Abstract
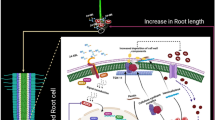
Brassinosteroids (BRs) can effectively alleviate the oxidative stress caused by Ca(NO3)2 in cucumber seedlings. The root system is an essential organ in plants due to its roles in physical anchorage, water and nutrient uptake, and metabolite synthesis and storage. In this study, 24-epibrassinolide (EBL) was applied to the cucumber seedling roots under Ca(NO3)2 stress, and the resulting chemical and anatomical changes were characterized to investigate the roles of BRs in alleviating salinity stress. Ca(NO3)2 alone significantly induced changes in the components of cell wall, anatomical structure, and expression profiles of several lignin biosynthetic genes. Salt stress damaged several metabolic pathways, leading to cell wall reassemble. However, EBL promoted cell expansion and maintained optimum length of root system, alleviating the oxidative stress caused by Ca(NO3)2. The continuous transduction of EBL signal thickened the secondary cell wall of casparian band cells, thus resisting against ion toxicity and maintaining water transport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil secondary salinization is an important factor that limits crop production in China. The high level of Ca(NO3)2 accumulation may lead to soil salinization. Too much Ca(NO3)2 in soil results in oxidative stress and metabolic disorders in plants, reducing their biomass yield (Zhang et al. 2008).
Plant cell walls have protective and structural functions and are therefore highly resistant to degradation. The secondary cell wall is mainly composed of three types of polymers: cellulose, hemicelluloses, and lignin, which primarily exists in xylem, fibers, and anther cells. Cellulose microfibrils along with hemicelluloses constitute the main load-bearing network, in which lignin imparts “waterproofing” capacity as well as mechanical strength, rigidity, and environmental protection. Secondary cell walls are thicker than the primary walls and are resistant to compressive forces (Doblin et al. 2010).
Cell walls protect cells from various environmental stresses such as wounding, mineral stress, osmotic stress, cold acclimation, drought tolerance, and salt stress (de Lima et al. 2014). Cell wall lignification occurs as a stress response and provides structural rigidity and durability to plant tissues (Kim and Triplett 2008). Salinity stress has been recognized as a factor contributing to increased lignin deposition in vascular tissues and/or xylem development (Srivastava et al. 2015; Sánchez-Aguayo et al. 2004).
Brassinosteroids (BRs) are plant hormones involved in a wide range of plant developmental processes including cell division and elongation, seed germination, and vascular differentiation (Schumacher and Chory 2000). 24-Epibrassinolide (EBL) is one of the most bioactive compounds among BRs, which are perceived by the plasma membrane receptor, brassinosteroid insensitive 1 (BRI 1) (Li and Chory 1997; Friedrichsen et al. 2000). The signal is then transmitted from the plasma membrane to the nucleus, where dephosphorylation of BES1 and BZR1 is triggered, allowing them to dimerize and bind DNA to regulate the expression of hundreds of genes (Hacham et al. 2011).
Numerous studies suggest that BR may be an essential regulator in cellulose and lignin biosynthesis and accumulation. The T-DNA insertional mutant of DIM1/DWF1/CBB1, which is involved in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis, leads to a deficiency in brassinosteroid accumulation and displays a dwarf phenotype with up to 38 and 23% reduction in total lignin and cellulose, respectively (Klahre et al. 1998; Hossain et al. 2012). The sterol-deficient Arabidopsis mutants, fackel, hydra1, and sterol methyltransferase1/cephalopod exhibit incomplete cell walls and aberrant cell wall thickenings, and are accompanied with ectopic lignin deposits (Schrick et al. 2004). EBL application resulted in significant modification in hemicellulose and cellulose biosynthesis in the secondary xylem of Liriodendron tulipifera (Jin et al. 2014). However, relatively few studies have been performed to determine the effect of EBL on the cell wall in cucumber seedlings subjected to salt stress.
Cucumber (Cucumis sativus L.) is an important vegetable crop grown under protected cultivation worldwide and is highly sensitive to salinity. In the present study, the effects of EBL on lignin biosynthesis and cell wall polysaccharide fractions in cucumber roots under Ca(NO3)2 stress were investigated. And we also attempted to determine the roles of EBL in the maintenance of normal water transport and plant growth under salt stress by observing lignin deposition regions and evaluating the expression of cell wall degradation enzymes at the transcription level.
Materials and methods
Plant materials and treatments
Cucumber (Cucumis sativus L., cv. “Jinyou No. 4”) seeds were obtained from the Tianjin Kernel Cucumber Research Institute, China. Seeds were placed on filter papers in Petri dishes in the dark at 29 ± 1 °C for 24 h. The germinated seeds were transferred into plastic trays (41 × 41 × 5 cm) containing quartz sand. The greenhouse conditions for plant growth were set as follows: 25–30 °C during the day and 15–18 °C during the night, natural light with relative humidity of 60–75%. When the second leaves were fully expanded, cucumber plants were transplanted into plastic containers containing half strength Hoagland solution with an electrical conductivity (EC) of 2.0–2.2 ds m−1 and renewed every 3 days. The nutrient solution was aerated using an air pump with an interval of 20 min to maintain the dissolved oxygen concentration of 8.0 ± 0.2 mg L−1 during the experiment.
When the third leaves were fully expanded, the cucumber seedlings were treated as follows: (1) control (Cont), half strength Hoagland solution; (2) CB, Cont +10−3 mg L−1 EBL (Sigma Aldrich, USA, applied to the solution); (3) N, half strength Hoagland solution containing 80 mM Ca(NO3)2; and (4) NB, N + 10−3 mg L−1 of EBL (applied to the solution). EBL was dissolved in ethanol, with Cont and N treatments containing the same ethanol level. Samples were collected after treatment for 72 h, immediately frozen in liquid nitrogen, and stored at − 80 °C. The experiment was carried out with three biological replicates, and each treatment consisted of 12 × 3 (repetitions) cucumber seedlings.
Lignin determination
Lignin was quantified according to the method of Liyama and Wallis with slight modifications (Iiyama and Wallis 1990). Approximately 40 mg of frozen tissues were placed and ground in a mortar. The samples were extracted three times with 3 mL of 80% (v/v) ethanol at 80 °C for 1.5 h, followed by extraction in 3 mL of chloroform at 62 °C for 1 h. The extracted samples were then dried at 50 °C for 2 days. Dried segments were dissolved in 2.6 mL of 25% (w/w) acetyl bromide in acetic acid containing 2.7% (v/v) perchloric acid. After digestion for 1 h, 100 μL of mixture was added to a test tube containing 580 μL of 17.24% (v/v) 2 N sodium hydroxide and 82.76% (v/v) acetic acid. About 20 μL of 7.5 mol/L hydroxylamine hydrochloride was added to terminate the reaction. The volume was corrected to 2 mL with acetic acid and the absorbance at A280 was recorded. The concentration of AcBr-soluble lignin was calculated by using an extinction coefficient (Iiyama and Wallis 1988).
Cell wall preparation and fractionation
Cell wall extraction and subsequent fractionation were performed according to the method provided by Zhu and her colleagues (Zhu et al. 2012). In brief, the collected samples were ground in liquid nitrogen with a mortar and pestle. The samples were then homogenized with 75% ethanol on the ice for 20 min and centrifuged at 7000 rpm for 12 min. The pellets were kept and successively washed with acetone, methanol-chloroform mixture (1:1, v/v), and methanol for 20 min. The remaining samples were freeze-dried and stored at 4 °C for further analysis.
The cell walls were divided into pectin, hemicellulose 1 (HC1), hemicellulose 2 (HC2), and cellulose, respectively, after fractionation. The pectin fraction was extracted twice with 5 cm3 0.5% ammonium oxalate buffer which contained 0.1% NaBH4 (pH 4) at 100 °C for 1 h. The supernatants and the sediment were separately harvested. The pellets were further extracted by three incubations with 5 mL of 4% KOH harboring 0.1% NaBH4 at room temperature for 10 h, followed by a similar extraction with 24% KOH/0.1% NaBH4. The pooled supernatants from the 4 and 24% KOH extractions were centrifuged at 14,000 rpm for 15 min to obtain HC1 and HC2 fractions, respectively. The sediment from the 24% KOH extraction was then dried by freezing in high vacuum, weighed and recognized as the cellulose fraction.
Uronic acid and total polysaccharide analysis
The content of uronic acid in pectin fraction was measured in accordance with the methods described by Blumenkrantz and Asboe-Hansen (Blumenkrantz and Asboe-Hansen 1973). Here, galacturonic acid (Sigma) was used as a standard. In simple terms, approximately 200 μL of pectin extracts was mixed with 1 mL of 98% H2SO4 that contained 0.0125 M Na2B4O7.10H2O in a boiled bath for 5 min. The mixture after cooling was added by 20 μL of M-hydro-diphenyl (0.15%) and was allowed to incubate at 25 °C for 25 min. The absorbance at 520 nm was recorded.
The total levels of polysaccharide in the hemicellulose fractions were measured according to the method by Dubois and his colleagues (Dubois et al. 1956). In brief, 200 μL of hemicellulose 1 (HC1) fractions was mixed with 1 mL of 98% H2SO4 and 10 μL of 80% phenol and placed at 25 °C for 20 min. The mixture was then incubated in a boiled bath for 15 min. The absorbance at 490 nm was recorded.
Anatomical structure analysis by histochemistry and autofluorescence
The anatomical structure of root tip was identified using a combination of histochemistry and fluorescence microscopy. The root tips were fixed in FAA (38% formaldehyde/glacial acetic acid/70% ethanol, 5:5:90, v/v/v) for over 24 h. The isolated root tips were subsequently dehydrated with ethanol and embedded in paraffin. The samples were then cut into sections and stained with toluidine blue-O (1%, w/v) to show the anatomical structure of root cross section.
For phloroglucinol staining, the sections were immersed in phloroglucinol solution [2% in ethanol:water (95:5)] for 5 min. Subsequently, the sections were subjected to concentrated HCl for 3 min. Lignified tissues were stained with red under an Olympus BX51 microscope.
Lignified tissues may be excited under ultraviolet (UV) radiation and produce fluorescence emission. To observe lignin autofluorescence, sections with lignified tissues were shown by fluorescence microscopy under UV excitation.
RT-PCR and qRT-PCR analysis
Genes were selected from NCBI and cucumber databases (cucumber.genomics.org.cn), and primers were designed by Primer Premier 6.0. Total RNA was isolated from cucumber roots using the TRI reagent as described in the manufacturer’s protocol. Subsequently, the total RNA (1 μg) was converted into cDNA using the PrimeScript™ 1st strand cDNA synthesis kit (TaKaRa, Dalian, China) according to the manufacturer’s protocol. Real-time quantitative PCR was carried out with SYBR PrimeScript RT-PCR Kit (TaKaRa, Dalian, China) and run on a StepOne™ real-time PCR system (Applied Biosystems, Singapore) following the manufacturer’s recommendations.
The PCR reactions were carried out in a mixture consisting of 2 μL of diluted cDNA, 10 μL of SYBR Premix ExTaq™ II (2×), 0.8 μL of each specific primer, 0.4 μL of ROX reference dye, and 6 μL of ddH2O, yielding a final volume of 20 μL. The reaction conditions were as follows: 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 1 min, and a final extension of 95 °C for 15 s. Gene expression fold changes were calculated using the delta-delta CT method, and the relative mRNA expression level was normalized against a reference gene, actin. Gene-specific primers used for real-time quantitative PCR are listed in Supplementary Table 1.
Statistical analysis
Data were collected and statistically analyzed using SAS software (SAS Institute, Cary, NC, USA) using Duncan’s multiple range test at a significance level of 0.05.
Results
Cell wall polysaccharides and lignin
Secondary cell walls are composites that contain pectin, cellulose, and hemicelluloses, often being encrusted with lignin. The polysaccharide was obtained through the sequential extraction of cell wall components in the roots of control, Ca(NO3)2-stressed and EBL-applied cucumber seedlings. The total sugar content of hemicelluloses ranged from 45 to 53 mg g−1, whereas values of 50 to 71 mg g−1 were observed for the total amount of pectic polysaccharides. The content of cellulose reached 514–615 mg g−1, but no significant difference was detected among different treatments for 3 days (data not shown). After 7 days, sugar content in the EBL-applied roots showed a decrease in hemicellulose I, hemicellulose II, and cellulose fractions compared with that in the control. Ca(NO3)2 treatments did not show any significant effect on sugar content of cell wall in cucumber seedling roots (Fig. 1).
Effect of exogenous EBL on sugar content in the cell wall of cucumber seedling roots under Ca(NO3)2 stress. Samples were collected on the seventh day after treatment. Cont, control (half strength Hoagland solution); CB, Con + 10−3 mg L−1 EBL; N, Con + 80 mM Ca(NO3)2 (half strength Hoagland solution containing 80 mM Ca(NO3)2); NB, N + 10−3 mg L−1 of EBL
Uronic acid and lignin accumulation in each cell wall fraction from the roots of control and treated seedlings was measured (Fig. 2). Ca(NO3)2 treatments considerably increased the uronic acid content in the pectin and hemicellulose fractions. EBL application further promoted uronic acid accumulation under Ca(NO3)2 stress. The application of EBL to the Ca(NO3)2 stress resulted in a remarkable enhancement in the pectin levels compared to the control plants (2.8-fold) and salt-stressed plants (1.7-fold) after 7 days. The content of lignin showed no significant change among different treatments after 24 h; after 3 days, a dramatic increase in lignin occurred in the presence of Ca(NO3)2, and finally after 7 days, the Ca(NO3)2-stressed plants had the highest value for lignin accumulation; however, the application of EBL could relieve this trend under Ca(NO3)2 stress.
Effect of exogenous EBL on lignin and uronic acid accumulation in the cell wall of cucumber seedling roots under Ca(NO3)2 stress. Cont, control (half strength Hoagland solution); CB, Con + 10−3 mg L−1 EBL; N, Con + 80 mM Ca(NO3)2 (half strength Hoagland solution containing 80 mM Ca(NO3)2); NB, N + 10−3 mg L−1 of EBL
Lignin distribution
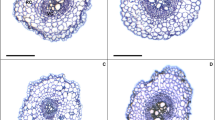
Lignin polymers are primarily derived from the guaiacyl (G) and syringyl (S) units, which can be reflected by Mäule reaction and phloroglucinol staining, respectively. As shown in Fig. 3, Ca(NO3)2 promoted the differentiation of the metaxylem as well as the accumulation of lignin in primary xylem of cucumber seedling roots after 7 days. Compared with the Ca(NO3)2-stressed plants, exogenous EBL altered lignin distribution under salt stress. The endodermal cells, which are closed to the pericycle, were stained with a bright violet-red color. However, no difference was detected in the cucumber roots exposed to different treatments when the root sections were stained with Mäule reaction (data not shown). The UV-excited fluorescence in the roots of cucumber was shown in Fig. 4. Autofluorescence was easily observed in the presence of Ca(NO3)2. Meanwhile, the images reflected by autofluorescence were in accordance with the results shown by phloroglucinol staining.
Effect of exogenous EBL on lignin G monomer accumulation in the root system of cucumber seedlings under Ca(NO3)2 stress. Yellow arrow shows the area of casparian band, and the green arrow shows xylem. Scale bars in N and NB at 7 days are 50 μm, whereas others are 100 μm in length. Cont, control (half strength Hoagland solution); CB, Con + 10−3 mg L−1 EBL; N, Con + 80 mM Ca(NO3)2 (half strength Hoagland solution containing 80 mM Ca(NO3)2); NB, N + 10−3 mg L−1 of EBL
Effect of exogenous EBL on lignin autofluorescence in the root system of cucumber seedlings under Ca(NO3)2 stress. Yellow arrow shows the area of casparian band, and the green arrow shows xylem. Scale bar = 50 μm. Cont, control (half strength Hoagland solution); CB, Con + 10−3 mg L−1 EBL; N, Con + 80 mM Ca(NO3)2 (half strength Hoagland solution containing 80 mM Ca(NO3)2); NB, N + 10−3 mg L−1 of EBL
Key gene expression of lignin biosynthesis
Exogenous EBL application resulted in significant changes in lignin content and altered the monomeric composition at the cellular level under Ca(NO3)2 stress. Therefore, expression of lignin biosynthesis genes was determined by qRT-PCR (Fig. 5).The expression of PAL, F5H783, and LFA POD in the Ca(NO3)2-stressed roots were significantly downregulated at the transcriptional level compared to that in the control after 24 h. By contrast, COMT788 and COMT109 were upregulated after salinity treatment for 24 h, suggesting that exogenously applied Ca(NO3)2 can induce lignin synthesis. As time was extended to 3 days, expression levels of PAL, COMT788, COMT109, and LAC525 in the Ca(NO3)2-stressed roots were significantly increased compared to those of controls. Meanwhile, expression of PAL, COMT109, COMT290, CCoAOMT121, LAC525, and LAC947 maintained a lower level by the application of EBL under Ca(NO3)2 stress, suggesting a dramatic reduction in the rate of lignin synthesis. After 7 days, the expression of most of the selected genes in the EBL-applied roots were significantly upregulated at the transcriptional level compared to that in the control plants, which may contribute to an increase in syringyl monolignols.
Effect of exogenous EBL on the expression of lignin biosynthesis related enzymes at the transcriptional level in cucumber seedling roots under Ca(NO3)2 stress. Cont, control (half strength Hoagland solution); CB, Con + 10−3 mg L−1 EBL; N, Con + 80 mM Ca(NO3)2 (half strength Hoagland solution containing 80 mM Ca(NO3)2); NB, N + 10−3 mg L−1 of EBL
Gene expression of cell wall modification enzymes
Ca(NO3)2-stressed plants exhibited marked expansion in root diameter and increased numbers of lateral roots, indicating the intensification of cell division and differentiation. Therefore, qRT-PCR was employed to detect the expression of three genes involved in cell wall modification in cucumber (Fig. 6). Compared with control plants, the expression of glycosyltransferase was significantly upregulated at the transcriptional level by Ca(NO3)2 treatment as time went on. In the roots treated with EBL and Ca(NO3)2, transcript levels of glycosyltransferase and β-D-galactosidase were highly expressed at 24 h, followed by a drastic decline.
Effect of exogenous EBL on the expression of cell wall degradation enzyme at the transcriptional level in cucumber seedling roots under Ca(NO3)2 stress. Cont, control (half strength Hoagland solution); CB, Con + 10−3 mg L−1 EBL; N, Con + 80 mM Ca(NO3)2 (half strength Hoagland solution containing 80 mM Ca(NO3)2); NB, N + 10−3 mg L−1 of EBL
MAPK signaling pathway plays important roles in cell division, initiation of developmental processes, and responses to abiotic and biotic stresses. It has been reported that BRs could control cell patterning via BIN2-mediated suppression of MKK4/5 activity (Khan et al. 2013). The expression patterns of MKK4/5 in Ca(NO3)2-stressed plants were similar to that of glycosyltransferase. The application of exogenous EBL resulted in a decrease in MKK4/5 expression compared to that in the control.
BR signaling cascade
The expression of positive and negative components in the BR signaling cascade was analyzed at the transcriptional level (Fig. 7). The expression of BRI1 and BIN2 changed at 24 h in the presence of Ca(NO3)2, suggesting that exogenously applied Ca(NO3)2 slightly induced BR signaling. However, as time went on, expression levels of BAK1 still maintained a lower level, whereas transcript levels of other positive BR signaling regulators (BRI1, BSU1, and BZR1) were significantly increased compared to those of control. By contrast, expression levels of BRI1 and BIN2 were significantly reduced by exogenous EBL under Ca(NO3)2 stress, suggesting that exogenously applied EBL regulated BR signaling. After 7 days, expression levels of BRI1, BSU1, and BZR1 in EBL-applied Ca(NO3)2-stressed roots still maintained a higher level compared with control plants.
Effect of exogenous EBL on the expression of BR signaling-related enzymes at the transcriptional level in cucumber seedling roots under Ca(NO3)2 stress. Cont, control (half strength Hoagland solution); CB, Con + 10−3 mg L−1 EBL; N, Con + 80 mM Ca(NO3)2 (half strength Hoagland solution containing 80 mM Ca(NO3)2); NB, N + 10−3 mg L−1 of EBL
Discussion
Salt stress is one of the major factors that retard crop yield and production. Here, we found that the number of lateral root and root diameter increased to adapt to the environmental stress. Cell wall plays an important role in the maintenance of tissue morphology and signal transduction. Secondary cell walls are restricted to specific types of differentiated cells and are composites of cellulose and hemicelluloses, often being encrusted with lignin (Cosgrove and Jarvis 2012). The polysaccharide is a principal component of plant cell wall (Agoda-Tandjawa et al. 2012). The total sugar content in the cell wall can indicate the amount of cell wall polysaccharides and further reflect the cell wall density. Previous studies have shown that cell wall polysaccharides may undergo an obvious alteration when exposed to salt stress (de Lima et al. 2014). Here, cell wall polysaccharide content was not significantly changed under Ca(NO3)2 stress in our experiment. However, the levels of hemicellulose and cellulose were decreased when exogenous EBL was applied (Fig. 1), suggesting that EBL has an effect on the cell wall component.
It was reported that hemicellulose can form a primary network via hydrogen bonding to cellulose microfibrils and may also be linked to acidic pectins by covalent attachment (Cosgrove 2005). Therefore, it is an essential component for maintaining wall framework. The structural and compositional change in hemicellulose is a way to adapt to wall extension and cell elongation. Ca(NO3)2 treatments as well as application of EBL considerably promoted the uronic acid content in the hemicellulose fractions, indicating expansion of cells (Fig. 2). Lignin, mainly distributed in secondarily thickened cell walls, is involved in many aspects of plant developmental processes (You et al. 2013). In this study, salt stress significantly increased lignin content; however, the increasing rate of lignin content in EBL-applied seedlings was higher than that in Ca(NO3)2-stressed seedling roots (Fig. 2). EBL may change the cell wall polysaccharide fractions and lignin accumulation, leading to cell wall reassembly under salt stress.
Lignin polymers are mainly derived from p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) monolignols, which are formed by the dehydrogenation of the hydroxycinnamyl alcohols (Zhao and Dixon 2011). An increased number of lignified tracheary elements was observed in tomato roots under salinity stress, which enhances water transport and ion uptake (Sánchez-Aguayo et al. 2004). In addition, a previous study reported that the width of the lignified region of casparian strip was increased under salinity stress in maize roots (Karahara et al. 2004). It is reported that the casparian band has minor effect on water movement, but it impedes the movement of ions (Peterson and Steudle 1993). Phloroglucinol staining showed a higher lignin accumulation closed to casparian strip when exogenous EBL was applied under Ca(NO3)2 stress (Fig. 3). Therefore, EBL could operate the degree of lignification around casparian strips and provide defense against osmotic stress.
Lignin biosynthesis pathway has been established, and the main structure enzymes involved in this pathway have been well isolated and characterized (Zhong and Ye 2007). Monolignols are derived from phenylalanine that are deaminated to cinnamic acid, followed by a series of enzyme reactions (Jia et al. 2015). It is reported that the proportion of S units was severely decreased when COMT transcription was suppressed (Tsai et al. 1998; Lapierre et al. 1999). Here, Ca(NO3)2 significantly increased the synthesis of S monomer, but this phenomenon was alleviated to a certain extent after EBL application. Lignin is finally formed by dehydrogenation polymerization (POD or LAC) after the synthesis of monolignols (Zhao and Dixon 2011). During the period of EBL-Ca(NO3)2 treatment, the expression of LAC and POD genes were also altered. These results suggested EBL can change lignin accumulation under Ca(NO3)2 stress.
β-galactosidase (β-Gal) is an enzyme involved in cell wall degradation, which can degrade the pectin and hemicellulose as well as glycoproteins and glycolipids, making components in the cell wall unstable (Dwevedi and Kayastha 2009; Bell et al. 2013). In our experiment, expression of β-D-galactosidase was significantly upregulated for 24 h and maintained at a relatively stable level. Golgi glycosyltransferases, which are involved in xylan biosynthesis, were markedly upregulated at the transcriptional level by Ca(NO3)2 treatment along with the increase of stress time (Fig. 6). MAP kinases have been identified as essential signal integration components in response to biotic and abiotic stress, hormone stimuli, and cell division (Marshall, 1994). It has been demonstrated that BIN2 and its homologs, GSK3/Shaggy-like kinases, can phosphorylate the MAPK kinases MKK4 and MKK5 (Khan et al. 2013). Here, transcription of MKK4/5 was decreased at first and then remained at a relatively stable level. Several studies have attributed the growth defects of BR mutants primarily to impaired cell expansion and cell division (Clouse and Sasse 1998; Savaldi-Goldstein et al. 2007; Hacham et al. 2011). Therefore, BR may maintain the elongation of root system by keeping normal expansion of root cells.
BRs signal through plasma membrane localized receptor BRI1 and other components including negatively acting BIN2 kinase to regulate BES1/BZR1 family transcription factors, which control the expression of downstream genes for various BR responses (Kim et al. 2009). Expression levels of BRI in Ca(NO3)2-stressed plant roots still maintained a higher level than in the control (Fig. 7), indicating that receptor-like kinases are induced by abiotic stress itself, thereby amplifying the signal for the necessary stress adaption response (Lindner et al. 2012).
In many dicot species, lateral root primordia are derived from the pericycle, a layer of cells that lies immediately within the endodermis (Robbins et al. 2014). Auxin signaling is also activated in endodermal cells to meet primordial growth (Gibbs and Coates 2014). Cell wall loosening is activated by auxin to allow the cells in the outer tissue layers to separate and grow as lateral root primordia (Robbins et al. 2014). As a result, the auxin signaling promotes the formation of lateral roots under salt stress and maintains normal water transport. However, the concrete effects of exogenous EBL on cell division and expansion of root cells, and the relationship between BRs and IAA in the process of lateral root formation need to be further researched.
Conclusions
Effects of applied EBL on the changes in cell wall components and anatomical structure of cucumber seedling roots subjected to salt stress were investigated. Phloroglucinol staining indicated increased S/G ratio after Ca(NO3)2 treatment. Expression analysis indicated that Ca(NO3)2 treatment promoted the expression of COMT genes, in consistence with the results shown by phloroglucinol staining. However, this phenomenon was alleviated by the addition of EBL. EBL may promote cell expansion and maintained optimum length of root system, alleviating the oxidative stress caused by Ca(NO3)2. The continuous transduction of EBL signal thickened the secondary cell wall of casparian band cells, which resisted ion toxicity, and maintained water transport.
Abbreviations
- BRs:
-
Brassinosteroids
- EBL:
-
24-Epibrassinolide
- BRI1:
-
Brassonosteroid insensitive 1
- BES1/BZR1:
-
bri1 EMS suppressor 1/brassinazole resistant 1
- PAL:
-
Phenylalanine ammonialyase
- F5H:
-
Ferulate-5-hydroxylas
- COMT:
-
Caffeicacid-3-O-methyltransferase
- CCoAOMT:
-
Caffeoyl-CoA-3-O-methyltransferase
- LAC:
-
Laccase
- LFA POD:
-
Lignin-forming anionic peroxidase
- BAK1:
-
BRI1-associated kinase 1
- BSU1:
-
bri1 suppressor 1
- BIN2:
-
Brassinosteroid insensitive 2
References
Agoda-Tandjawa G, Durand S, Gaillard C, Garnier C, Doublier JL (2012) Properties of cellulose/pectins composites: implication for structural and mechanical properties of cell wall. Carbohydr Polym 90(2):1081–1091. https://doi.org/10.1016/j.carbpol.2012.06.047
Bell AN, Magill E, Hallsworth JE, Timson DJ (2013) Effects of alcohols and compatible solutes on the activity of β-galactosidase. Appl Biochem Biotechnol 169(3):786–794. https://doi.org/10.1007/s12010-012-0003-3
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54(2):484–489. https://doi.org/10.1016/0003-2697(73)90377-1
Clouse SD, Sasse JM (1998) Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol 49(1):427–451. https://doi.org/10.1146/annurev.arplant.49.1.427
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6(11):850–861. https://doi.org/10.1038/nrm1746
Cosgrove DJ, Jarvis MC (2012) Comparative structure and biomechanics of plant primary and secondary cell walls. Front Plant Sci 3:204. https://doi.org/10.3389/fpls.2012.00204
de Lima RB, dos Santos TB, Vieira LG, de Lourdes Lúcio Ferrarese M, Ferrarese-Filho O, Donatti L, Boeger MR, de Oliveira Petkowicz CL (2014) Salt stress alters the cell wall polysaccharides and anatomy of coffee (Coffea arabica L.) leaf cells. Carbohydr Polym 112:686–694. https://doi.org/10.1016/j.carbpol.2014.06.042
Doblin MS, Pettolino F, Bacic A (2010) Plant cell walls: the skeleton of the plant world. Funct Plant Biol 37(5):357–381. https://doi.org/10.1071/FP09279
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Dwevedi A, Kayastha AM (2009) A beta-galactosidase from pea seeds (PsBGAL): purification, stabilization, catalytic energetics, conformational heterogeneity, and its significance. J Agric Food Chem 57(15):7086–7096. https://doi.org/10.1021/jf900874p
Friedrichsen DM, Joazeiro CA, Li J, Hunter T, Chory J (2000) Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase 1. Plant Physiol 123(4):1247–1255. https://doi.org/10.1104/pp.123.4.1247
Gibbs DJ, Coates JC (2014) AtMYB93 is an endodermis-specific transcriptional regulator of lateral root development in Arabidopsis. Plant Signal Behav 9(10):e970406. https://doi.org/10.4161/15592316.2014.970406
Hacham Y, Holland N, Butterfield C, Ubeda-Tomas S, Bennett MJ, Chory J, Savaldi-Goldstein S (2011) Brassinosteroid perception in the epidermis controls root meristem size. Development 138(5):839–848. https://doi.org/10.1242/dev.061804
Hossain Z, Mcgarvey B, Amyot L, Gruber M, Jung J, Hannoufa A (2012) DIMINUTO 1 affects the lignin profile and secondary cell wall formation in Arabidopsis. Planta 235(3):485–498. https://doi.org/10.1007/s00425-011-1519-4
Iiyama K, Wallis AFA (1988) An improved acetyl bromide procedure for determining lignin in woods and wood pulps. Wood Sci Technol 22(3):271–280. https://doi.org/10.1007/BF00386022
Iiyama K, Wallis AFA (1990) Determination of lignin in herbaceous plants by an improved acetyl bromide procedure. J Sci Food Agric 51(2):145–161. https://doi.org/10.1002/jsfa.2740510202
Jia XL, Wang GL, Xiong F, XR Y, ZS X, Wang F, Xiong AS (2015) De novo assembly, transcriptome characterization, lignin accumulation, and anatomic characteristics: novel insights into lignin biosynthesis during celery leaf development. Sci Rep 5(1):8259. https://doi.org/10.1038/srep08259
Jin H, Do J, Shin SJ, Choi JW, Choi YI, Kim W, Kwon M (2014) Exogenously applied 24-epi brassinolide reduces lignification and alters cell wall carbohydrate biosynthesis in the secondary xylem of Liriodendron tulipifera. Phytochemistry 101:40–51. https://doi.org/10.1016/j.phytochem.2014.02.003
Karahara I, Ikeda A, Kondo T, Uetake Y (2004) Development of the Casparian strip in primary roots of maize under salt stress. Planta 219(1):41–47. https://doi.org/10.1007/s00425-004-1208-7
Khan M, Rozhon W, Bigeard J, Pflieger D, Husar S, Pitzschke A, Teige M, Jonak C, Hirt H, Poppenberger B (2013) Brassinosteroid-regulated GSK3/shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. J Biol Chem 288(11):7519–7527. https://doi.org/10.1074/jbc.M112.384453
Kim HJ, Triplett B (2008) Involvement of extracellular Cu/Zn superoxide dismutase in cotton fiber primary and secondary cell wall biosynthesis. Plant Signal Behav 3(12):1119–1121. https://doi.org/10.4161/psb.3.12.7039
Kim TW, Guan S, Sun Y, Deng Z, Tang W, Shang JX, Sun Y, Burlingame AL, Wang ZY (2009) Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol 11(10):1254–1260. https://doi.org/10.1038/ncb1970
Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua NH (1998) The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell 10(10):1677–1690. https://doi.org/10.1105/tpc.10.10.1677
Lapierre C, Pollet B, Petit-Conil M, Toval G, Romero J, Pilate G, Leple JC, Boerjan W, Ferret VV, De Nadai V, Jouanin L (1999) Structural alterations of lignins in transgenic poplars with depressed cinnamyl alcohol dehydrogenase or caffeic acid O-methyltransferase activity have an opposite impact on the efficiency of industrial kraft pulping. Plant Physiol 119(1):153–163. https://doi.org/10.1104/pp.119.1.153
Li J, Chory J (1997) A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90(5):929–938. https://doi.org/10.1016/S0092-8674(00)80357-8
Lindner H, Müller LM, Boisson-Dernier A, Grossniklaus U (2012) CrRLK1L receptor-like kinases: not just another brick in the wall. Curr Opin Plant Biol 15(6):659–669. https://doi.org/10.1016/j.pbi.2012.07.003
Marshall CJ (1994) MAP kinase kinase kinase, MAP kinase kinase and MAP kinase. Curr Opin Genet Dev 4(1):82–89. https://doi.org/10.1016/0959-437X(94)90095-7
Peterson CA, Steudle E (1993) Lateral hydraulic conductivity of early metaxylem vessels in Zea mays L. roots. Planta 189:288–297
Robbins NE, Trontin C, Duan L, Dinneny JR (2014) Beyond the barrier: communication in the root through the endodermis. Plant Physiol 166(2):551–559. https://doi.org/10.1104/pp.114.244871
Sánchez-Aguayo I, Rodríguez-Galán JM, García R, Torreblanca J, Pardo JM (2004) Salt stress enhances xylem development and expression of S-adenosyl-l-methionine synthase in lignifying tissues of tomato plants. Planta 220(2):278–285. https://doi.org/10.1007/s00425-004-1350-2
Savaldi-Goldstein S, Peto C, Chory J (2007) The epidermis both drives and restricts plant shoot growth. Nature 446(7132):199–202. https://doi.org/10.1038/nature05618
Schrick K, Fujioka S, Takatsuto S, Stierhof YD, Stransky H, Yoshida S, Jürgens G (2004) A link between sterol biosynthesis, the cell wall, and cellulose in Arabidopsis. Plant J 38(2):227–243. https://doi.org/10.1111/j.1365-313X.2004.02039.x
Schumacher K, Chory J (2000) Brassinosteroid signal transduction: still casting the actors. Curr Opin Plant Biol 3(1):79–84. https://doi.org/10.1016/S1369-5266(99)00038-2
Srivastava S, Vishwakarma RK, Arafat YA, Gupta SK, Khan BM (2015) Abiotic stress induces change in Cinnamoyl CoA reductase (CCR) protein abundance and lignin deposition in developing seedlings of Leucaena leucocephala. Physiol Mol Biol Plants 21(2):197–205. https://doi.org/10.1007/s12298-015-0289-z
Tsai CJ, Popko JL, Mielke MR, WJ H, Podila GK, Chiang VL (1998) Suppression of O-methyltransferase gene by homologous sense transgene in quaking aspen causes red-brown wood phenotypes. Plant Physiol 117(1):101–112. https://doi.org/10.1104/pp.117.1.101
You TT, Mao JZ, Yuan TQ, Wen JL, Xu F (2013) Structural elucidation of the lignins from stems and foliage of Arundo donax linn. J Agric Food Chem 61(22):5361–5370. https://doi.org/10.1021/jf401277v
Zhang GW, Liu ZL, Zhou JG, Zhu YL (2008) Effects of Ca(NO3)2 stress on oxidative damage, antioxidant enzymes activities and polyamine contents in roots of grafted and non-grafted tomato plants. Plant Growth Regul 56(1):7–19. https://doi.org/10.1007/s10725-008-9281-8
Zhao Q, Dixon RA (2011) Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci 16(4):227–233. https://doi.org/10.1016/j.tplants.2010.12.005
Zhong R, Ye Z (2007) Regulation of cell wall biosynthesis. Curr Opin Plant Biol 10(6):564–572. https://doi.org/10.1016/j.pbi.2007.09.001
Zhu XF, Lei GJ, Jiang T, Liu Y, Li GX, Zheng SJ (2012) Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 236(4):989–997. https://doi.org/10.1007/s00425-012-1652-8
Funding
This work was supported by the National Natural Science Foundation of China (No. 31471869, No. 31401919 and No. 31272209), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PDPA), China Agriculture Research System (CARS-25-C-03), and sponsored by the Research Fund for the Doctoral Program of Higher Education (20130097120015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Bhumi Nath Tripathi
Electronic supplementary material
Supplementary Table 1
(XLS 38 kb)
Rights and permissions
About this article
Cite this article
An, YH., Zhou, H., Yuan, YH. et al. 24-Epibrassinolide-induced alterations in the root cell walls of Cucumis sativus L. under Ca(NO3)2 stress. Protoplasma 255, 841–850 (2018). https://doi.org/10.1007/s00709-017-1187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1187-8