Abstract
Key message
The ripening-induced MaBSD1 acts as a transcriptional activator, and might be involved in banana fruit ripening partly through directly activating the expression of two ripening-associated genes, MaEXP1/2.
Abstract
BSD (BTF2-like transcription factors, synapse-associated proteins and DOS2-like proteins) transcription factors are characterized by a typical BSD domain. However, little information is available concerning their possible roles in plant growth and development, especially in fruit ripening. In the present study, one BSD gene, designated as MaBSD1, was isolated from banana fruit. MaBSD1 has an open reading frame (ORF) of 921 bp which encodes a polypeptide of 306 amino acid residues with molecular weight of 34.80 kDa, and isoelectric point (pI) of 4.54. Subcellular localization and transcriptional activation assays showed that MaBSD1 was localized in both the nucleus and cytoplasm and possessed transcriptional activity. RT-qPCR and promoter activity analysis indicated that MaBSD1 was ethylene and ripening inducible, and the accumulation of MaBSD1 transcript was correlated well with the evolution of ethylene production and ripening process. Moreover, transient assay showed that MaBSD1 could activate the expression of two cell wall modification-related genes, MaEXP1/2, via directly interacting with their promoters. Together, these data suggest that ripening-induced MaBSD1 acts as a transcriptional activator and might be associated with banana fruit ripening, at least partially through directly activating the expression of MaEXP1/2, expanding the limited information concerning the BSD transcription factor in relation to fruit ripening.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcription factors (TFs) play important roles in gene regulation through their interaction with cis-elements and/or other TFs, resulting in the regulated transcription of downstream genes during various developmental stages or responses of plant to environmental stimuli (Ye et al. 2004). About 2000 TFs have been identified to regulate the first step of the expressions of over 26,000 genes located in the genome of Arabidopsis thaliana (Mitsuda and Ohme-Takagi, 2009). The BSD (named from BTF2-like transcription factors, synapse-associated proteins and DOS2-like proteins) TFs contain a typical BSD domain, which is characterized by three α-helices probably involved in DNA binding, and by conserved tryptophan and phenylalanine residues located at the C-terminus of the domain (Doerks et al. 2002). The BSD domain-containing proteins are found in a variety of species ranging from primal protozoans to humans. In Arabidopsis, for example, there are at least ten genes encoding BSD domain, among which AtBSD1 was constitutively expressed in all tissues. Moreover, the expression level of the AtBSD1 transcript was not significantly affected by abiotic stresses or plant hormones (Park et al. 2009). However, limited data regarding the characterization and function of BSD TFs, as well as their direct targets, are available. In addition, whether BSD TFs are involved in fruit ripening is largely unknown.
Banana (Musa acuminate) ranks as the world’s second largest fruit crop and is listed among the world’s ten most important food commodities (Sreedharan et al. 2012). However, banana being a typical climacteric fruit is highly sensitive to ethylene and ethylene can easily trigger ripening, leading to reduced shelf life. Therefore, gaining a better understanding of the mechanism of fruit ripening is important for maintaining quality and extending shelf life of the fruit. Recently, several ripening-related TFs, including MADS-box, EIN3, NAC, ERF, and LBD, have been identified in banana fruit, as well as their transcriptional control of ripening (Mbéguié-A-Mbéguié et al. 2008; Elitzur et al. 2010; Choudhury et al. 2012; Shan et al. 2012; Xiao et al. 2013; Ba et al. 2014). However, it is clear that multiple TFs are involved in the ripening process (Klee and Giovannoni 2011), indicating that the full complexity of transcriptional regulatory mechanism remains elusive. Therefore, functional characterization of other TFs is essential for understanding transcriptional regulatory networks of banana fruit ripening. In the present study, one BSD TF designated as MaBSD1 was isolated and identified from banana fruit. The expression patterns of MaBSD1 during natural, ethylene-induced and 1-methylcyclopropene (1-MCP)-delayed fruit ripening were monitored. Moreover, the interactions of MaBSD1 with promoters of two cell wall-modifying genes, MaEXP1/2, were investigated. Our results suggest that MaBSD1 acts as an ethylene- and ripening-inducible transcriptional activator, and might be associated with banana fruit ripening, at least partially through directly activating the expression of MaEXP1/2.
Materials and methods
Plant materials
Preclimacteric banana (Musa acuminata, AAA group, cv. Cavendish) fruits at 75–80 % maturation were obtained from a local commercial plantation near Guangzhou, China. Three postharvest treatments, including a control (natural ripening), ethylene-induced ripening (100 μlL−1 ethylene, 18 h), and 1-methylcyclopropene (1-MCP)-delayed ripening (0.5 μlL−1 1-MCP, 18 h) were performed to create three different ripening characteristics, and samples were taken as described previously (Shan et al. 2012). All assessments were conducted using three biological replicates.
RNA extraction, gene isolation, and sequence analysis
Total RNA was extracted using the hot borate method of Wan and Wilkins (1994). Total RNA extract was treated with DNAse I (Promega, Madison, WI, USA), and the resulting DNA-free total RNA was used as the template for reverse transcription PCR (RT-PCR). According to gene annotation, bioinformatics and RNA-Seq analysis, one ripening-related BSD gene termed MaBSD1 (GSMUA_Achr3T24850_001) with complete start and stop codons was selected from banana whole-genome sequence (D’Hont et al. 2012), and the sequence was verified by further cloning from banana fruit pulp and sequencing (primers are listed in Supplementary Table S1). Alignments were carried out on ClustalX (version 1.83) and GeneDoc software.
Quantitative real-time PCR analysis
Synthesis of first-strand cDNA and all RT-qPCR analysis were performed as described previously (Chen et al. 2011; Shan et al. 2012). The sequences of primers used for RT-qPCR analysis are listed in Supplementary Table S1. MaRPS4 (ribosomal protein 4) was selected as a reference gene according to our previous study on the selection of reliable reference genes under different experimental conditions (Chen et al. 2011). All RT-qPCR reactions were normalized using Ct value corresponding to the reference gene. The relative expression levels of target gene were calculated with the formula 2−ΔΔCT. Three independent biological replicates were used in the analysis.
Subcellular localization of MaBSD1
The coding sequence of MaBSD1 without the stop codon was amplified by PCR (primers are listed in Supplementary Table S1) and subcloned into the pUC-GFP vector, in frame with the green fluorescent protein (GFP) sequence, resulting in 35S::GFP–MaBSD1 vector under the control of the cauliflower mosaic virus (CaMV) 35S promoter. The fusion construct and the control GFP vector were transformed into tobacco BY-2 suspension culture cell protoplasts by PEG methods as described previously (Shan et al. 2012; Ba et al. 2014). GFP fluorescence was observed with a fluorescence microscopy (Zeiss Axioskop 2 Plus). All transient expression assays were repeated at least three times.
Promoter isolation
Genomic DNA was extracted from banana leaves using the DNeasy Plant Mini Kit (Qiagen). The promoter of MaBSD1 gene was isolated using a Genome Walker Kit (Clontech) with nested PCR according to the manufacturer’s instructions. The nested PCR analysis was performed with two sets of primers, including two adaptor primers that were obtained from the kit and two gene-specific primers listed in Supplementary Table S1. After sequencing, conserved cis-element motifs of promoter were predicted using PLACE (http://www.dna.affrc.go.jp/PLACE/signalscan.html) and Plant-CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) databases.
Protoplast transfection assay
A dual luciferase assay was performed in the transient assay, and all primers used for the following constructs are listed in Supplementary Table S1. For promoter activity assay, the MaBSD1 promoter region was amplified by PCR. The PCR product was inserted into the pGreenII 0800-LUC double-reporter vector (Hellens et al. 2005) to fuse it with the Firefly luciferase (LUC) reporter gene (MaBSD1 pro-LUC), and a Renilla luciferase (REN) under the control of the 35S promoter at the same vector was used as a positive control. The construct CaMV35S–REN/MaBSD1 pro-LUC was transformed into tobacco BY-2 protoplasts as described above. The transformed protoplasts were subjected to 0 (control) or 0.8 mM ethrel (ethylene releaser) treatment and then incubated at 23 °C for 16 h.
For transactivation analysis of MaBSD1, the coding sequence of MaBSD1 without the stop codon was cloned into the constructed GAL4 DBD vector driven by the 35S promoter plus the translation enhancer Ω sequence as effector. The double-reporter vector includes a GAL4–LUC and an internal control REN driven by the 35S promoter. GAL4–LUC contains five copies of GAL4-binding element and minimal TATA region of the 35S promoter of CaMV, and these sequences are located upstream of the LUC. For the assay of the binding activity of MaBSD1 to the MaEXPs promoter, the MaEXPs promoter was cloned into pGreenII 0800-LUC double-reporter vector, while MaBSD1 was inserted into the pGreenII 62-SK vector as effector (Hellens et al. 2005). The constructed effector and reporter plasmids were co-transformed into BY-2 protoplasts and incubated as described above.
LUC and REN luciferase activities were measured using the dual luciferase assay kit (Promega). The analysis was carried out using the Luminoskan Ascent Microplate Luminometer (Thermo) according to the manufacturer’s instructions, with a 5-s delay and 15-s integrated measurements. The promoter activity, transactivation ability of MaBSD1, and the binding activity of MaBSD1 to the MaEXPs promoter are indicated by the ratio of LUC to REN. At least six transient assay measurements were included for each assay.
Results and discussion
Characterization of MaBSD1 from banana fruit
There existed 14 BSD genes in banana genome, while only 1 BSD is specifically up-regulated during ripening stages through RNA-Seq analysis (D’Hont et al. 2012). Thus, we selected this BSD gene, termed MaBSD1, and isolated its cDNA from banana fruit using RT-PCR method. The open reading frame (ORF) of MaBSD1 is 921 bp in length, encoding a polypeptide of 306 amino acid residues with molecular mass of 34.80 kDa and isoelectric point (pI) of 4.54. GenBank blast showed that MaBSD1 is holomogous to BSDs from other plants, such as XP_003591620.1 from Medicago truncatula (44 % amino acid identity), XP_006358434.1 from Solanum tuberosum (43 % amino acid identity), and NP_563683.1 from Arabidopsis (41 % amino acid identity). Multiple alignments with plants BSD proteins clearly confirmed the presence of BSD domain in MaBSD1 (Fig. 1). In addition, two conserved amino residues including phenylalanine (F) and tryptophan (W), which are the most striking sequence features of the domain, are also found in the C-terminus of BSD domain in all the analyzed BSD proteins, including MaBSD1 (Fig. 1). These results indicate that MaBSD1 from banana fruit is a novel member of the BSD gene family.
Alignment of MaBSD1 and other plant BSD proteins, including Arabidopsis AtBSD (NP_563683.1), barrel medic MtBSD (XP_003591620.1), and tomato SlBSD1 (XP_004247485.1). The BSD domain is underlined, and two conserved amino residues including phenylalanine (F) and tryptophan (W) within the BSD domain are indicated by asterisk. Identical and similar amino acids are presented by black and gray shading, respectively. Multiple alignments were done by Clustal W and viewed with GenDoc program
Subcellular localization and transcriptional activation of MaBSD1
To assess the subcellular localization of MaBSD1, we fused the full-length ORF of MaBSD1 without the stop codon to the green fluorescence protein (GFP) reporter gene under the control of the CaMV35S promoter to generate the construct 35S::GFP–MaBSD1. The construct and the empty vector (control) were transiently expressed in tobacco BY2 protoplasts by PEG methods. Fluorescence microscopy revealed that there was no difference between the localization of control GFP and 35S::GFP–MaBSD1, and the fluorescence distribution of the 35S::GFP–MaBSD1 signal, similar to the GFP control, was observed not only at the cell membrane but also in the cytoplasm and nucleus (Fig. 2). Similar localization was also observed when 35S::GFP–MaBSD1 was transiently expressed in tobacco (Nicotiana benthamiana) leaf epidermal cells (Supplementary Fig. S1). Although many reported transcription factors are localized in the nucleus, some are localized in other compartments of the cell. For example, GmMYB176 was localized in the cytoplasm and nucleus (Li et al. 2012). Interestingly, NAC089 is an endoplasmic reticulum (ER) membrane-associated protein and it relocates from the ER membrane to the nucleus in response to ER stress (Yang et al. 2014).
Subcellular localization of MaBSD1 in tobacco BY2 protoplasts. The tobacco BY2 protoplasts were transiently transformed with GFP–MaBSD1 or GFP vector by a modified polyethylene glycol method. GFP fluorescence was observed with fluorescence microscopy. Images were taken in a dark field for green fluorescence, while the outline of the cell and the combination were photographed in a bright field. The length of the bar (25 μm) is indicated in the photos
We then examined the transactivation activity of MaBSD1 using transient expression assay in BY-2 protoplasts. Full-length MaBSD1 fused to the GAL4 DNA-binding domain (GAL4-BD) was used as the effector, and the dual luciferase reporter harboring five copies of the GAL4 DNA-binding element and minimal TATA region of 35S promoter fused to the Firefly luciferase (LUC) reporter and a Renilla luciferase (REN) reporter under the control of the 35S promoter at the same vector was used as an internal control for successful transfection (Fig. 3a). The empty GAL4-BD (pBD) vector was used as a negative control (Fig. 3a). As shown in Fig. 3b, compared with the pBD negative control, MaBSD1 strongly activated the LUC reporter gene, and the LUC/REN ratio of MaBSD1 was 2.2-fold higher than that of the negative control, suggesting that MaBSD1 may act as a transcriptional activator. Similarly, in Arabidopsis, AtBSD1 also functions as a transcriptional activator and the transcriptional activation domain locates at the N-terminal region of the AtBSD1 protein (Park et al. 2009).
Transcriptional activation of MaBSD1 in tobacco BY-2 protoplasts. a Reporter and effector constructs. The dual luciferase reporter construct contained the LUC reporter gene driven by the mini-35S (TATA box) plus five GAL4-binding elements. Each of the effectors contained a GAL4 DNA-binding domain (GAL4-BD), and pBD was used as a negative control. MaBSD1 was fused with the GAL4-BD and driven by the 35S promoter plus the translation enhancer Ω sequence. b Transactivation ability of MaBSD1. Plasmid combinations of dual REN/LUC reporter, and effectors were cotransformed into BY-2 protoplasts. The protoplasts were incubated for 16 h, and the transactivation ability of MaBSD1 is indicated by the ratio of LUC to REN. Each value represents the means of six biological replicates, and vertical bars represent the S.E. The asterisk indicates a significant difference at the 5 % level compared to the negative control pBD
Expression of MaBSD1 during banana fruit ripening
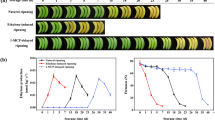
To understand the possible role of MaBSD1 during banana fruit ripening, the expression pattern of MaBSD1 in fruit with different ripening characteristics, including natural ripening, ethylene-induced and 1-MCP-delayed ripening, was investigated by RT-qPCR. In the pulp of naturally ripening fruit, MaBSD1 was almost at a constant level from 0 to 7 days of storage, then began to increase at 12 days when the production of ethylene appeared, and reached a maximum at 18 days, approximately 40-fold higher than the level at day 0, but slightly decreased thereafter (Fig. 4). Ethylene obviously increased the expression of MaBSD1, with significant increase observed in 3–7 days after ethylene treatment. On the contrary, there was a delay in accumulation of MaBSD1 transcript in 1-MCP-treated fruit, with enhanced accumulation on 30–36 days (Fig. 4). Collectively, the results that the accumulation of MaBSD1 transcript correlated well with the evolution of ethylene production during ripening process suggest that MaBSD1 might be associated with banana fruit ripening.
Expression of MaBSD1 in pulp during three ripening characteristics: natural (control), ethylene-induced, and 1-MCP-delayed ripening. The expression levels of MaBSD1 are expressed as a ratio relative to the harvest time (0 days of control), which was set at 1. Each value represents the mean ± S.E. of three biological replicates. The broken arrow and full arrow represent the time point at which ethylene production began to increase and its peak for each treatment, respectively. The physiology data related with fruit ripening and softening, including changes in fruit firmness and ethylene production in banana fruit with these three different ripening characteristics, have been described in Shan et al. (2012)
Promoter activity of MaBSD1 in response to ethylene
The upstream promoter region sequences with 1467 bp from the transcription start site of MaBSD1 was isolated from the genome of Musa acuminata using genome walking PCR method. Analysis of the MaBSD1 promoter using the PLACE and Plant-CARE databases, as shown in Supplementary Table S2, revealed that the MaBSD1 promoter contained the core cis-acting elements such as TATA and CAAT, several cis-acting elements for light response, zein metabolism regulation, heat stress responsiveness, and low-temperature responsiveness, as well as other potential cis-regulatory elements involved in the activation of hormone -responsiveness (ABA, MeJA, and ethylene). Interestingly, two sites for ethylene-responsive element (ERE) were found in the promoter at positions −403 and −1226 upstream of ATG, indicating that the promoter activity of MaBSD1 may be regulated by ethylene.
To investigate MaBSD1 promoter activity in response to ethylene, a transient protoplast assay was conducted using a dual luciferase reporter vector containing the Firefly LUC driven by the MaBSD1 promoter and the REN driven by the CaMV35S promoter (CaMV35S–REN/MaBSD1 pro-LUC, Fig. 5a). As shown in Fig. 5b, after transient expression in tobacco BY2 protoplasts with CaMV35S–REN/MaBSD1 pro-LUC, MaBSD1 pro-LUC/REN ratio apparently increased after ethylene treatment, revealing that MaBSD1 promoter activity was induced by ethylene. These data, together with its mRNA accumulation pattern during fruit ripening, provide further evidence that MaBSD1 is ethylene inducible and might be involved in banana fruit ripening.
MaBSD1 promoter activity in response to ethylene. a Schematic of the dual luciferase reporter vector containing MaBSD1 promoter (CaMV35S–REN/MaBSD1 pro-LUC). b Promoter activity of MaBSD1 in response to ethylene. The reporter construct containing MaBSD1 promoter was transiently transformed into tobacco BY-2 protoplasts using a modified PEG method, and the transformed protoplasts were subjected to 0 (control) or 0.8 mM ethrel (ethylene releaser) treatment. After incubation for 14 h, LUC and REN luciferase activities were assayed, and the promoter activity is indicated by the ratio of LUC to REN. The asterisk indicates a significant difference at the 5 % level compared to the control. Each value represents the means of six biological replicates, and vertical bars represent the S.E
Interaction of MaBSD1 and promoters of MaEXP1/2
It has been well documented that expansins are the cellular proteins that are associated with cell wall loosening during fruit ripening (Rose et al. 2000). Previous studies have shown that MaEXP1 and MaEXP2 were induced by ethylene and their transcript levels in fruit increased in accordance with the progression of ripening (Trivedi and Nath, 2004; Sane et al. 2007; Ba et al. 2014). Moreover, some ripening-associated TFs such as MaMADS5 and MaLBDs are able to interact with the promoters of MaEXP1 and MaEXP2 and transcriptionally regulate their expressions (Choudhury et al. 2012; Ba et al. 2014). To examine whether MaBSD1 could act as a transcriptional activator to directly activate MaEXP1 and MaEXP2 promoters in plant cells, a dual luciferase-based transactivation assay was performed in tobacco BY2 protoplasts. The dual luciferase reporter plasmid harbored the MaEXP1/2 promoter fused to LUC, and the REN driven by the CaMV35S promoter (CaMV35S–REN/MaEXP1/2 pro-LUC), while an effector plasmid carried MaBSD1 expressed under the control of the CaMV 35S promoter (Fig. 6a). As exhibited in Fig. 6b, the LUC/REN ratio was significantly increased when either the MaEXP1 pro-LUC or the MaEXP2 pro-LUC reporter construct was co-transfected with the effector of CaMV 35S–MaBSD1, 2.2- and 2.6-fold higher than when co-transformed with the empty control, respectively. These findings demonstrate that MaBSD1 is a transcriptional activator that could regulate MaEXP1/2 gene expressions through directly activating their promoters. It has been reported that differential gene expression is transcriptional regulation which involves the binding of TFs to promoters of downstream genes during fruit ripening. For example, AdEIL2 and AdEIL3 TFs activated transcription of the ripening-related genes AdACO1 and AdXET5 during the ripening of kiwifruit (Yin et al. 2010). In our previous study, it was found that MaERF11, a ripening repressor in banana fruit, bound to MaACS1 and MaACO1 promoters to suppress their activities, while MaERF9 activated MaACO1 promoter activity (Xiao et al. 2013). More interestingly, tomato ripening regulator RIN transcriptionally regulates more than 241 direct targets, including lycopene accumulation, ethylene production, chlorophyll degradation, and many other physiological processes (Fujisawa et al. 2013). However, in the present study, further experiments such as electrophoresis mobility shift assay (EMSA) or chromatin immunoprecipitation (ChIP) are needed to confirm the binding of MaBSD1 to the promoters of MaEXP1/2.
MaBSD1 activates the MaEXP1/2 promoter in a dual luciferase assay. a Schematic representation of the double reporters and effector plasmids used in the assay. The double-reporter plasmid contained the MaEXP1/2 promoter fused to LUC luciferase and REN luciferase drove by CaMV35S. The effector plasmid contained the MaBSD1 drove by the CaMV35S. b MaBSD1 activates the MaEXP1/2 promoter. The reporter and effector vectors, as indicated, were co-transformed into tobacco BY-2 protoplasts. The protoplasts were incubated for 16 h, and the activation of MaEXP1/2 promoter by MaBSD1 was indicated by the ratio of LUC to REN. The asterisk indicates a significant difference at the 5 % level compared to the empty effector. Each value represents the means of six biological replicates, and vertical bars represent the S.E
In conclusion, a BSD gene named MaBSD1 was isolated from banana fruit. MaBSD1 was localized in both the nucleus and cytoplasm and possessed transcriptional activity. Moreover, MaBSD1 was ethylene and ripening inducible, and was able to activate the expression of two cell wall modification-related genes, MaEXP1/2, via directly interacting with their promoters. Together, these data suggest that MaBSD1 acts as a transcriptional activator and might be associated with banana fruit ripening, at least partially through directly activating the expression of MaEXP1/2.
Abbreviations
- BSD:
-
BTF2-like transcription factors, synapse-associated proteins and DOS2-like proteins
- EXP:
-
Expansin
- GFP:
-
Green fluorescence protein
- ORF:
-
Open reading frame
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- TF:
-
Transcription factor
References
Ba LJ, Shan W, Kuang JF, Feng BH, Xiao YY, Lu WJ, Chen JY (2014) The banana MaLBD (lateral organ boundaries domain) transcription factors regulate EXPANSIN expression and are involved in fruit ripening. Plant Mol Biol Rep. doi:10.1007/s11105-014-0720-6
Chen L, Zhong HY, Kuang JF, Li JG, Chen JY, Lu WJ (2011) Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 234:377–390
Choudhury SR, Roy S, Nag A, Singh SK, Sengupta DN (2012) Characterization of an AGAMOUS-like MADS box protein, a probable constituent of flowering and fruit ripening regulatory system in banana. PLoS One 7:e44361
D’Hont A, Denoeud F, Aury JM, Baurens FC, Carreel F et al (2012) The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488:213–217
Doerks T, Huber S, Buchner E, Bork P (2002) BSD: a novel domain in transcription factors and synapse-associated proteins. Trends Biochem Sci 27:168–169
Elitzur T, Vrebalov J, Giovannoni JJ, Goldschmidt EE, Friedman H (2010) The regulation of MADS-box gene expression during ripening of banana and their regulatory interaction with ethylene. J Exp Bot 61:1523–1535
Fujisawa M, Nakano T, Shima Y, Ito Y (2013) A large-scale identification of direct targets of the tomato MADS box transcription factor RIPENING INHIBITOR reveals the regulation of fruit ripening. Plant Cell 25:371–386
Hellens R, Allan A, Friel E, Bolitho K, Grafton K, Templeton M, Karunairetnam S, Gleave A, Laing W (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1:13
Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59
Li X, Chen L, Dhaubhadel S (2012) 14-3-3 proteins regulate the intracellular localization of the transcriptional activator GmMYB176 and affect isoflavonoid synthesis in soybean. Plant J 71:239–250
Mbéguié-A-Mbéguié D, Hubert O, Fils-Lycaon B, Chillet M, Baurens FC (2008) EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande Naine). Physiol Plant 133:435–448
Mitsuda N, Ohme-Takagi M (2009) Functional analysis of transcription factors in Arabidopsis. Plant Cell Physiol 50:1232–1248
Park J, Kim MJ, Jung SJ, Suh MC (2009) Identification of a novel transcription factor, AtBSD1, containing a BSD domain in Arabidopsis thaliana. J Plant Biol 52:141–146
Rose JKC, Cosgrove DJ, Albersheim P, Darvill AG, Bennett AB (2000) Detection of expansin proteins and activity during tomato fruit ontogeny. Plant Physiol 123:1583–1592
Sane AVA, Sane AP, Nath P (2007) Multiple forms of a-expansin genes are expressed during banana fruit ripening and development. Postharvest Biol Tec 45:184–192
Shan W, Kuang JF, Chen L, Xie H, Peng HH, Xiao YY, Li XP, Chen WX, He QG, Chen JY, Lu WJ (2012) Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J Exp Bot 63:5171–5187
Sreedharan S, Shekhawat UKS, Ganapathi TR (2012) MusaSAP1, a A20/AN1 zinc finger gene from banana functions as a positive regulator in different stress responses. Plant Mol Biol 80:503–517
Trivedi PK, Nath P (2004) MaEXP1, an ethylene-induced expansin from ripening banana fruit. Plant Sci 167:1351–1358
Wan CY, Wilkins TA (1994) A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.). Anal Biochem 223:7–12
Xiao YY, Chen JY, Kuang JF, Shan W, Xie H, Jiang YM, Lu WJ (2013) Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. J Exp Bot 64:2499–2510
Yang ZT, Wang MJ, Sun L, Lu SJ, Bi DL et al (2014) The membrane-associated transcription factor NAC089 controls ER-stress-induced programmed cell death in plants. PLoS Genet 10(3):e1004243
Ye R, Yao QH, Xu ZH, Xue HW (2004) Development of an efficient method for the isolation of factors involved in gene transcription during rice embryo development. Plant J 38:348–357
Yin XR, Allan AC, Chen KS, Ferguson IB (2010) Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol 153:1280–1292
Acknowledgments
We thank Professor Seiichiro Hasezawa (Department of Integrated Biosciences, the University of Tokyo), Professor Shouyi Chen (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences), and Professor Junping Gao (Department of Ornamental Horticulture, China Agricultural University) for the generous gift of tobacco BY-2 suspension cells and the transient expression vectors, respectively. This work was supported in part by the China Agriculture Research System (grant No. CARS-32-09) and Guangdong Modern Agricultural Industry Technology System (grant No. LNSG2011-12).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Prakash Lakshmanan.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ba, Lj., Shan, W., Xiao, Yy. et al. A ripening-induced transcription factor MaBSD1 interacts with promoters of MaEXP1/2 from banana fruit. Plant Cell Rep 33, 1913–1920 (2014). https://doi.org/10.1007/s00299-014-1668-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-014-1668-6