative interferences promoted by O2− in PSII, with subsequent inhibition of the photosynthesis process (Hussain and Reigosa 2011). In contrast, steroid protects the photosynthetic apparatus from ROS and attenuates the photoinhibition induced by excess NaCl in PSII reaction centres (Ahammed et al. 2012). In other words, steroid used probably interfered on translation of the psbA gene, which is required for transcription of pre-D1, a precursor of D1 protein in PSII. Salinity often inhibits the degradation of D1 protein during photoinhibition by blocking the interaction between FtsH, DegP2 proteases, and D1 protein (Ohnishi and Murata 2006). In addition, this study described that EBR caused increases in ΦPSII, ETR, and qp, while NPQ, EXC, and ETR/PN declined in plants pretreated with EBR and exposed to Na+. There results demonstrate the positive effects of this steroid on ETR and better utilization of light energy via qp, improving ΦPSII (Qin et al. 2011; Qiu et al. 2013; Kahlaoui et al. 2014). Wang et al. (2015) investigated chlorophyll fluorescence, leaf surface morphology, and cell ultrastructure in Vitis vinifera plants treated with EBR and exposed to water deficit and detected beneficial outcomes on Fv/Fm and ΦPSII, similar to the results found in this research.
Gas exchange was maximized, with increases in PN, E, gs, WUE, and PN/Ci and a decrease in Ci; these results indicate positive effects associated with exogenous application of EBR. Plants treated with EBR and Na+ had increases in SD and SI as previously reported, suggesting beneficial effects of EBR on the stomatal mechanism (Chaves et al. 2009). This led to an increase in the gs values, verified in this study in plants under the effect of 100 nM EBR and 150 mM NaCl, which led to higher CO2 absorption (PN/Ci) and a probable increase in the activity of RuBisCo, an enzyme responsible for CO2 fixation in the Calvin Cycle, and an ensuing increase in PN (Karlidag et al. 2011; Allel et al. 2018). The increase observed in WUE indicates a better utilization of the water resource, a part of the adaptive mechanisms against the deleterious effects caused by the high concentration of Na+ in the cells of the roots and leaves. In addition, the increase observed in E is intrinsically linked to gs, suggesting that EBR improves water inflow through stomatal adaptations, modulating tolerance to the osmotic and ionic imbalance induced by NaCl (Gupta et al. 2016; Hasanuzzaman et al. 2018). Our research corroborates the results of Shahbaz et al. (2008), who found increases in PN, E, and gs of two Triticum aestivum genotypes treated with EBR (0, 0.0125, 0.025, and 0.0375 mg L−1) and exposed to NaCl (0 and 150 mM).
Functional and anatomical characteristics of the stomata were improved upon treatment with EBR, resulting in increases in SD, SF, and SI values and decreases in PDS and EDS. It is conceivable that EBR stimulated transporters with a high affinity to K+, therefore, enhancing the absorption of this ion and attenuating the imbalance in the K+/Na+ ratio, which is important for the osmoregulation of cells and stomatal functioning (Chen et al. 2005). A consequent increase in SF may have improved the efficiency of the gas exchange process, as previously verified in this study. The absorption and assimilation of Na+ ions directly interfere with the functionality and anatomy of the stomata, resulting in reduction of SD and SF. However, plants sprayed with EBR had decreases in PDS and EDS, resulting in higher SD and SI. Khan et al. (2003) reported that smaller and elliptical stomata present higher density and greater functionality compared to large and cylindrical stomata, which has positive effects on WUE, CO2 absorption, and gas exchange (Souza et al. 2018). Our results confirm the study conducted by Maia et al. (2018) working with two Lycopersicon esculentum genotypes (BR-efficient and BR-deficient) sprayed with two EBR concentrations (0 and 100 nM) that showed that EBR increased SD, SF, and SI and decreased PDS and EDS values.
Plants treated with EBR and exposed to Na+ increased the activities of the SOD, CAT, APX, and POX enzymes, demonstrating the action of EBR in the cellular homeostatic processes linked to the reduced production of oxygen reactive species (ROS). Salinity frequently induces cellular redox imbalance, resulting in the accelerated accumulation of ROS (Parihar et al. 2015); however, our results revealed that EBR positively modulated the activity of enzymes responsible for the elimination of ROS through the antioxidant system (Fita et al. 2015). This system is composed of reactions that promote the detoxification of toxic radicals, such as O2− and H2O2, with the enzyme SOD being the first defence line by dismutation of O2− to H2O2 (Apel and Hirt 2004). Dong et al. (2017) studied EBR functions in relation to salt tolerance in Triticum aestivum seedlings subjected to three NaCl concentrations (0, 100, and 120 nM) and sprayed with 1, 10, and 100 nM EBR and found increases in the enzymatic activities of SOD and POX after spraying with 10 nM EBR when compared to the same treatment without EBR.
Steroid induced reductions in O2−, H2O2, MDA, and EL levels in plants under 150 mM NaCl and maximized the activities of the antioxidant enzymes intrinsically related to ROS removal previously described in this study. Paralelly, Oliveira et al. (2018) found that EBR application improves K+ uptake, which implies a reduction in the K+/Na+ ratio. K+ ions are responsible for cell osmoregulation, contributing among others to mitigate the production of ROS in chloroplasts in adverse conditions (Pang and Wang 2008; Khoshbakht et al. 2018). Our research corroborates the results described by Sun et al. (2015) investigating the application of EBR in Lolium perene plants under salt stress, observing reductions of 29% and 21% for H2O2 and MDA, respectively, when sprayed with 10 nM EBR.
Treatment with exogenous EBR attenuated the impacts on biomass in plants exposed to 150 mM NaCl, increasing LDM, RDM, SDM, and TDM. This may be directly related to the improvement promoted by EBR on photosynthetic pigments, PSII efficiency, stomatal characteristics, and antioxidant enzymes. Under salt stress, reduced rates of light absorption (Latrach et al. 2014), insufficient function of the photosynthetic machinery (Forieri et al. 2016a), cellular damage (Hu et al. 2016), and negative reflexes on leaf anatomy (Oliveira et al. 2018) are normally detected, and they result in lower biomass (Sharma et al. 2013). Interestingly, our study discovered multiple positive effects of this steroid; it mitigated the degradation of photosynthetic pigments via cell osmoregulation due to the reduced production of ROS and photochemical dissipation as demonstrated by reductions in NPQ, EXC, and ETR/PN. In addition, it favoured cell division and expansion, as verified in the anatomical variables PPT and SPT, resulting in increase in growth. Fariduddin et al. (2014) studied the physiological, biochemical, and morphological responses in Cucumis sativus plants under salt stress and observed an increase of 47% in TDM after spraying with 10−8 M EBR. Lima and Lobato (2017) studied Vigna unguiculata plants under water deficit combined with the application of EBR (0, 50, and 100 nM) and reported benefits on biomass, obtaining increases of 11%, 7%, 10%, and 10% for LDM, SDM, RDM, and TDM, respectively.
Conclusions
This research confirmed that pretreatment with EBR in tomato plants attenuated the deleterious effects associated with Na+ stress. EBR modulated protection and vascularization to root structures, demonstrated by the increases in epidermis thickness and metaxilem diameter, respectively. Concomitantly, this steroid relieved oxidative damage, which was clearly associated with elevation of the activities of antioxidant enzymes, such as superoxide ascorbate peroxidase and guaiacol peroxidase. EBR also had beneficial effects on photosynthetic pigments, alleviating the degradation of chlorophylls, concomitant with reductions in malondialdehyde and electrolyte leakage. In addition, pretreatment with EBR favoured higher biomass accumulation due to positive effects on leaf and root tissues, including improved performance of the photosynthetic apparatus. Therefore, these results suggest that pretreatment with 100 nM EBR clearly provided protection in tomato plants under salt stress.
Data Availability
Data are available upon request to the corresponding author.
Abbreviations
- APX:
-
Ascorbate peroxidase
- BRs:
-
Brassinosteroids
- CAR:
-
Carotenoids
- CAT:
-
Catalase
- Chl a :
-
Chlorophyll a
- Chl b :
-
Chlorophyll b
- C i :
-
Intercellular CO2 concentration
- CO2 :
-
Carbon dioxide
- E :
-
Transpiration rate
- EBR:
-
24-Epibrassinolide
- EDS:
-
Equatorial diameter of the stomata
- EL:
-
Electrolyte leakage
- ETAb:
-
Epidermis thickness from abaxial leaf side
- ETAd:
-
Epidermis thickness from adaxial leaf side
- ETR:
-
Electron transport rate
- ETR/P N :
-
Ratio between the apparent electron transport rate and net photosynthetic rate
- EXC:
-
Relative energy excess at the PSII level
- F0 :
-
Minimal fluorescence yield of the dark-adapted state
- Fm :
-
Maximal fluorescence yield of the dark-adapted state
- Fv :
-
Variable fluorescence
- Fv/Fm :
-
Maximal quantum yield of PSII photochemistry
- g s :
-
Stomatal conductance
- H2O2 :
-
Hydrogen peroxide
- K:
-
Potassium
- LDM:
-
Leaf dry matter
- MDA:
-
Malondialdehyde
- Mg:
-
Magnesium
- Na+ :
-
Sodium
- NPQ:
-
Nonphotochemical quenching
- O2 − :
-
Superoxide
- PDS:
-
Polar diameter of the stomata
- P N :
-
Net photosynthetic rate
- P N/C i :
-
Instantaneous carboxylation efficiency
- POX:
-
Peroxidase
- PPT:
-
Palisade parenchyma thickness
- PSII:
-
Photosystem II
- qP :
-
Photochemical quenching
- RCD:
-
Root cortex diameter
- RDM:
-
Root dry matter
- RDT:
-
Root endodermis thickness
- RET:
-
Root epidermis thickness
- ROS:
-
Reactive oxygen species
- RUBISCO:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- SD:
-
Stomatal density
- SDM:
-
Stem dry matter
- SF:
-
Stomatal functionality
- SI:
-
Stomatal index
- SOD:
-
Superoxide dismutase
- SPT:
-
Spongy parenchyma thickness
- TDM:
-
Total dry matter
- Total Chl:
-
Total chlorophyll
- VCD:
-
Vascular cylinder diameter
- WUE:
-
Water-use efficiency
- Φ PSII :
-
Effective quantum yield of PSII photochemistry.
References
Abbruzzese G, Beritognolo I, Muleo R et al (2009) Leaf morphological plasticity and stomatal conductance in three Populus alba L. genotypes subjected to salt stress. Environ Exp Bot 66:381–388. https://doi.org/10.1016/j.envexpbot.2009.04.008
Ahammed GJ, Choudhary SP, Chen S et al (2013) Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213. https://doi.org/10.1093/jxb/ers323
Ahammed GJ, Gao C-J, Ogweno JO et al (2012) Brassinosteroids induce plant tolerance against phenanthrene by enhancing degradation and detoxification in Solanum lycopersicum L. Ecotoxicol Environ Saf 80:28–36. https://doi.org/10.1016/j.ecoenv.2012.02.004
Ahammed GJ, Li X, Liu A, Chen S (2020) Brassinosteroids in plant tolerance to abiotic stress. J Plant Growth Regul. https://doi.org/10.1007/s00344-020-10098-0
Akhtar N, Hameed M, Nawaz F, et al (2017) Leaf anatomical and biochemical adaptations in Typha domingensis Pers. ecotypes for salinity tolerance. Bot Sci 95:807–821. https://doi.org/10.17129/botsci.886
Alam P, Albalawi TH, Altalayan FH et al (2019) 24-epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules. https://doi.org/10.3390/biom9110640
Allel D, Ben-Amar A, Abdelly C (2018) Leaf photosynthesis, chlorophyll fluorescence and ion content of barley (Hordeum vulgare) in response to salinity. J Plant Nutr 41:497–508. https://doi.org/10.1080/01904167.2017.1385811
Alzahrani S, Alaraidh I, Migdadi H, et al (2019) Physiological, biochemica, and antioxidant properties of two genotypes os Vicia faba grown under salinity stress. Pakistan J Bot 51:786–798. https://doi.org/10.30848/PJB2019
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Aragão RM, Silva EN, Vieira CF, Silveira JAG (2012) High supply of NO3 − mitigates salinity effects through an enhancement in the efficiency of photosystem II and CO2 assimilation in Jatropha curcas plants. Acta Physiol Plant 34:2135–2143. https://doi.org/10.1007/s11738-012-1014-y
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16. https://doi.org/10.1016/j.plantsci.2003.10.024
Badawi GH, Yamauchi Y, Shimada E et al (2004) Enhanced tolerance to salt stress and water deficit by overexpressing superoxide dismutase in tobacco (Nicotiana tabacum) chloroplasts. Plant Sci 166:919–928. https://doi.org/10.1016/j.plantsci.2003.12.007
Bai Y, Lindhout P (2007) Domestication and breeding of Tomatoes: what have we gained and what can we gain in the future? Ann Bot 100:1085–1094. https://doi.org/10.1093/aob/mcm150
Bouksila F, Bahri A, Berndtsson R et al (2013) Assessment of soil salinization risks under irrigation with brackish water in semiarid tunisia. Environ Exp Bot 92:176–185. https://doi.org/10.1016/j.envexpbot.2012.06.002
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Cai Z, Yang W, Zhang H et al (2018) Ethylene participates in the brassinolide-regulated asymmetric growth of O. sativa root. South African J Bot 119:86–93. https://doi.org/10.1016/j.sajb.2018.08.017
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468. https://doi.org/10.1111/j.1399-3054.1991.tb00121.x
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227. https://doi.org/10.1104/pp.98.4.1222
Cao Y, Gao Y, Li J, Tian Y (2019) Straw composts, gypsum and their mixtures enhance tomato yields under continuous saline water irrigation. Agric Water Manag 223:105721. https://doi.org/10.1016/j.agwat.2019.105721
Carmo CAFS, Araújo WS, Bernardi ACC, Saldanha MFC (2000) Métodos de análise de tecidos vegetais utilizados na Embrapa Solos. Embrapa Solos, Rio de Janeiro. http://www.infoteca.cnptia.embrapa.br/bitstream/doc/337672/1/Metododeanalisedetecido.pdf. (in portuguese)
Castro EM, Pereira FJ, Paiva R (2009) Plant histology: structure and function of vegetative organs. 234
Céccoli G, Ramos JC, Ortega LI et al (2011) Salinity induced anatomical and morphological changes in Chloris gayana Kunth roots. Biocell 35:9–17
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Chen M (2014) Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu Rev Biochem 83:317–340. https://doi.org/10.1146/annurev-biochem-072711-162943
Chen Z, Newman I, Zhou M et al (2005) Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant, Cell Environ 28:1230–1246. https://doi.org/10.1111/j.1365-3040.2005.01364.x
Choudhary SP, Yu J-Q, Yamaguchi-Shinozaki K et al (2012) Benefits of brassinosteroid crosstalk. Trends Plant Sci 17:594–605. https://doi.org/10.1016/j.tplants.2012.05.012
Cui H (2016) Middle cortex formation in the root: an emerging picture of integrated regulatory mechanisms. Mol Plant 9:771–773. https://doi.org/10.1016/j.molp.2016.05.002
Dalio RJD, Pinheiro HP, Sodek L, Haddad CRB (2011) The effect of 24-epibrassinolide and clotrimazole on the adaptation of Cajanus cajan (L.) Millsp. to salinity. Acta Physiol Plant 33:1887–1896. https://doi.org/10.1007/s11738-011-0732-x
Debnath M, Ashwath N, Midmore DJ (2019) Physiological and morphological responses to abiotic stresses in two cultivars of Stevia rebaudiana (Bert.) Bertoni. South Afr J Bot 123:124–132. https://doi.org/10.1016/j.sajb.2019.01.025
Dong YJ, Wang WW, Hu GQ et al (2017) Role of exogenous 24-epibrassinolide in enhancing the salt tolerance of wheat seedlings. J Soil Sci Plant Nutr 17:554–569. https://doi.org/10.4067/S0718-95162017000300001
El-Mashad AAA, Mohamed HI (2012) Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 249:625–635. https://doi.org/10.1007/s00709-011-0300-7
Elstner EF, Heupel A (1976) Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70:616–620. https://doi.org/10.1016/0003-2697(76)90488-7
Fariduddin Q, Mir BA, Yusuf M, Ahmad A (2014) 24-epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica 52:464–474. https://doi.org/10.1007/s11099-014-0052-7
Farooq M, Hussain M, Wakeel A, Siddique KHM (2015) Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron Sustain Dev 35:461–481. https://doi.org/10.1007/s13593-015-0287-0
Fita A, Rodríguez-Burruezo A, Boscaiu M et al (2015) Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front Plant Sci 6:1–14. https://doi.org/10.3389/fpls.2015.00978
Forieri I, Hildebrandt U, Rostás M (2016) Salinity stress effects on direct and indirect defence metabolites in maize. Environ Exp Bot 122:68–77. https://doi.org/10.1016/j.envexpbot.2015.09.007
Gerszberg A, Hnatuszko-Konka K (2017) Tomato tolerance to abiotic stress: a review of most often engineered target sequences. Plant Growth Regul 83:175–198. https://doi.org/10.1007/s10725-017-0251-x
Gerszberg A, Hnatuszko-Konka K, Kowalczyk T, Kononowicz AK (2015) Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant Cell, Tissue Organ Cult 120:881–902. https://doi.org/10.1007/s11240-014-0664-4
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Gong M, Li Y-J, Chen S-Z (1998) Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J Plant Physiol 153:488–496. https://doi.org/10.1016/S0176-1617(98)80179-X
González-García MP, Vilarrasa-Blasi J, Zhiponova M et al (2011) Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 138:849–859. https://doi.org/10.1242/dev.057331
Guerrero YR, Martínez González L, Dell`Amico J et al (2015) Reversion of deleterious effects of salt stress by activation of ROS detoxifying enzymes via foliar application of 24-epibrassinolide in rice seedlings. Theor Exp Plant Physiol 27:31–40. https://doi.org/10.1007/s40626-014-0029-8
Gupta P, Srivastava S, Seth CS (2016) 24-epibrassinolide and sodium nitroprusside alleviate the salinity stress in Brassica juncea L. cv. varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 411:483–498. https://doi.org/10.1007/s11104-016-3043-6
Hacham Y, Holland N, Butterfield C et al (2011) Brassinosteroid perception in the epidermis controls root meristem size. Development 138:839–848. https://doi.org/10.1242/dev.061804
Hafsi C, Falleh H, Saada M et al (2017) Potassium deficiency alters growth, photosynthetic performance, secondary metabolites content, and related antioxidant capacity in Sulla carnosa grown under moderate salinity. Plant Physiol Biochem 118:609–617. https://doi.org/10.1016/j.plaphy.2017.08.002
Hameed M, Ashraf M, Naz N (2009) Anatomical adaptations to salinity in cogon grass [Imperata cylindrica (L.) Raeuschel] from the Salt Range. Pakistan Plant Soil 322:229–238. https://doi.org/10.1007/s11104-009-9911-6
Hansen M, Chae HS, Kieber JJ (2009) Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J 57:606–614. https://doi.org/10.1111/j.1365-313X.2008.03711.x
Hasanuzzaman M, Shabala L, Zhou M et al (2018) Factors determining stomatal and non-stomatal (residual) transpiration and their contribution towards salinity tolerance in contrasting barley genotypes. Environ Exp Bot 153:10–20. https://doi.org/10.1016/j.envexpbot.2018.05.002
Hasegawa PM (2013) Sodium (Na+) homeostasis and salt tolerance of plants. Environ Exp Bot 92:19–31. https://doi.org/10.1016/j.envexpbot.2013.03.001
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol 84:450–455. https://doi.org/10.1104/pp.84.2.450
Hayat S, Maheshwari P, Wani AS et al (2012) Comparative effect of 28 homobrassinolide and salicylic acid in the amelioration of NaCl stress in Brassica juncea L. Plant Physiol Biochem 53:61–68. https://doi.org/10.1016/j.plaphy.2012.01.011
He J, Wang Y, Ding H, Ge C (2016) Epibrassinolide confers zinc stress tolerance by regulating antioxidant enzyme responses, osmolytes, and hormonal balance in Solanum melongena seedlings. Brazilian J Bot 39:295–303. https://doi.org/10.1007/s40415-015-0210-6
Hohmann-Marriott MF, Blankenship RE (2011) Evolution of Photosynthesis. Annu Rev Plant Biol 62:515–548. https://doi.org/10.1146/annurev-arplant-042110-103811
Hu Y, Schmidhalter U (2005) Drought and salinity: a comparison of their effects on mineral nutrition of plants. J Plant Nutr Soil Sci 168:541–549. https://doi.org/10.1002/jpln.200420516
Hu Y, Xia S, Su Y et al (2016) Brassinolide increases potato root growth in vitro in a dose-dependent way and alleviates salinity stress. Biomed Res Int 2016:1–11. https://doi.org/10.1155/2016/8231873
Huang HY, Jiang WB, Hu YW et al (2013) BR signal influences arabidopsis ovule and seed number through regulating related genes expression by BZR1. Mol Plant 6:456–469. https://doi.org/10.1093/mp/sss070
Hussain MI, Reigosa MJ (2011) A chlorophyll fluorescence analysis of photosynthetic efficiency, quantum yield and photon energy dissipation in PSII antennae of Lactuca sativa L. leaves exposed to cinnamic acid. Plant Physiol Biochem 49:1290–1298. https://doi.org/10.1016/j.plaphy.2011.08.007
Ibanes M, Fabregas N, Chory J, Cano-Delgado AI (2009) Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc Natl Acad Sci 106:13630–13635. https://doi.org/10.1073/pnas.0906416106
Johansen DA (1940) Plant microtechnique, 1th edn. New York
Johnson MP (2016) Photosynthesis. Essays Biochem 60:255–273. https://doi.org/10.1042/EBC20160016
Kahlaoui B, Hachicha M, Rejeb S et al (2014) Response of two tomato cultivars to field-applied proline under irrigation with saline water: growth, chlorophyll fluorescence and nutritional aspects. Photosynthetica 52:421–429. https://doi.org/10.1007/s11099-014-0053-6
Kalaji HM, Bąba W, Gediga K et al (2018) Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth Res 136:329–343. https://doi.org/10.1007/s11120-017-0467-7
Kalaji HM, Govindjee BK et al (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot 73:64–72. https://doi.org/10.1016/j.envexpbot.2010.10.009
Kang HK, Nam KH (2016) Reverse function of ROS-induced CBL10 during salt and drought stress responses. Plant Sci 243:49–55. https://doi.org/10.1016/j.plantsci.2015.11.006
Karlidag H, Yildirim E, Turan M (2011) Role of 24-epibrassinolide in mitigating the adverse effects of salt stress on stomatal conductance, membrane permeability, and leaf water content, ionic composition in salt stressed strawberry (Fragaria×ananassa). Sci Hortic (amsterdam) 130:133–140. https://doi.org/10.1016/j.scienta.2011.06.025
Khan PSSV, Kozai T, Nguyen QT et al (2003) Growth and water relations of Paulownia fortunei under photomixotrophic and photoautrophic conditions. Biol Plant 46:161–166. https://doi.org/10.1023/A:1022844720795
Khoshbakht D, Asghari MR, Haghighi M (2018) Influence of foliar application of polyamines on growth, gas-exchange characteristics, and chlorophyll fluorescence in Bakraii citrus under saline conditions. Photosynthetica 56:731–742. https://doi.org/10.1007/s11099-017-0723-2
Latrach L, Farissi M, Mouradi M et al (2014) Growth and nodulation of alfalfa-rhizobia symbiosis under salinity: electrolyte leakage, stomatal conductance, and chlorophyll fluorescence. Turkish J Agric for 38:320–326. https://doi.org/10.3906/tar-1305-52
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Current Protocols in Food Analytical Chemistry. John Wiley & Sons Inc, Hoboken, NJ, USA, pp 431–438
Lima JV, Lobato AKS (2017) Brassinosteroids improve photosystem II efficiency, gas exchange, antioxidant enzymes and growth of cowpea plants exposed to water deficit. Physiol Mol Biol Plants 23:59–72. https://doi.org/10.1007/s12298-016-0410-y
Ma JF, Mitani N, Nagao S et al (2004) Characterization of the silicon uptake system and molecular mapping of the silicon transporter gene in rice. Plant Physiol 136:3284–3289. https://doi.org/10.1104/pp.104.047365
Maia CF, Silva BRS, Lobato AKS (2018) Brassinosteroids positively modulate growth: physiological, biochemical and anatomical evidence using two tomato genotypes contrasting to dwarfism. J Plant Growth Regul 37:1–14. https://doi.org/10.1007/s00344-018-9802-2
Maita S, Sotomayor C (2015) The effect of three plant bioregulators on pollen germination, pollen tube growth and fruit set in almond prunus dulcis (Mill.) D.A. Webb. Cvs. Non pareil and carmel. Electron J Biotechnol 18:381–386. https://doi.org/10.1016/j.ejbt.2015.07.004
Mao J, Zhang D, Li K et al (2017) Effect of exogenous Brassinolide (BR) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Plant Growth Regul 82:391–401. https://doi.org/10.1007/s10725-017-0264-5
Mazorra LM, Oliveira MG, Souza AF et al (2013) Involvement of brassinosteroids and ethylene in the control of mitochondrial electron transport chain in postharvest papaya fruit. Theor Exp Plant Physiol 25:203–212. https://doi.org/10.1590/s2197-00252013005000003
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Müssig C (2005) Brassinosteroid-PROMOTED GROWTH. Plant Biol 7:110–117. https://doi.org/10.1055/s-2005-837493
Mussig C, Shin G, Altmann T (2003) Brassinosteroids promote root growth in Arabidopsis. Plant Physiol 133:1261–1271. https://doi.org/10.1104/pp.103.028662.However
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nishiyama Y, Allakhverdiev SI, Yamamoto H et al (2004) Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43:11321–11330. https://doi.org/10.1021/bi036178q
Ohnishi N, Murata N (2006) Glycinebetaine counteracts the inhibitory effects of salt stress on the degradation and synthesis of D1 protein during photoinhibition in Synechococcus sp. PCC 7942. Plant Physiol 141:758–765. https://doi.org/10.1104/pp.106.076976
Oliveira VP, Lima MDR, Silva BRS et al (2018) Brassinosteroids confer tolerance to salt stress in Eucalyptus urophylla plants enhancing homeostasis, antioxidant metabolism and leaf anatomy. J Plant Growth Regul 38:557–573. https://doi.org/10.1007/s00344-018-9870-3
Pang C-H, Wang B-S (2008) Oxidative stress and salt tolerance in plants. Prog Bot 69:231–245. https://doi.org/10.1007/978-3-540-72954-9_9
Parihar P, Singh S, Singh R et al (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075. https://doi.org/10.1007/s11356-014-3739-1
Pereira MP, de Rodrigues LC, Corrêa FF et al (2016) Cadmium tolerance in Schinus molle trees is modulated by enhanced leaf anatomy and photosynthesis. Trees 30:807–814. https://doi.org/10.1007/s00468-015-1322-0
Pereira YC, Rodrigues WS, Lima EJA, et al (2019) Brassinosteroids increase electron transport and photosynthesis in soybean plants under water deficit. Photosynthetica 57:181–191. https://doi.org/10.32615/ps.2019.029
Qadir M, Quillérou E, Nangia V et al (2014) Economics of salt-induced land degradation and restoration. Nat Resour Forum 38:282–295. https://doi.org/10.1111/1477-8947.12054
Qin LQ, Li L, Bi C et al (2011) Damaging mechanisms of chilling- and salt stress to Arachis hypogaea L. leaves. Photosynthetica 49:37–42. https://doi.org/10.1007/s11099-011-0005-3
Qiu Z, Wang L, Zhou Q (2013) Effects of bisphenol A on growth, photosynthesis and chlorophyll fluorescence in above-ground organs of soybean seedlings. Chemosphere 90:1274–1280. https://doi.org/10.1016/j.chemosphere.2012.09.085
Qureshi AS, McCornick PG, Qadir M, Aslam Z (2008) Managing salinity and waterlogging in the Indus Basin of Pakistan. Agric Water Manag 95:1–10. https://doi.org/10.1016/j.agwat.2007.09.014
Rattan A, Kapoor D, Kapoor N et al (2020) Brassinosteroids regulate functional components of antioxidative defense system in salt stressed maize seedlings. J Plant Growth Regul 39:1465–1475. https://doi.org/10.1007/s00344-020-10097-1
Rengasamy P (2010) Soil processes affecting crop production in salt-affected soils. Funct Plant Biol 37:613–620. https://doi.org/10.1071/FP09249
Ribeiro DGS, Silva BRS, Lobato AKS (2019) Brassinosteroids induce tolerance to water deficit in soybean seedlings: contributions linked to root anatomy and antioxidant enzymes. Acta Physiol Plant 41:1–11. https://doi.org/10.1007/s11738-019-2873-2
Risopatron JPM, Sun Y, Jones BJ (2010) The vascular cambium: molecular control of cellular structure. Protoplasma 247:145–161. https://doi.org/10.1007/s00709-010-0211-z
Ruban AV (2015) Evolution under the sun: optimizing light harvesting in photosynthesis. J Exp Bot 66:7–23. https://doi.org/10.1093/jxb/eru400
Sarabi B, Bolandnazar S, Ghaderi N, Ghashghaie J (2017) Genotypic differences in physiological and biochemical responses to salinity stress in melon (Cucumis melo L.) plants: Prospects for selection of salt tolerant landraces. Plant Physiol Biochem 119:294–311. https://doi.org/10.1016/j.plaphy.2017.09.006
Sasse JM (2003) Physiological actions of brassinosteroids: an update. J Plant Growth Regul 22:276–288. https://doi.org/10.1007/s00344-003-0062-3
Segatto FB, Bisognin DA, Benedetti M et al (2004) A technique for the anatomical study of potato leaf epidermis. Ciência Rural 34:1597–1601. https://doi.org/10.1590/S0103-84782004000500042
Shahbaz M, Ashraf M, Athar HUR (2008) Does exogenous application of 24-epibrassinolide ameliorate salt induced growth inhibition in wheat (Triticum aestivum L.)? Plant Growth Regul 55:51–64. https://doi.org/10.1007/s10725-008-9262-y
Shahzad B, Tanveer M, Che Z et al (2018) Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol Environ Saf 147:935–944. https://doi.org/10.1016/j.ecoenv.2017.09.066
Sharma I, Ching E, Saini S et al (2013) Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol Biochem 69:17–26. https://doi.org/10.1016/j.plaphy.2013.04.013
Sheikh-Mohamadi MH, Etemadi N, Nikbakht A et al (2017) Antioxidant defence system and physiological responses of Iranian crested wheatgrass (Agropyron cristatum L.) to drought and salinity stress. Acta Physiol Plant 39:1–16. https://doi.org/10.1007/s11738-017-2543-1
Shelke DB, Pandey M, Nikalje GC et al (2017) Salt responsive physiological, photosynthetic and biochemical attributes at early seedling stage for screening soybean genotypes. Plant Physiol Biochem 118:519–528. https://doi.org/10.1016/j.plaphy.2017.07.013
Shu S, Tang Y, Yuan Y et al (2016) The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol Biochem 107:344–353. https://doi.org/10.1016/j.plaphy.2016.06.021
Silva BRS, Batista BL, da Silva Lobato AK (2020) Anatomical changes in stem and root of soybean plants submitted to salt stress. Plant Biol plb.13176. https://doi.org/10.1111/plb.13176
Souza P, Lima L, Soares T et al (2018) Biometric, physiological and anatomical responses of Passiflora spp. to controlled water deficit. Sci Hortic (amsterdam) 229:77–90. https://doi.org/10.1016/j.scienta.2017.10.019
Steel RG, Torrie JH, Dickey DA (2006) Principles and procedures of statistics: a biometrical approach, 3rd edn. Academic Internet Publishers, Moorpark
Stevens J, Senaratna T, Sivasithamparam K (2006) Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul 49:77–83. https://doi.org/10.1007/s10725-006-0019-1
Sun S, An M, Han L, Yin S (2015) Foliar application of 24-epibrassinolide improved salt stress tolerance of perennial ryegrass. HortScience 50:1518–1523
Suresh BV, Roy R, Sahu K et al (2014) Tomato genomic resources database: an integrated repository of useful tomato genomic information for basic and applied research. PLoS ONE 9:e86387. https://doi.org/10.1371/journal.pone.0086387
Tang W, Yuan M, Wang R et al (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol 13:124–131. https://doi.org/10.1038/ncb2151
Tanveer M, Shahzad B, Sharma A et al (2018) 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: a review. Plant Physiol Biochem 130:69–79. https://doi.org/10.1016/j.plaphy.2018.06.035
Tavakkoli E, Fatehi F, Coventry S et al (2011) Additive effects of Na+ and Cl- ions on barley growth under salinity stress. J Exp Bot 62:2189–2203. https://doi.org/10.1093/jxb/erq422
Tong H, Xiao Y, Liu D et al (2014) Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in ricec. Plant Cell 26:4376–4393. https://doi.org/10.1105/tpc.114.132092
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vogler F, Schmalzl C, Englhart M et al (2014) Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reprod 27:153–167. https://doi.org/10.1007/s00497-014-0247-x
Wang B, Zhang J, Xia X, Zhang W-H (2011) Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regul 65:407–413. https://doi.org/10.1007/s10725-011-9595-9
Wang Z, Zheng P, Meng J, Xi Z (2015) Effect of exogenous 24-epibrassinolide on chlorophyll fluorescence, leaf surface morphology and cellular ultrastructure of grape seedlings (Vitis vinifera L.) under water stress. Acta Physiol Plant 37:1729. https://doi.org/10.1007/s11738-014-1729-z
Wu Q-S, Xia R-X, Zou Y-N (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 163:1101–1110. https://doi.org/10.1016/j.jplph.2005.09.001
Yu X, Liang C, Chen J et al (2015) The effects of salinity stress on morphological characteristics, mineral nutrient accumulation and essential oil yield and composition in Mentha canadensis L. Sci Hortic (amsterdam) 197:579–583. https://doi.org/10.1016/j.scienta.2015.10.023
Zheng Q, Liu J, Liu R et al (2016) Temporal and spatial distributions of sodium and polyamines regulated by brassinosteroids in enhancing tomato salt resistance. Plant Soil 400:147–164. https://doi.org/10.1007/s11104-015-2712-1
Acknowledgements
This research received financial support from Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), and Universidade Federal Rural da Amazônia (UFRA/Brazil) to Lobato AKS. In addition, VQ Sousa and WFS Messias were supported with scholarships from Programa de Educação Tutorial (PET/Brazil). The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/180), King Saud University, Riyadh, Saudi Arabia.
Funding
The funder was funded by Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Universidade Federal Rural da Amazônia (UFRA/Brazil), Programa de Educação Tutorial (PET/Brazil), Researchers Supporting Project Number (RSP-2020/180), Grant Number (RSP-2021/180).
Author information
Authors and Affiliations
Contributions
AKSL and PA were the advisors of this project, planning all phases of the research and critically revised the manuscript. VQS, WFSM, YCP, BRSS, and EMSGL conducted the experiment and performed physiological, biochemical, anatomical, and morphological determinations, as well as wrote and edited the manuscript. MNA critically revised the manuscript. All authors read and approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Andrzej Bajguz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sousa, V.Q., Messias, W.F.S., Pereira, Y.C. et al. Pretreatment with 24-Epibrassinolide Synergistically Protects Root Structures and Chloroplastic Pigments and Upregulates Antioxidant Enzymes and Biomass in Na+-Stressed Tomato Plants. J Plant Growth Regul 41, 2869–2885 (2022). https://doi.org/10.1007/s00344-021-10481-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-021-10481-5