Abstract
Purpose
To analyze with diffusion-weighted magnetic resonance imaging (DW-MRI) the evolution and progress to resolution of acute pyelonephritis (APN) foci over a period of 3 months after onset.
Methods
30 women (age 22–51 years) with clinical, laboratory (white blood cell and C-reactive protein), and DW-MRI (4b-values 0, 50, 600, 1000 s/mm2) diagnosis of APN were prospectively enrolled. Two double-blinded radiologists evaluated the number of APN foci, and for each of them dimension (D), absolute diffusion coefficient (ADC), and its ratio R to the ADC of unaffected parenchyma. Signature of radiological recovery was focus no longer visible (DW−) and ADC of its site not inferior to the ADC of the unaffected parenchyma, i.e., R ≥ 0.9. Clinical and DW-MRI follow-ups (FU) were performed at 1 and 3 months.
Results
At the acute stage (t 0), 187 APN foci were found, with ADC0 = 1.3 ± 0.2 × 10−3 mm2/s, R 0 = 0.65 ± 0.12, and D 0 = 14 ± 7.5 mm. By the 1-month FU (t 1), all patients had no symptoms and physiological laboratory values; despite this, only 80 (43%) foci were solved, increasing to 138 (74%) by at the 3-month FU. The ROC curve (AUC ≥ 0.80) identified R 0 ≤ 0.6 and D 0 > 15 mm as forecast of slow radiologic resolution. About 80% of foci unsolved at 1 month but with R 1 ≥ 0.8 and D 1 ≤ 10 mm reached solution at 3 months.
Conclusions
DW-MRI recovery of APN foci does not always coincide with clinical recovery. The evolution of an APN focus is shaped by its initial values R 0 and D 0. About half of the foci still visible at 1 month reached radiological resolution in the two following months.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Urinary tract infections (UTI) are a common problem faced by many primary care and emergency physicians and by urologists [1]. The incidence of acute pyelonephritis (APN) is at its highest in otherwise healthy women between 15 and 29 years (18–22 cases per 10,000 population), followed by infants (15–20 cases per 10,000 population) and people over 80 years of age (10–15 cases per 10,000 population) [2].
Contrast-enhanced computed tomography (CT) is the gold standard for diagnosis of APN and its complications [3], but it is associated with an amount of radiation that may be perceived dangerous for young women, for the possible impact on the reproductive system, and for infants since they are inherently more radiosensitive and because they have more remaining years of life during which a radiation-induced cancer could develop [4]. Contrast-enhanced ultrasound (CEUS) has been shown to be an excellent tool in the work-up of APN [5], but its use requires specific expertise, not available in every institution. Contrast-enhanced magnetic resonance imaging was proven to be a valid alternative to CT in terms of sensitivity and specificity [6]. Imaging is important also for confirming treatment effectiveness during follow-up [6,7,8,9,10]. Piccoli et al. [7] using both MR and CT with contrast media evaluated the relationship between clinical, laboratory, and imaging data and the evolution of APN, demonstrating that the features of lesions were highly correlated with the development of kidney scars.
Several studies fostered the emerging role of DW-MR in confirming a clinical suspicion of APN and impacting treatment decisions [8, 10,11,12,13]. DW-MR can in fact be safely used in case of contraindications to administration of paramagnetic contrast medium (e.g., renal insufficiency, pregnancy, lactation, etc.), in uncooperative subjects, or slightly sedated claustrophobic ones (since DWI is respiration-triggered); furthermore, not using contrast media is both time and money saving.
There are a number of papers dealing with the role of DWI in APN identification both in native [8, 11,12,13,14] and in transplanted kidneys [10]. As to the follow-up, there is the paper by Faletti et al. [10] about transplanted kidneys, but, to the best of our knowledge, there are none dealing with the information given by DW-MRI on the evolution of APN foci in native kidneys and its correlation with clinical status and laboratory parameters.
The purpose of this study was to analyze the information given by DW-MRI on the evolution of APN foci over a three-month follow-up. We used the results of our trial to find an answer to the following questions:
-
(i)
How does the radiological signature of foci evolve in time?
-
(ii)
Does the diagnosis of “clinical recovery” coincide in time with “radiological recovery,” i.e., assessment of a complete restitutio ad integrum of the kidney regions where the APN foci were originally observed?
-
(iii)
If not so, is it possible to identify from the acute stage warning signs for a presumably delayed radiological solution?
Materials and methods
Study design
The roots of the study lay in our institution’s protocol for the diagnostic pathway in case of APN suspicion. This includes, besides the standard clinical and laboratory exams, a series of imaging exams: first of all, the execution of ultrasound (US) to identify the presence of anatomical predisposing factors and, if this is negative, two DW-MR examinations. The first DW-MR, performed at the acute stage, at the same time as the clinical exams, is used by our nephrologists for planning the most appropriate initial antibiotic therapy on the basis of the number, characteristics, extension, and distribution of the APN foci. The second DW-MR, performed after 1 month, has the task to photograph the evolution of the APN foci in order to complement the second set of clinical and laboratory results and give the nephrologists direct information on whether continuing, incrementing, or dismissing the therapy.
Over the years, we came to observe a mismatch between clinical and radiological findings at the 1-month follow-up (FU), i.e., DW-MR persistence of some APN foci despite the absence of clinical symptoms, in a not negligible number of patients. We thus designed a prospective study including also a DW-MR at 3 months: the hypothesis was that a longer observation period could yield more information on the background of this mismatch, eventually allowing the identification of predictors that could be used by nephrologists in the therapy planning. As routinely done at the APN onset and the 1-month FU, also the 3-month DW-MR exam was to be performed at the same time as the laboratory tests for white blood cell (WBC) and C-reactive protein (CRP) and a complete nephrologist visit.
The trial was approved by the institutional ethical review board (n. 58/2016, January 20th, 2016). The hospital agreed to assume the financial burden of the third DW-MR exam for 30 consecutive patients meeting the inclusion and exclusion criteria.
Study sample
The study sample was extracted from the population of consecutive patients referred by the nephrologists of our institution to our center for DW-MRI examination between March and September 2016. The request was for confirming the clinical suspicion of APN based on the presence of symptoms (such as fever, costovertebral angle tenderness or pain, recent or present UTI) and signs of systemic infection (high white blood cells or C-reactive protein) without predisposing condition found at US. Inclusion criteria for enrollment in the trial were MR confirmation of APN and willingness to cooperate with the study protocol and follow-up program. Exclusion criteria were pregnancy (if women), contraindication to MR (including claustrophobia), APN episodes in the previous 24 months, and presence of vesicoureteral reflux (VUR), since it could affect the DW-MRI. During this period, no male was referred to our Institution with an APN scenario, so the thirty patients who met the criteria and were thus enrolled in the trial turned out to be all females.
Nine patients had a history of recurrent UTI, eight of previous APN, and six of renal calculi. One patient suffers from multiple sclerosis treated with immunotherapy and another with interferon for essential thrombocytosis.
All patients were hospitalized at diagnosis. Parenteral antibiotics was administered for 14–21 days, followed by oral therapy for 1–2 weeks. 18 out of 30 patients were treated with an aminoglycoside and a third-generation cephalosporin, nine with third-generation cephalosporin, and the other three patients were administered, respectively, a carbapenem, a quinolone, or a fourth-generation cephalosporin.
MR protocol
All MR examinations were performed at 1.5 T (Achieva, version 2.6, Philips Medical Systems, Eindhoven, The Netherlands) with body coil phased array (16-channel Sense XL Torso). The MR protocol with its detailed sequences and its technical parameters is shown in Table 1.
According to the consensus on DW-MRI [15], we used a protocol with 4 b values (b = 0, 50, 600, 1000 s/mm2) and the ADC quantification of the APN foci was calculated with a monoexponential decay between 50 and 1000 s/mm2. This is fundamental for calculating perfusion-insensitive ADC values, because the degree of perfusion bias in ADC measurement increases with the volume fraction of flow and decreases with the b value range [15].
Imaging analysis
MR Extended Work Space 2.6.3.2 2009 software (Philips Medical Systems) was used for imaging interpretation.
Qualitative analysis was based on visual assessment of the morphologic T1-weighted images (T1WI), T2-weighted images (T2WI), and DWI (DWI+), as compared with the corresponding ADC map. The typical APN focus is revealed by a low-intensity signal on T1WI and a high-intensity signal on T2WI, due to interstitial edema, and is identified as an area with higher signal on DWI than in healthy parenchyma, because of reduced diffusivity of water molecules. The healthy renal parenchyma was defined as the parenchyma without signal abnormalities in all sequences.
For quantitative analysis, the ADC maps were generated (b 50–1000 s/mm2). Oval ROIs as small as possible were manually drawn on the ADC maps near the center of each APN focus for reducing the possibility of signal contamination caused by the partial volume effect. At the FU examinations, if the foci were still visible (DWI+), a ROI of the same dimension was depicted near the center of the hypointense area on the ADC maps; if the foci were no longer visible (DWI−), an equal size ROI was depicted in the site of the APN focus detected at the acute phase. At every DW-MR examination, the ADC of the background parenchyma was measured by placing freehand-oval regions of interest (ROIs) with the diameter of about 1 cm on the upper, middle, and lower poles of cortical parenchyma not interested by foci.
The largest diameter of each lesion (D) was measured. All lesions were included, regardless of their size.
Two observers (S.B. and M.G. with 4-year experience in MR) independently reviewed all images in a double-blinded way, recorded existence and location of foci, and measured the corresponding ADC and D values.
Data presentation and statistical analysis
In the following, the data relative to three steps of the APN evolution, onset, FU at 1 month, and FU at 3 months, are represented with the subscript relative to the time of observation: t 0, t 1, t 3.
Continuous variables satisfied the normality test of Shapiro–Wilks and were thus expressed as average ± standard deviation. Matched variables were compared with Wilcoxon’s test (2 variables) or Friedman’s test (>2 variables) and independent variables with Mann–Whitney’s test (2 variables) or Kruskal–Wallis’s test (>2 variables). The association between two sets of data was explored with the eta-squared coefficient for unordered pairs and Pearson’s linear correlation coefficient r. The discriminating ability of continuous variables was measured by the ROC curve procedure, through the Area Under the Curve (AUC). The most appropriate cut-offs between the two regions of interest were derived through maximization of the harmonic mean of sensitivity and specificity and of Youden’s index and minimization of the curve distance from the upper left vertex (0,1).
Categorical variables, reported as counts and percentages, were arranged in cross-correlation tables and studied with the χ 2 test (with Yates’ correction for 2 × 2) or Fisher’s exact test.
Statistical significance was set at p ≤ 0.05 and RR 95% CI not including 1. Statistical tests were run on StatPlus: Mac v.6 (AnalysisSoft. Walnut. CA. USA).
Results
Laboratory and clinical data
The age of the 30 patients was 36 ± 10 (minimum 22, maximum 51) years. At the time of the first MR examination (t 0), all presented severe and painful symptoms and laboratory values significantly above the physiological range: white blood cell count WBC0 = 13 ± 4×109/l and C-protein Reactive CPR0 = 124±64 mg/l.
At the 1st month FU (t 1), all patients were free from symptoms and with laboratory values restored to the physiological range: WBC1 = 6 ± 2109/l and CPR1 = 2.0 ± 1.5 mg/l. This situation was confirmed at the 3rd month check-up: no symptoms and stable physiological laboratory values: WBC3 = 6 ± 1 × 109/l and CPR3 = 1.8 ± 1.7 mg/l.
DWI-MR data: detection of APN foci
The inter-reader agreement, evaluated at the time of the first exam, was very good as to the identification of foci (93%) and values of ADC and D (Wilcoxon’s p = 0.81 and 0.67 and eta-squared = 0.96 and 0.94, respectively). In the few cases of disagreement, the decision was reached by consultation with a senior radiologist (RF with 10 years’ experience in MR).
At the acute stage, the total number of detected foci was N 0 = 187, irregularly distributed from 1 (3 patients) to 18 per patient. Two patients had bilateral APN.
The average dimension of detected foci over all patients was D 0 = 14.0 ± 7.5 (minimum 1.2–maximum 40.0) mm. The corresponding ADC0 was 1.3 ± 0.2 (0.7–1.8) × 10−3mm2/s.
The ADC of the healthy parenchyma of each patient was computed as the mean of the three measurements performed on the upper, middle, and lower poles (no statistical difference between the three sites for all patients, with p ≥ 0.70). Its value was ADCpar = 2.0 ± 0.1 × 10−3 mm2/s (1.7–2.2), significantly higher (p < 0.0001) than the values for APN foci.
The value for the focus-free parenchyma was used to compute for each focus the ratio R = ADCfocus/ADCpar. The use of the ratio R instead of ADC has the double advantage of granting uniformity to the results in our study, since it smoothens out biological differences among patients, and of allowing comparisons with other studies, since the ADC values depend on the technical parameters and diffusion gradient used in the DW-MRI. The average value of the ratio was R 0 = 0.65 ± 0.12 (0.32–0.95). R 0 showed a modest but significant inverse correlation with D 0 (r = −0.36; p < 0.0001), witnessing the variability of presentation of the various foci.
The signature of APN radiological resolution, defined as achieved restitutio ad integrum of the renal tissue site of the original inflammatory focus, included two conditions: focus no longer visible (DW−) and ADC of the site not significantly inferior to the ADC of the surrounding healthy parenchyma, i.e., R ≥ 0.9.
DWI-MR data: resolution of APN foci
At the 1-month examination, 80/187 (42.8%) foci were radiologically resolved, i.e., they were no longer visible (DWI−) and with R 1 = 0.96 ± 0.05. By the 3-month examination, other 58 (31%) foci had reached complete radiological resolution (DW− and R 3 = 0.94 ± 0.05), increasing the number of resolved foci to 138 (73.8%). The 49 foci (26.2%) not resolved by the 3-month FU had two different presentations: 37 were visible (DW+, D 3 = 7 ± 3 mm) and with R 3 = 0.72 ± 0.08, whereas 12 were not visible (DW−), but with R 3 = 0.80 ± 0.04, still far from the resolution value.
We explored the possibility that the different evolution of foci toward remission could be related to differences in their initial conditions, as expressed by the values of R 0 and D 0. Table 2 shows the values of the time-zero variables for the two extreme situations: foci radiologically resolved at 1 month (matching the clinical and laboratory results) and foci not yet resolved at 3 months (despite the 2-month permanence of satisfying clinical and laboratory data).
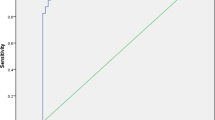
The significant differences between the initial features R 0 and D 0 for these two samples suggested for them a potential predicting role. The ROC curve procedure identified as the threshold for delayed radiological solution R 0 = 0.6 (AUC = 0.83; Sensitivity SNS = 74%, Specificity SPC = 78%, Positive Predictive Value PPV = 65%, Negative Predictive Value NPV = 85%; Likelihood Ratio for Positive test LR+ = 3.6) and D 0 = 0.15 mm (AUC = 0.80; SNS = 64%, SPC = 79%, PPV = 75%, NPV = 72%, LR+ = 3.0) (Fig. 1).
Height of the columns represents the percentage of APN foci solved by the 3rd month follow-up DW-MR exam: the bottom and top segments of the columns represent, respectively, the percentage of foci solved by the 1st month FU and the percentage of foci solved in the interval between the 1st and 3rd month FU
The presence at APN onset of both risk factors R 0 ≤ 0.6 and D 0 > 15 mm, or only one of them (either R 0 ≤ 0.6 or D 0 > 15 mm) or none, was associated with three different evolution paces over the next 3 months, as shown in Table 3 and Fig. 2. Radiological resolution paralleled clinical recovery for 80/187(43%) of all diagnosed foci, ranging from 63% in the absence of risk factors down to 17.5% in the presence of both risk factors. Among the 58 foci which managed to achieve the restitutio ad integrum target by the 3-month FU, about 81% had R 1 ≥ 0.8 and D 1 ≤ 10 mm. The incidence of unresolved foci for the group with both risk factors was 52.5%, about ten times higher than that for the group without risk factors.
A typical case of restitution ad integrum post APN. The top panels refer to the acute DW-MRI (on the left DWI: 1000 s/mm2 and on the right the corresponding ADC map) of an APN focus in the right kidney. It appears as an area of high signal on the high-b-value image corresponding to an area of low signal intensity on the ADC map. The middle and bottom panels show the 1- and 3-month follow-up: the APN focus is no longer visible (DWI−)
Figures 2, 3, and 4 show the DWI-MR outcome for the different evolution paces. Figure 2 illustrates a case of restitution ad integrum post APN observed at the 1-month FU, Fig. 3 a case of foci solved at the 3-month FU, and Fig. 4 a typical case of failed radiologic resolution by the 3-month FU.
A typical case of radiologic resolution at 3 months. The top panels refer to the acute DW-MRI (on the left DWI: 1000 s/mm2 and on the right the corresponding ADC map) of an APN focus in the left kidney. The middle and bottom panels show the 1- and 3-month follow-up: at 1 month the APN focus is still visible (DWI+) and its ADC value is lower than the ADC of parenchyma, whereas at 3 months the APN focus is no longer visible (DWI−)
A typical case of slow radiologic resolution. The top panels refer to the acute DW-MRI (on the left DWI: 1000 s/mm2 and on the right the corresponding ADC map) of an APN focus in the left kidney. The middle and bottom panels show the 1- and 3-month follow-up: the APN focus is still visible (DWI+) and its ADC value is lower than the ADC of parenchyma
Discussion
This paper explores the different evolution paces of 187 APN foci detected by DWI-MR in 30 patients, comparing clinical and radiological resolution during the various steps of a 3-month follow-up.
At the acute stage, when all patients presented severe and painful symptoms and laboratory values well above the physiological range, the 187 APN foci had ADC0 = 1.3 ± 0.2 (minimum 0.7–maximum 1.8) × 10−3 mm2/s, corresponding to R 0 = ADCfocus/ADCpar = 0.66 ± 0.12 (0.32–0.95). This value is consistent with the outcome of recent studies with technical characteristics not too different from ours. For De Pascale et al. [8], R ≈ 0.58 (ratio of reported 1.4 × 10−3 to 2.4 × 10−3 mm2/s); for Rathod et al. [12], R ≈ 0.62 (ratio of reported 1.3 × 10−3 to 2.1 × 10−3 mm2/s); and for Faletti et al., R ≈ 0.64 (ratio of reported 1.5 × 10−3 to 2.2 × 10−3 mm2/s) in native kidneys [11] and ≈0.67 (ratio of reported 1.4 × 10−3 to 2.1 × 10−3 mm2/s) in transplanted kidneys [10].
The maximum diameter of the foci at the acute stage was D 0 = 14 ± 8 mm, with a modest inverse correlation (r = −0.36) with R 0. The weakness of the correlation is due to the wide variability of R 0 values for each given D 0, which creates a cloud-shaped graph.
At the 1-month follow-up, “clinical recovery,” defined as the normalization of clinical and laboratory data was diagnosed by nephrologists for all patients. The DW-MRI outcome presented instead a less positive picture: only 80 of the 187 original foci (43%) had achieved the restitutio ad integrum target, defined as DW− and R 1 ≥ 0.9. It is not clear why some foci persist on DW-MRI despite clinical and laboratory data resolution; as hypothesized in [10], a viable theory is that the lingering of edema (mainly) and fibrosis may reduce the interstitial spaces hampering molecular diffusion.
The percentage of 1-month visible foci was approximately similar to previous findings on the native kidney [7] but more frequent than for the transplanted ones [10] (41 vs. 14%). This could be related to the fact that transplanted patients, being aware of UTI risks and having a close follow-up, are promptly treated at the early beginning of the symptoms; differently, healthy women most often do not recognize the symptoms and thus have a later diagnosis when the disease has become more severe.
At the 3-month FU, DW-MRI declared fully resolved 138/187 (74%) foci. Among the 49 (26%) foci still missing radiological resolution, 37 were visible whereas 12 were no longer visible but with R 3 significantly below the target value. In this case, it is plausible that the reduction of the interstitial spaces be mostly determined by fibrosis rather than edema. Fibrosis in fact appears high in signal intensity on DW-MRI and returns low ADC values [15].
The ROC curve procedures, applied separately to R 0 and D 0, associated the foci with the slowest radiological resolution to R 0 ≤ 0.6 (AUC = 0.83) and D 0 > 15 mm (AUC = 0.80), respectively. Because of the poor correlation between R and D, it is the full set of initial conditions [R 0, D 0] which influences the foci evolution: a low R value may be reinforced by a high D value (damping the already slow healing) or compensated by a low D value (speeding up the pace of healing). The focus evolution pace toward radiological solution is thus more or less slowed down according to the presence of both risk factors R 0 ≤ 0.6 and D 0 > 15 mm.
Radiological solution paralleled clinical recovery at 1 month for 63% of foci with no risk factors, for 37% of foci with one risk factor, and for 17% of foci with both risk factors. Among the 58 foci unresolved at one month, which managed to achieve the restitutio ad integrum target in the following two months, about 80% had R 1 ≥ 0.8 and D 1 ≤ 10 mm.
The lack of contrast does not allow us to go beyond a suspicion of abscess based on particularly low values of ADC and R. It is thus interesting to fit our results in the scenario created by previous studies on native kidneys using contrast media, i.e., with the actual possibility of distinguishing abscesses from “simple” foci. The condition R 0 ≤ 0.6, characterizing our foci with the slowest radiological resolution, is consistent with the threshold for abscesses in native kidneys given by Faletti [11]: ADCabs ≤ 1.2 × 10−3 mm2/s, corresponding to R abs ≤ 0.54 (ratio of 1.2 × 10−3 mm2/s to the ADC of healthy parenchyma 2.2 ± 0.21 × 10−3 mm2/s). Piccoli et al. [7], using an integrated analysis of imaging, classified APN foci into “simple,” “with colliquative tendency,” and “abscesses,” evaluating their relationship with the development of kidney scars. Our 138 foci that achieved full recovery by the end of the FU might correspond to their “simple” foci, whereas the unresolved 49 foci might correspond to their “abscesses” with the highest tendency to develop fibrosis.
In clinical practice, these results can be very useful to a clinician requesting a MR examination on a patient with lingering APN symptoms despite the treatment completion; at the same time, the knowledge of a possibly slow focus evolution gives radiologists the tools to avoid a false-positive diagnosis in case of persistence of a focus which had initially been diagnosed with one or two risk factors.
In addition, it is intriguing to speculate that in the future the APN therapeutic treatment could be tailored, not only relying on the acute phase (e.g., the presence of abscesses or bilateral infection), but also considering the imaging evolution of the foci. Based on the results of the 1-month follow-up, the antibiotics therapy might be dismissed in case of disappearance of all foci and increased/prolonged in the event of persistence of visible foci.
This study has some limitations. First, it covers a limited number of patients, even with a substantial number of foci (and in fact all statistical tests had good power). Second, the diagnosis of pyelonephritis was based on the clinical scenario and the radiologic findings, without confirmation by histopathological findings. Third, the lack of contrast media and dynamic study forbade a direct identification of complicated foci.
In summary, our study evidenced the existence and cohabitation within the same patient of foci with fast (1 month) and slow (≥3 months) radiological recovery and that their path to radiological solution is shaped by the values of R and D at the acute stage. The 1-month FU is important because it routinely coincides with the end of the ATB treatment and is thus crucial to avoid radiological false positive, which may mislead the therapeutic decision of the nephrologist. Foci still visible at one month but with a significant increase in R and decrease in D have in fact good chances to reach radiological resolution, with restitutio ad integrum of the renal tissue, in the two following months. The 3-month FU did not yield results to impact the ongoing clinical management: its value laid in having confirmed that several foci not yet resolved at the 1-month FU, were nevertheless on the path to resolution, with a decreasing dimension and an increasing ADC. On the basis of this result, we deem that our institution protocol which includes DWI-MR at onset and at 1 month already offers a thorough diagnostic package for the majority of patients: however, were the 1-month exam to evidence no improvement in the initial scenario, then a 3-months FU exam could be useful to reach a better understanding of the evolution of the disease.
We hope that the scenario sketched by this study may foster the interaction between radiologists and nephrologists for the most appropriate diagnostic–therapeutic management.
References
Neal DE (2008) Complicated urinary tract infections. Urol Clin North Am 35(1):13–22
Czaja CA, Scholes D, Hooton TM, Stamm WE (2007) Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis 45(3):273–280
Das CJ, Ahmad Z, Sharma S, Gupta AK (2014) Multimodality imaging of renal inflammatory lesions. World J Radiol 6(11):865–873
Brenner DJ, Hall EJ (2007) Computed tomography—an increasing source of radiation exposure. N Engl J Med. 357(22):2277–2284
Fontanilla T, Minaya J, Cortés C, et al. (2012) Acute complicated pyelonephritis: contrast-enhanced ultrasound. Abdom Imaging 37(4):639–646
Martina MC, Campanino PP, Caraffo F, et al. (2010) Dynamic magnetic resonance imaging in acute pyelonephritis. Radiol Med 115(2):287–300
Piccoli GB, Colla L, Burdese M, et al. (2006) Development of kidney scars after acute uncomplicated pyelonephritis: relationship with clinical, laboratory and imaging data at diagnosis. World J Urol 24(1):66–73
De Pascale A, Piccoli GB, Priola SM, et al. (2013) Diffusion-weighted magnetic resonance imaging: new perspectives in the diagnostic pathway of non-complicated acute pyelonephritis. Eur Radiol 23(11):3077–3086
Piccoli G, Colla L, Maass J, et al. (2005) Acute pyelonephritis: a new approach to an old clinical entity. J Nephrol 18(4):474–496
Faletti R, Cassinis MC, Gatti M, et al. (2016) Acute pyelonephritis in transplanted kidneys: can diffusion-weighted magnetic resonance imaging be useful for diagnosis and follow-up? Abdom Radiol N Y 41(3):531–537
Faletti R, Cassinis MC, Fonio P, et al. (2013) Diffusion-weighted imaging and apparent diffusion coefficient values versus contrast-enhanced MR imaging in the identification and characterisation of acute pyelonephritis. Eur Radiol 23(12):3501–3508
Rathod SB, Kumbhar SS, Nanivadekar A, Aman K (2015) Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 56(2):244–249
Vivier P-H, Sallem A, Beurdeley M, et al. (2014) MRI and suspected acute pyelonephritis in children: comparison of diffusion-weighted imaging with gadolinium-enhanced T1-weighted imaging. Eur Radiol 24(1):19–25
Aoyagi J, Odaka J, Kuroiwa Y, et al. (2014) Utility of non-enhanced magnetic resonance imaging to detect acute pyelonephritis. Pediatr Int 56(3):e4–e6
Padhani AR, Liu G, Mu-Koh D, et al. (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia N Y N 11(2):102–125
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faletti, R., Gatti, M., Bassano, S. et al. Follow-up of acute pyelonephritis: what causes the diffusion-weighted magnetic resonance imaging recovery to lag clinical recovery?. Abdom Radiol 43, 639–646 (2018). https://doi.org/10.1007/s00261-017-1242-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1242-0