Abstract
This study was performed to determine if a high supply of N-NO3 − is capable of mitigating negative salinity effects on photosynthesis and growth through the stimulation of nitrate assimilation, which could act as an sink from photosynthetic electron transport chain and restrict the over reduction in thylakoid membrane in Jatropha curcas leaves. The experiment was arranged in a factorial design with two nitrate concentrations (1 and 10 mM) and two NaCl levels (0 and 100 mM). Salt-stressed plants supplied with high NO3 − demonstrated a higher nitrate uptake rate, nitrate reductase activity and soluble-protein content when compared with plants that presented low nitrate uptake. High nitrate assimilation was associated with higher leaf growth, CO2 assimilation and lower membrane damage in salt-stressed plants. The superior performance of salt-stressed plants grown with high NO3 − was indicated by a higher effective quantum yield of PSII and electron transport rate and lower energy excess at the PSII level and non-photochemical quenching. Interestingly, a high NO3 − level in the absence of NaCl did not alter the leaf growth, photochemical activity and gas exchange parameters when compared with plants supplied with low nitrate. The proline and glycinebetaine contents were similarly increased in both low- and high-NO3 − salt-stressed plants. Our data suggest that the favorable effects induced by high nitrate supply were possibly associated with stimulation in the nitrate assimilatory pathway. This process might have acted as a sink of electrons from the thylakoid membranes minimizing photo-damage and stimulating CO2 assimilation under salinity in J. curcas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High salinity and low soil nitrogen (N) availability are important growth-limiting factors for most plants species (Villa-Castorena et al. 2003). Increasing the availability of N in soils through fertilizer utilization can improve crop productivity and mitigate some stressful factors (Albassam 2001). In saline soil, by mechanisms yet unknown, the supply of N might alleviate the adverse effects of salinity (Flores et al. 2003). Nitrate is often the main nitrogen source in agricultural soil, and the uptake and assimilation of nitrogen are strongly affected by salinity (Silveira et al. 2001). Nitrate reductase (NR, E.C.1.7.1.1) is the most important enzyme involved in the nitrate assimilation pathway (Campbell 1999). The second step, nitrite reduction to ammonia, occurs in chloroplasts and is a strong consumer of electrons from reduced ferredoxin (Campbell 1999).
The final step of N assimilation involves the glutamine synthetase/glutamate synthase activities (the GS/GOGAT cycle), which also occurs in chloroplasts. The reactions of this cycle consume ATP and reduced ferredoxin (Lea and Azevedo 2007). The GS/GOGAT cycle produces glutamine and glutamate, which can act as amino-acids initiators for several pathways involved with the synthesis of amino acids, proteins and other compounds essential for plant growth (Rocha et al. 2012). Most of these reactions are consumers of energy and electrons as NAD(P)H, reduced ferredoxin and ATP. Thus, the whole nitrate assimilation process is a strong consumer of electrons in the photosynthetic electron-transport chain, and can thus, alleviate the “electron pressure” on photosystem II (Osmond and Forster 2006).
The electron excess and over-reduction of the thylakoid membranes is frequent under the combined conditions of high light and abiotic stress. Stress conditions, such as salinity, induce stomatal closure and impairment in CO2 assimilation (Adams et al. 2006). Under these conditions, the plants can deploy several mechanisms to avoid or minimize photo-damage, photo-inhibition and oxidative stress (Silva et al. 2010a). The most common processes utilized are photorespiration, heat dissipation and the xanthophyll cycle. Recently, Osmond and Forster (2006) have suggested other processes, such as N metabolism, growth and respiration. However, the mechanisms associated with nitrate assimilation and energy dissipation in photosystems are currently unknown.
Because a large fraction of leaf nitrogen is allocated to the photosynthetic apparatus, especially for Rubisco content, the leaf N content can also influence the photosynthetic capacity (Hikosaka and Hirose 2000). An N deficit necessarily promotes the remobilization of nitrogen from Rubisco (Paul and Foyer 2001) the salinity might decrease the nitrate acquisition, and as a consequence, the Rubisco content and the photosynthesis rates could decrease (Soussana et al. 2000). There are reports that nitrate improves the growth of salt-stressed citrus by improving photosynthetic activity and reducing chloride accumulation (Iglesias et al. 2004). However, in maté plants supplied with different forms of N, a higher photosynthetic efficiency was observed in the plants supplied with N-NH4 + when compared to plants supplied with N-NO3 − (Gaiad et al. 2006).
Another important strategy used by plants in response to abiotic stress is the accumulation of compatible solutes (Silva et al. 2010b). High levels of N might stimulate the accumulation of nitrogenous solutes, such as proline and glycinebetaine (Chen et al. 2007). Thus, the N-induced accumulation of these solutes could mitigate the negative effects of salinity by increments of osmotic adjustment and the protection of proteins and membranes against damage caused by reactive oxygen species (ROS) (Silveira et al. 2009). Our recent studies have demonstrated that amino acids, glycinebetaine and soluble sugars are effective and important mediators of the osmotic adjustment of J. curcas young plants under conditions of high salinity and drought (Silva et al. 2009, 2010b).
Jatropha curcas is a species that grows in marginal areas where other crop species are not able to survive (Francis et al. 2005). In addition, J. curcas has high economic potential due to its seed-oil quality, which can be converted to biodiesel for industrial use (King et al. 2009). Although this species has shown satisfactory yield under the constraining conditions of semiarid regions, such as drought and high temperature (Silva et al. 2010a), there are few studies regarding the salt tolerance associated with nitrate nutrition. In this study, we tested the hypothesis that a high supply of NO3 − can mitigate the negative effects of the salinity by increasing the nitrate assimilation rate. This process might contribute to an improvement in the photosynthetic electron transport and CO2 assimilation, because it acts as an electron sink, thus attenuating thylakoid over-reduction. Our study evaluated changes in nitrate assimilation, photosystem II efficiency and CO2 assimilation, and it evaluated proline and glycinebetaine accumulation in the absence and presence of salinity in J. curcas plants cultivated under high and low exogenous nitrate levels.
Materials and methods
Plant material and experimental conditions
The experiment was conducted in a greenhouse under natural conditions (3°44′S; 38°33′W, at sea level), and the environmental conditions were as follows: an average air temperature of 29 °C, a mean air relative humidity of 65 %, an average maximum photosynthetic photon flux density (PPFD) of approximately 1,300 μmol m−2 s−1 and a photoperiod of 12 h. Jatropha curcas seeds were supplied by the Fazenda Tamanduá (Santa Terezinha, PB, Brazil), and they were selected by taking into account the seed size and weight. Eight days after the germination in sand, the seedlings were transferred to plastic pots (2 L) containing quarter-strength Hoagland and Arnon (1950) nutrient solution (pH 6.0) in the first week and were given half-strength nutrient solution for the remainder of the experiment.
Twenty-three days after germination, the NO3 − and NaCl treatments were applied. The plants were divided into two groups and supplied with 1 mM NO3 − as 0.25 mM Ca(NO3 −)2 and 0.5 mM KNO3, which represented the N1 treatment, or 10 mM NO3 − in the form of 2.5 mM Ca(NO3 −)2 and 5 mM KNO3, which represented the N10 treatment, dissolved in a complete nutrient solution. The Ca2+ and K+ concentrations were kept at 3.0 mM and 6.0 mM, respectively, in all nutrient solutions by utilization of CaCl2 and KCl. The other nutrients were utilized according to a half-strength Hoagland and Arnon (1950) nutrient solution. These two groups of plants were simultaneously subjected to salt stress over 10 days by the dissolution of 100 mM NaCl in the nutritive solution. To avoid osmotic shock, the NaCl was added to the solution in two subsequent steps (50 mmol NaCl L−1 day−1). Two new treatments were then initiated (N1+Salt and N10+Salt). The N10 treatment was used as the control or reference. Nitrate concentration was monitored daily and adjusted to 1 or 10 mM as necessary. The in vivo photosynthesis measurements were performed at 10:00 a.m. in a fully expanded leaf. A similar leaf was subsequently used to determine the NR activity. After 5 hours of sunshine, the plant material was harvested at 11:00 a.m. to allow for the induction of nitrate reductase.
Relative water content, membrane damage, dry weight and chlorophyll content in leaves
The leaf relative water content (RWC) was calculated as follows: RWC = [(FW − DW)/(TW − DW)] × 100, where FW is the fresh weight, TW is the turgid weight measured after 6 h of saturation in deionized water at 4 °C in the dark, and DW is the dry weight determined after 48 h in an oven at 75 °C (Silveira et al. 2009). The electrolyte leakage (EL) in the leaves was determined as previously described (Silva et al. 2010a). At the end of the experiment, the plants were collected, and the total leaf dry matter was obtained by drying the leaves in an oven at 75 °C for 48 h. The total chlorophyll concentration was calculated as previously described (Silva et al. 2010c).
Leaf gas exchange and chlorophyll fluorescence measurements
The leaf gas exchange and chlorophyll fluorescence parameters were measured using a portable photosynthesis system (LI-6400-XT) and a leaf chamber fluorometer (6400-40), respectively (LI-COR, USA). The measurements were performed in fully expanded and mature leaves under constant CO2 concentration and PPFD (~380 μmol mol−1 CO2 and 1,000 μmol photons m−2 s−1, respectively). The air-flow rate was 300 μmol s−1. The actinic light intensity was 1,000 μmol photons m−2 s−1. Measurements were recorded when the total coefficient of variation (CV) was <5 %. To reduce the time for measurement stabilization, the air pumped into the LI-6400-XT was passed through a buffering gallon (5 L). There was an approximate 1- to 2-min time lag to acquire the steady-state level of fluorescence. Measurements of the leaf CO2 assimilation rate (P N, in μmol m−2 s−1), transpiration (E, in mmol m−2 s−1), stomatal conductance (g S, in mol m−2 s−1) and intercellular CO2 concentration (C I) in Pa were taken, and the instantaneous carboxylation efficiency (P N/C I, in μmol m−2 s−1 Pa−1) and the ratio between apparent electron-transport rate (ETR) and leaf CO2 assimilation rate (ETR/P N in μmol μmol−1) were calculated. For leaves that were in the light or that had adapted to the dark for 30 min, the chlorophyll fluorescence measurements were taken using the saturation pulse method. Prior to this determination, a test subjecting the leaf to different dark periods (10, 20, 30, 40, 50 and 60 min) was performed to obtain the best time for the F v/F m measurement. After the optimization of the dark adaptation time, the measured values of F m and F m′ were used to calculate the non-photochemical quenching (NPQ). The intensity and duration of the saturation light pulse were 8,000 μmol m−2 s−1 and 0.7 s, respectively. To maximize the stomatal opening, the amount of blue light was 10 % of the PPFD (Flexas et al. 2007).
The following parameters were assessed: the maximum quantum yield of PSII in dark-adapted leaves [F v/F m = (F m − F o)/F m], the effective quantum yield of PSII [∆F/F m′ = (F m′ − F s)/F m′], the photochemical [qP = (F m′ − F s)/(F m′ − F o′)] and non-photochemical [NPQ = (F m − F m′)/F m′] quenching, the apparent ETR [ETR = (∆F/F m′ × PPFD × 0.5 × 0.84)], and the relative energy excess at the PSII level [EXC = (F v/F m) − (∆F/F m′)/(F v/F m)]. To evaluate the ETR, 0.5 was used as the fraction of the excitation energy distributed to PSII, and 0.84 was used as the fraction of incoming light absorbed by the leaves. PPFD is the PPFD. The minimum (F o), maximum (F m) and variable (F v = F m − F o) fluorescence intensities were sampled in dark-adapted leaves. In addition, measurements were taken under light-adapted conditions, with a sampling of the minimum (F o′) and maximum (F m′) fluorescence intensities. The F o′ signal was measured after PSI excitation using far-red light. The measurement of F m′ was performed by supplying far-red illumination after the actinic light flash removal. The fluorescence signal measured immediately before the saturation pulse is referred to as F s′, and the variable fluorescence signal under light conditions is ΔF′ = F m′ − F s′ (Flexas et al. 2007; Silva et al. 2010a).
Net nitrate uptake by roots and nitrate reductase activity, nitrate content and soluble-protein content in leaves
The net nitrate uptake was evaluated by NO3 − depletion in the nutrient solution after a 24-h interval of root uptake (Silveira et al. 2001). The nitrate concentration was measured by the Cawse (1967) method. Over the experimental period (10 days), the nitrate concentration was measured daily in the nutrient solution and the initial concentrations of each treatment (1 and 10 mM) were restored by addition of CaCl2 1 M and KNO3. The nitrate reductase activity was measured by an in vivo method according to Hageman and Hucklesby (1971), with minor modifications described in details by Silveira et al. (2001). The leaf nitrate was extracted with hot water (100 °C), and the concentration was determined using the method of Cataldo et al. (1975). The total soluble protein was extracted with a 100 mM Tris–HCl buffer (pH 8.0) containing 30 mM DTT, 20 % (v/v) glycerol and 3 % (w/v) PEG-6000 (Zimmermam et al. 2006). The total soluble-protein concentration was measured using the Bradford (1976) method with a standard curve obtained using bovine serum albumin (BSA).
Determination of organic solutes
Lyophilized leaf samples were transferred to hermetically closed tubes containing deionized water, and the tubes were placed in a 100 °C water bath for 1 h. After the supernatant extraction, the total soluble sugar was determined using the phenol–sulfuric method (Dubois et al. 1956). Sucrose determination was performed using the method described by van Handel (1968). The total free amino acids (TFAA) and the proline and glycinebetaine (GB) concentrations in the leaves were determined as previously described (Silveira et al. 2009).
Experimental design and data analysis
The experiment was arranged in a factorial (2 × 2) design. The experiment had two nitrate concentrations (1 and 10 mM) and two NaCl levels (0 and 100 mM) with four replicates (an individual pot containing one plant was one replicate). The data were analyzed by an ANOVA, and the means were compared with Tukey’s test at the 0.05 level of confidence. The standard deviation is plotted in each of the tables and figures.
Results
Leaf dry weight accumulation and membrane damage
Salt stress promoted strong changes in the physiological parameters of the J. curcas plants under high and low nitrate concentrations during medium growth. For example, the leaf dry weight was reduced by 57 and 43 % in the N1+Salt and N10+Salt treatments, respectively, compared to their respective controls (Table 1). In contrast, the electrolyte leakage (EL) and membrane damage were significantly increased by 57 and 33 %, respectively, in the same salt treatments compared to the controls (Table 1). The degree of leaf hydration, expressed as the relative water content and the concentrations of photosynthetic pigments in the salt-stressed plants, was not affected by salinity or nitrate (Table 1). It is important to note that, in terms of growth and membrane integrity, a high NO3 − level (10 mM) in the nutrient solution was able to minimize the damage caused by NaCl.
Leaf gas exchange and chlorophyll fluorescence
All of the leaf gas exchange parameters evaluated in this study were also affected by salinity. The leaf CO2 assimilation was decreased by 55 and 30 % in the N1+Salt and N10+Salt treatments, respectively, compared to their respective references (Table 2). Similarly, transpiration (E), stomatal conductance (g S) and carboxylation instantaneous efficiency (P N/C I) showed reductions of 20, 53 and 65 %, respectively, in the N1+Salt and 25, 37 and 35 %, respectively, in the N10+Salt treatment compared to the respective references (Table 2). In contrast, the ETR/P N ratio increased by 42 and 28 %, respectively, in the N1+Salt and N10+Salt treatments when compared to the reference plants (Table 3). Based on the leaf DW and EL results, the plants supplied with 10 mM NO3 − suffered less-severe salt stress than the plants treated with 1 mM NO3 −.
Regarding the chlorophyll fluorescence parameters, when compared to the reference plants, the effective quantum yield of PSII (∆F/F m′) and the photochemical quenching were reduced by 28 and 18 %, respectively, in the N1+Salt treatment and by 13 and 20 %, respectively, in the N10+Salt treatment (Table 3). Similarly, in the N1+Salt and N10+Salt treatments, the apparent ETR was reduced by 36 and 17 %, respectively, when compared to the reference plants (Table 3). However, the non-photochemical quenching coefficient (NPQ) and the relative energy excess at the PSII level (EXC) reached values approximately 200 and 120 % higher than those of the reference plants, respectively, in the N1+Salt treatment, and they reached values approximately 130 and 40 % higher, respectively, in the N10+Salt treatment (Table 3). In addition, neither stressed nor non-stressed plants showed significant changes (p > 0.05) in the maximum quantum yield of PSII in dark-adapted leaves (F v/F m) (Table 3) and minimum florescence (F o) (data not shown).
Nitrate assimilation and nitrogenous compounds accumulation
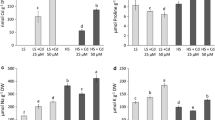
Regardless of the salt present, the nitrate uptake was strongly enhanced in plants supplied with high NO3 − concentration when compared to those that received a low nitrate level (Fig. 1a). The leaf nitrate concentration was slightly higher in both salt-treated and non-treated (reference) plants grown under high nitrate levels compared to the respective plants cultivated in the presence of a low nitrate level (Fig. 1b). Nitrate reductase activity was strongly decreased by salinity in both low and high NO3 − fed plants (Fig. 1c). Moreover, compared with the plants grown under a low nitrate level, a high supply of nitrate strongly increased the nitrate reductase only in salt-stressed plants. The soluble-protein concentrations indicated a similar pattern to those observed by nitrate uptake and nitrate reductase activity. That is, in both salt-treated and non-treated conditions, the plants treated with 10 mM NO3 − had higher leaf soluble-protein concentrations than the plants grown under low nitrate levels (Fig. 1d). The contents of the TFAA in leaves were slightly increased by the effect of salinity under a low supply of nitrate, but the contents were significantly increased by salinity under a high supply of nitrate. However, when compared with the plants grown under a low nitrate level, the plants grown under a high level of nitrate alone demonstrated a discrete increase in the concentrations of amino acids.
Net nitrate uptake (a), nitrate content (b), nitrate reductase activity (c) and total soluble-protein levels (d) in the J. curcas leaves exposed to salt stress under high and low nitrate conditions. The values are the means of four replicates ± SD. The bars represent the mean values (n = 4) ± SD. The same letters represent differences that are not significant based on a significance cutoff of 0.05, as assessed by Tukey’s test
Proline, glycinebetaine and soluble-sugar accumulation
In regard to the studied osmo-solutes, proline and glycinebetaine (GB), the salinity significantly increased the proline levels in low and high nitrate-treated plants compared to their respective references (Fig. 2b) Nitrate alone did not exhibit any effect on the proline concentration. However, the salt stress promoted moderate increases in the GB content in both high and low nitrate-treated plants. In addition, in the absence of NaCl, the exogenous nitrate levels had no effect on the GB concentrations (Fig. 2c). It is important to note the low levels of all obtained proline concentrations (from 0.25 to 0.38 μmol g−1 DW when compared with GB, which changed from approximately 205–245 μmol g−1 DW). In spite of this high GB concentration, salt stress was slightly stimulated at both nitrate levels. In contrast to other solutes, neither the nitrate levels nor the salinity changed the soluble-carbohydrate contents (Fig. 2d).
The levels of total free amino acids (a), proline (b), glycinebetaine (c) and soluble sugar (d) in the J. curcas leaves exposed to salt stress under high and low nitrate conditions. The values are the means of four replicates ± SD. The bars represent the mean values (n = 4) ± SD. The same letters represent differences that are not significant based on a significance cutoff of 0.05, as assessed by Tukey’s test
Discussion
In this study, we demonstrated that a high supply of exogenous NO3 − was more effective in mitigating, at least partially, the negative effects caused by salinity when compared with the low NO3 − in the root medium of J. curcas. Interestingly, because the endogenous NO3 − concentrations in the leaf tissues were almost similar among the two exogenous nitrate levels studied, these effects were essentially triggered by the nitrate flux. These results allow us to propose the following hypothesis: in alleviating the negative effects of salinity, the nitrate flux from the roots to the leaves is more important than the nitrate endogenous level or N status in leaf tissues. As the nitrate flux might control the nitrate reductase activity (Abd-ElBaki et al. 2000; Bybordi 2010), it is plausible to assume that the nitrate assimilatory reduction process, by acting as a sink of electrons in the photosynthetic electron-transport chain, is essential in minimizing the deleterious effects caused by salinity on the photosynthesis of J. curcas.
This current hypothesis is reasonably supported by our obtained data. First, the highest supply of nitrate favored leaf growth and photosynthesis (CO2 assimilation and photochemical activity) only in salt-stressed plants. Second, the concentrations of the two nitrogenous solutes involved in osmotic adjustment and cell protection, proline and glycinebetaine, were similarly increased following both the low and high NO3 − supply. The free amino acids and soluble proteins had their concentrations increased by high nitrate concentrations in both salt- and non-stressed plants. It is important to note that both low and high NO3 −-fed plants exhibited similar leaf growth and photosynthesis. Thus, low NO3 −-fed plants previously stored sufficient amounts of N, which, when combined with 1 mM NO3 − supplied by the nutrient solution, was likely sufficient to maintain an adequate rate of growth and photosynthesis.
The nitrate assimilatory reduction by nitrate reductase and nitrite reductase are the reactions that consume high amounts of electrons in the cytosol from NAD(P)H (two electrons) and in the chloroplasts from reduced ferredoxin (six electrons), respectively (Campbell 1999). This fact can explain the more severe effects on photosynthesis, growth and membrane damage caused by NaCl on the plants grown under low nitrate concentrations, which exhibited low nitrate uptake and assimilation. In other words, the higher rates of nitrate assimilation could contribute to the consummation of a part of the electron excess in the thylakoid membrane, thus inducing a lower NADPH/NADP+ ratio. This process might allow a higher electron-transport rate from photosystem II to CO2 assimilation under conditions of restrictions in the stomatal opening caused by salt stress (Kato et al. 2003). Nitrate assimilation could then act as an additional chloroplast electron sink under low CO2 assimilation conditions.
Although Kato et al. (2003) have demonstrated that a high N level favors photochemical activity, these authors neither discussed nor explained the underlying mechanisms involved with photo-inhibition and photosynthesis improvement by N. Thus, to the best of our knowledge, our study is the first to demonstrate a proposed mechanism for explaining the favorable effects triggered by high N in photo-acclimation under salt stress. Indeed, in an excellent review on the mechanisms involved with the protection of photosystems, Osmond and Forster (2006) suggested that N utilization might attenuate over-reduction of the photosystem II and to improve photo-acclimation. However, these authors did not propose any explanation of the biochemical mechanism involving N and the improvement in photosystem II efficiency and CO2 assimilation.
All photochemical and gas exchange parameters obtained in the current study corroborate with the notion that high rates of NO3 − flux and assimilation might attenuate the adverse effects caused by an imbalance between the high rates of electron supply from photosystems I and II and the low rates of utilization for the most important electron sink, CO2 assimilation. Moreover, as our experiments were conducted under natural conditions with high light levels, high temperature and a high vapor pressure deficit, these factors might have interacted strongly with the salinity, thus allowing for a large accumulation of electrons in the photosystem II and over-reduction in the thylakoid membrane due to low rates of CO2 assimilation (Silva et al. 2010a). Under these conditions, J. curcas frequently suffers membrane damage and oxidative stress (Silva et al. 2010a). Interestingly, as revealed by the high values of F o and F v/F m, the significant photochemical alterations induced in the J. curcas salt-stressed plants were not sufficient to cause photo-inhibition and photo-damage in PSII.
In our current study, the higher NO3 − uptake and nitrate reductase activity in salt-stressed plants grown under high NO3 − occurred in parallel to the high nitrate assimilation and amino-acids synthesis. Nitrate assimilation and amino-acids synthesis are processes that consume considerable amounts of energy, reducing power (electrons) and ATP (Cabello-Pasini et al. 2011). Of course, protein synthesis requires amino acids that have originated from nitrate and ammonia assimilation, followed by an intense interconversion among amino acids. Thus, nitrate assimilation, amino acids and protein synthesis are important processes involved in the utilization of electrons and ATP production by the photochemical machinery reducing the “energy pressure” on the photosystems and the thylakoid membrane. As the nitrate and ammonia assimilation depends on the supply of carbon skeletons, electrons and ATP produced by the Calvin cycle and photochemical reactions, it is important to emphasize that both CO2 and NO3 − assimilation might operate in balance (Robredo et al. 2011).
Although decreases in photochemical activity and increases in the ETR/P N ratio (an indicator of an alternative sink for photosynthetic electrons) have been observed in J. curcas plants under conditions of high salinity (evidenced by reductions in ΔF/F m′, qP and ETR), such conditions suggest that these changes are an acclimation mechanism rather than an indicator of dangerous effects on the chloroplast’s machinery (Ribeiro et al. 2009a, b; Silva et al. 2011). In the current study, the NPQ, a parameter associated with heat dissipation, was strongly increased, especially in low-nitrate grown plants. These results again reinforce our hypothesis that high rates of nitrate assimilation might act as an important sink for the electron excess in the PSII, and thus, protect chloroplasts against photo-inhibition and oxidative damages.
The reduction in the actual quantum yield of PSII and apparent electron-transport rates might be associated with a down-regulation in the electron-transport rate at the PSII level (Ribeiro et al. 2009a, b), especially in high NO3 −-fed salt-stressed plants. Down-regulating the linear electron transport between the two photosystems to match the demand of CO2 fixation could decrease the electron transport to O2 on the acceptor side of PSII (i.e., in the Mehler reaction), thus minimizing the production of reactive oxygen species (Drodzova et al. 2004; Foyer et al. 2009). Alternatively, an up-regulation of other electron sinks, such as photorespiration and nitrate assimilation, also could minimize photo-damage, photo-inhibition and/or oxidative stress (Foyer et al. 2009).
Conclusion
Our results demonstrate that a high supply of NO3 − and the nitrate assimilation process can mitigate the negative effects of salinity. Nitrite assimilatory reduction and ammonia assimilation in the chloroplast might act as a sink of electrons from the thylakoid membrane, thus minimizing photo-inhibition and photo-damage and stimulating CO2 assimilation under conditions of stomatal limitations imposed by salt stress in J. curcas.
Author contributions
J. A. G. Silveira was the mastermind of this project, planning all of the experiments and writing the manuscript. R. M. Aragão conducted all of the experiments in the greenhouse and performed chemical and biochemical determinations. E. N. Silva measured all of the parameters of leaf gas exchange and chlorophyll fluorescence and helped in drafting the manuscript and in interpreting the results. C. F. Vieira performed the statistical analysis and helped with both the chemical measurements and the experiments in the greenhouse.
References
Abd-ElBaki GK, Siefritz F, Man HM, Weiner H, Haldenhoff R, Kaiser WM (2000) Nitrate reductase in Zea mays L. under salinity. Plant Cell Environ 23:515–521
Adams WW III, Zarter CR, Mueh KE, Amiard V, Demmig-Adams B (2006) Energy dissipation and photoinhibition: a continuum of photoprotection. In: Demmig-Adams B, Adams W, Mattoo A (eds) Photoprotection, photoinhibition, gene regulation, and environment. Springer, Netherlands, pp 11–22
Albassam BA (2001) Effect of nitrate nutrition on growth and nitrogen assimilation of pearl millet exposed to sodium chloride stress. J Plant Nutr 24:1325–1335
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bybordi A (2010) Effects of salinity and N on the growth, photosynthesis and N status of canola (Brassica napus L.). Not Sci Biol 2:92–97
Cabello-Pasini A, Macías-Carranza V, Abdala R, Korbee N, Figueroa FL (2011) Effect of nitrate concentration and UVR on photosynthesis, respiration, nitrate reductase activity, and phenolic compounds in Ulva rigida (Chlorophyta). J Appl Phycol 23:363–369
Campbell WH (1999) Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Annu Rev Plant Physiol Mol Biol 50:277–303
Cataldo DA, Haroon M, Schrader LE, Yougs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80
Cawse PA (1967) The determination of nitrate in soil solution by ultraviolet spectrophotometry. Analyst 92:311–315
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J Exp Bot 58:4245–4255
Drodzova IS, Pustovoitova TN, Dzhibladze TG, Barabanshchikova NS, Zhdanova NE, Maevskaya SN, Bukhov NG (2004) Endogenous control of photosynthetic activity during progressive drought: influence of final products of photosynthesis. Rus J Plant Physiol 51:668–675
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Flexas J, Diaz-Espejo A, Galme′s J, Kaldenhoff R, Medrano H, Ribas-Carbo M (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30:1284–1298
Flores P, Navarro JM, Carvajal M, Cerdá M, Martínez V (2003) Tomato yield and quality as affected by nitrogen source and salinity. Agronomie 23:249–256
Foyer CH, Bloom AJ, Queval G, Noctor G (2009) Photorespiratory metabolism: genes, mutants, energetics and redox signaling. Annu Rev Plant Biol 60:455–484
Francis G, Edinger R, Becker K (2005) A concept for simultaneous wasteland reclamation, fuel production, and socioeconomic development in degraded areas in India: need, potential and perspectives of Jatropha plantations. Nat Res Forum 29:12–24
Gaiad S, Rakocevic M, Reissmann CB (2006) N sources affect growth, nutrient content, and net photosynthesis in Maté (Ilex paraguariensis St. Hil.). Braz Arch Bio Tech 49:689–697
Hageman RH, Hucklesby DP (1971) Nitrate reduction from higher plants. Meth Enzymol 23:491–503
Hikosaka K, Hirose T (2000) Photosynthetic nitrogen-use efficiency in evergreen broad-leaved woody species coexisting in a warm-temperate forest. Tree Physiol 20:1249–1254
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Sta Circ 347:1–39
Iglesias DJ, Levy Y, Cadenas AG, Tadeo FR, Primo-Millo E, Talon M (2004) Nitrate improves growth in salt-stressed citrus seedlings through effects on photosynthetic activity and chloride accumulation. Tree Physiol 24:1027–1034
Kato MC, Hikosaka K, Hirotsu N, Makino A, Hirose T (2003) The Excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in photosystem II. Plant Cell Physiol 44:318–325
King AJ, He W, Cuevas JA, Freudenberger M, Ramiaramana D, Graham IA (2009) Potential of Jatropha curcas as a source of renewable oil and animal feed. J Exp Bot 60:2897–2905
Lea PJ, Azevedo RA (2007) Nitrogen use efficiency II: amino acid metabolism. Ann App Biol 151:269–275
Osmond CB, Forster B (2006) Photoinhibition: then and now. In: Demmig-Adams B, Adams W, Mattoo A (eds) Photoprotection, photoinhibition, gene regulation, and environment. Springer, Netherlands, pp 11–22
Paul MJ, Foyer CH (2001) Sink regulation of photosynthesis. J Exp Bot 52:1383–1400
Ribeiro RV, Machado EC, Santos MG, Oliveira RF (2009a) Seasonal and diurnal changes in photosynthetic limitation of young sweet orange trees. Environ Exp Bot 66:203–211
Ribeiro RV, Machado EC, Santos MG, Oliveira RF (2009b) Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 47:215–222
Robredo A, Pérez-López U, Miranda-Apodaca J, Lacuesta M, Mena-Petite A, Munoz-Rueda A (2011) Elevated CO2 reduces the drought effect on nitrogen metabolism in barley plants during drought and subsequent recovery. Environ Exp Bot 71:399–408
Rocha IMA, Vitorello VA, Silva JS, Ferreira-Silva SL, Silva EN, Silveira JAG (2012) Exogenous ornithine is an effective precursor and the δ-ornithine amino transferase pathway contributes to proline accumulation under high N recycling in salt-stressed cashew leaves. J Plant Physiol 169:41–49
Silva EN, Silveira JAG, Rodrigues CRF, Lima CS, Viégas RA (2009) Contribution of organic and inorganic solutes to osmotic adjustment of physic nut under salinity. Pesq Agrop Bras 44:437–445
Silva EN, Ferreira-Silva SL, Fontenele AV, Ribeiro RV, Viégas RA, Silveira JAG (2010a) Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol 167:1157–1164
Silva EN, Ferreira-Silva SL, Viégas RA, Silveira JAG (2010b) The role of organic and inorganic solutes in the osmotic adjustment of drought-stressed Jatropha curcas plants. Environ Exp Bot 69:279–285
Silva EN, Ribeiro RV, Ferreira-Silva SL, Viégas RA, Silveira JAG (2010c) Comparative effects of salinity and water stress on photosynthesis, water relations and growth of Jatropha curcas plants. J Arid Environ 74:1130–1137
Silva EN, Ribeiro RV, Ferreira-Silva SL, Viégas RA, Silveira JAG (2011) Salt stress induced damages on the photosynthesis of physic nut young plants. Sci Agric 68:62–68
Silveira JAG, Melo ARB, Viégas RA, Oliveira JTA (2001) Salinity-induced effects on nitrogen assimilation related to growth in cowpea plants. Environ Exp Bot 46:171–179
Silveira JAG, Araújo SAM, Lima JPMS, Viégas RA (2009) Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in Atriplex nummularia. Environ Exp Bot 66:1–8
Soussana JF, Teyssonneyre F, Thiery JM (2000) Un modèle dynamique d′allocation base sur l′hypothèse d′une co-limitation de la croissance végétale par les absorptions de lumière et l′azote. In: Maillard P, Bonhomme R (eds) Fonctionnement des peuplements végétaux sous contraintes environnementales. Paris, France
Van Handel E (1968) Direct microdetermination of sucrose. Anal Biochem 22:280–283
Villa-Castorena M, Ulery AL, Catalán-Valencia EA, Remmenga MD (2003) Salinity and nitrogen rate effects on the growth and yield of chile pepper plants. Soil Sci Soc Am J 67:1781–1789
Zimmermam P, Heinlein C, Orendi G, Zentgra U (2006) Senescence-specific regulation of catalases in Arabidopsis thaliana (L.) Heynh. Plant Cell Environ 29:1049–1060
Acknowledgments
We would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support. The authors acknowledge the CNPq for their fellowships (J.A.G.S. and E.N.S.) and give thanks to the Tamandua Farm Institute, Santa Terezinha-PB (Brazil), and especially, to Prof. Ricardo Almeida Viégas for supplying the J. curcas seeds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klobus.
Rights and permissions
About this article
Cite this article
Aragão, R.M., Silva, E.N., Vieira, C.F. et al. High supply of NO3 − mitigates salinity effects through an enhancement in the efficiency of photosystem II and CO2 assimilation in Jatropha curcas plants. Acta Physiol Plant 34, 2135–2143 (2012). https://doi.org/10.1007/s11738-012-1014-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1014-y