Abstract
Purpose
To assess reliability of diffusion-weighted magnetic resonance imaging (DW-MRI) in the management of acute pyelonephritis (APN) foci in transplanted kidneys.
Materials and methods
In the 2012–2014 period, 24 kidney-transplanted patients underwent MR screening for clinical suspicion of APN. Two readers independently analyzed all images, establishing presence and location of APN foci. The 22 patients who were positive at the MR exam constituted the study population. For each patient the apparent diffusion coefficient (ADC) was measured in the APN foci and in three sites of the healthy parenchyma (case–control comparison). The data were matched to the laboratory measurements for white blood cell, C-reactive protein, and serum creatinine.
Results
Forty-six APN foci were found in 22/24 patients. At the acute stage, the difference in ADC between healthy parenchyma and APN foci was significant (2.06 ± 0.16 vs. 1.43 ± 0.32 × 10−3 mm2/s; p < 0.0001). The performance of ADC as APN indicator was tested by the receiving operating characteristics (ROC) curve: the area under curve AUC = 0.99 witnessed an excellent discriminatory ability, with threshold APN/normal parenchyma 1.9 × 10−3 mm2/s. At the 1-month follow-up 43/46 APN foci were no longer visible, with ADC values significantly higher than at the acute stage; all laboratory data were physiological, with WBC significantly reduced from the acute phase (5.2 ± 1.6 × 109/L vs. 10.6 ± 4.8 × 109/L; p < 0.0001). The other 3 patients underwent further therapy and exams, including a third MR.
Conclusions
DW-MRI with ADC measurement seems to be a reliable tool in diagnosing and monitoring APN foci in transplanted kidneys, with clinical impact on patient management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Kidneys are the most frequently transplanted organs since renal transplantation is the preferred method for treating patients with end-stage renal disease. Post-transplantation urinary tract infection (UTI) is a source of morbidity and graft failure [1]. Renal transplant recipients develop UTIs more frequently than the general population; in particular, UTIs are more common during the first months after transplantation, and can predispose the patient to allograft pyelonephritis [1–4]. Early and specific detection of such medical problems is thus of utmost importance to warrant the appropriate treatment to be started on time or the treatment approach modified, if necessary [3–5].
Imaging of the kidneys in these patients is challenging because the administration of contrast medium ought to be limited to patients with normal renal function. Contrast-enhanced computed tomography (CT) is the gold standard for diagnosis and assessment of severity of acute pyelonephritis (APN) and its complications [6]; it is however burdened by the well-known risk of contrast medium-induced nephropathy [7–9] CEUS has been shown to perform well in the diagnosis of APN for both native and transplanted kidneys [10, 11], but its use requires specific expertise not so widely diffused. Magnetic resonance imaging (MRI) is indicated for patients with contraindications to iodinated contrast media such as transplant recipients. Contrast medium administration for MRI has, however, been associated with nephrogenic systemic fibrosis in patients with renal function impairment [12, 13].
MR without contrast media, particularly Diffusion-weighted magnetic resonance imaging (DW-MRI) and the apparent diffusion coefficient (ADC) value have already been shown capable to distinguish healthy kidney functions from pathologic ones in native kidneys [14–17]. In particular one study on the diffusion and microcirculation properties of tissues in native kidneys identified three ADC value brackets corresponding, respectively, to healthy parenchyma, acute pyelonephritis (APN) foci, and abscesses, suggesting that ADC maps could be a reasonable alternative to contrast-enhanced MR imaging when contrast media are contraindicated [14].
To assess reliability of DW-MRI as an alternative approach to routine renal MR imaging protocol in the management of Acute Pyelonephritis for transplanted kidneys, we resorted to a retrospective study with the objective of answering to four questions:
-
i)
Is it possible to confirm the clinical suspicion of acute pyelonephritis in transplanted kidneys with DW-MRI without the use of contrast media?
-
ii)
Is it possible to derive for transplanted kidneys reference ADC values for distinguishing between healthy parenchyma and APN foci?
-
iii)
Is there a relation between ADC values and the clinical and laboratory data used to advance suspicion of APN or state its progress toward healing?
-
iv)
How do the ADC reference values for transplanted kidneys compare to those for native kidneys?
Materials and methods
Study population
The kidney transplant center in our institution is currently following 342 patients who underwent transplant in the period from January 2012 to December 2014. In this 3-year period, 50 of these patients were submitted to MR in our department under request of the clinicians of the transplant center. The time interval from transplant to MR spanned between 12 and 509 days.
Twenty-four patients (48%) were sent with suspicion of APN on the basis of their clinical scenarios: all of them had at least one of the three main symptoms (temperature, pain, recent, or present UTI) and abnormally high values of white blood cell (WBC), C-reactive protein (CRP), and serum creatinine (CSR).
Twenty-two patients were found positive at MR and they formed our study population, with no exclusion. As for the two patients negative at MR, the first one continued the therapy prescribed by the nephrologists’ protocol, i.e., 14 days of broad-spectrum therapy per os, until the temperature disappeared. The second patient, on the basis of the result of the MR examination, was considered fit to interrupt the ATB therapy preventively started and thus avoided unnecessary treatment.
The 26/50 patients sent for reasons other than suspected APN or reduced renal function were used only as an additional control group for ADC in healthy parenchyma.
At the moment of the MR examination, all 50 patients were informed about the possible use of their data for study purposes, and signed an informed consent form. The study is a retrospective trial without any study-related clinical intervention and conforms to the Helsinki Declaration of 1975 and subsequent modifications.
MR protocol
The MR examination was performed at 1.5 T (Achieva, version 2.6, Philips Medical Systems, Eindhoven, The Netherlands) with body coil phased array (16-channel Sense XL Torso). The MR protocol with its detailed sequences and its technical parameters is shown in Table 1.
The protocol used for all 50 patients, whether they were sent for APN suspicion or other reasons, was the same used in the renal evaluation of native kidneys except for the dynamic study with contrast media administration, which could not be performed because of the low value of glomerular filtration rate (eGFR) of our transplanted population: eGFR ≤ 40 mL/min in 49/50 (98%) of transplanted patients and ≤30 mL/min in 42/50 (84%).
Imaging analysis
MR Extended Work Space 2.6.3.2 2009 software (Philips Medical Systems) was used for imaging interpretation.
Qualitative analysis was based on visual assessment of the morphologic T1WI, T2WI, and DWI, as compared with the corresponding ADC map. The APN focus is revealed by a low-intensity signal on T1-weighted and a high-intensity signal on T2-weighted images due to interstitial edema and is identified as an area with higher signal on DWI than in healthy parenchyma, as a consequence of reduced diffusivity of water molecules. The healthy renal parenchyma was defined as the parenchyma without signal abnormalities in all sequences. There were no cases of diffuse renal involvement.
For quantitative analysis, the ADC maps were generated. Oval regions of interest (ROIs) were manually drawn as small as possible near the center of each APN focus for reducing the possibility of signal contamination caused by the partial volume effect. A freehand-oval ROI (with diameter of about 1 cm), including the majority of healthy cortical parenchyma on an axial plane, was drawn in the upper, middle, and lower pole to better reproduce the ADC value of the whole renal cortical parenchyma and reduce the standard deviation. All lesions were included, regardless of their size.
Two observers (C.G. and M.G., with 3-year experience in MR) independently reviewed all images in a randomized and blinded way, recorded existence and location of foci and measured the corresponding ADC values.
Statistical analysis
Continuous variables, reported as mean ± standard deviation and range (minimum–maximum value) were analyzed by non-parametric tests. Correlated distributions were compared with the Wilcoxon test (k = 2 distributions) or Friedman’s test (k > 2); independent distributions with the Mann–Whitney test (k = 2).
Binary and categorical variables, reported as counts and percentages, were arranged in contingency tables and studied with the Chi-square test (with Yates’ correction for 2×2) or, when appropriate, with Fisher’s exact test. Statistical significance was set at two-tails p < 0.05.
The discriminatory ability of the ADC value as indicator of APN was assessed by the ROC curve. The area under the curve (AUC) measures the quality of discrimination: conventionally 0.6 corresponds to poor and 1 to excellent. The threshold for APN was set at the ADC value for which two parameters associated with the ROC curve—harmonic mean HM of specificity and sensitivity and Cohen’s coefficient k-reached contemporarily their maximum.
The relation between two sets of data was explored with η 2 coefficient and Pearson’s linear correlation coefficient r with its associated regression coefficient.
Results
ADC: acute stage
MR-DWI was positive for 22 of the 24 patients with suspected APN (92%): their baseline at dismissal after transplantation is summarized in Table 2. The 22 patients with confirmed APN were scheduled to undergo a second follow-up exam after 1 month, at the end of the clinical treatment prescribed by nephrologists.
The 22 patients found positive for APN had a total of 46 APN foci, so distributed: 11 patients had one, 4 patients had two, 2 patients had three, 4 patients had four, and 1 patient had five. The two readers agreed in recognizing APN foci in 44/46 (96%) of cases: in the other cases, the final verdict was reached by consensus with a senior radiologist (R.F). The ADC values measured by the two readers were in agreement according to both Wilcoxon’s test for matched data (p = 0.92) and η 2 coefficient (0.97).
The analysis was carried out as a case–control comparison within the same patient at the same time, the 46 pairs being constituted by the ADC value in each APN focus (study) and the ADC value in the healthy parenchyma (control). The latter was computed as the average of ADC measured at the upper, middle, and lower pole of normal renal cortical parenchyma, since the Friedman test for matched distribution assessed for each patient the lack of significant differences among the three measures (p = 0.84).
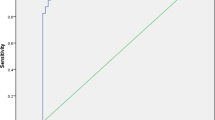
The analysis of the 46 pairs of ADC measured in this first exam evidenced that APN foci and healthy parenchyma constitute two significantly different populations, as shown in the first row of Table 3; Fig. 1, top panel, evidences how the two ADC distributions are remarkably well separated with only a tiny overlapping region. The third column of Table 3 shows that the healthy parenchyma of the 22 patients with confirmed APN had ADC value similar to the one measured for the 26 patients who underwent the MR examinations for reasons other than suspected APN.
ROC curve procedure to test the discriminating ability of ADC between APN foci and healthy parenchyma. Top panel ADC distributions (0.05 × 10−3 mm2/s sampling interval). Middle panel ROC curve (AUC = 0.999). Bottom panel Plot of HM (harmonic mean of sensitivity and specificity) and k (Cohen’s coefficient) as a function of ADC: their maximum indicates the ADC threshold. (The ADC values are × 10−3 mm2/s)
The ROC computed on the basis of the two ADC distributions is plotted in the middle panel of Fig. 1 and is striking for its almost perfectly squared shape: AUC = 0.999 (95% CI 0.995-1.00), corresponding to an excellent discriminating performance of the ADC indicator. The bottom panel of Fig. 1 clearly shows that the harmonic mean of sensitivity and specificity (HM) and Cohen’s coefficient (k) reach their maximum for ADC = 1.9 × 10−3 mm2/s, thus unanimously identifying this value as the most appropriate threshold, with sensitivity, specificity, and diagnostic accuracy >95%. ADC ≤ 1.9 × 10−3 mm2/s held for all APN foci and for 3/46 healthy parenchyma sites (values between 1.81 and 1.86 × 10−3 mm2/s): the difference is highly significant (p < 0.0001), with 94% positive predicting value and 100% negative predictive value.
ADC: 1-month follow-up
The qualitative analysis of the 22 patients follow-up exams evidenced that 43 out of the 46 APN foci (93%) detected at the first diagnostic exam were no longer visible. The ADC values measured at the site of the original foci were significantly higher than the values measured during the acute stage (1.94 ± 0.15 × 10−3 vs. 1.43 ± 0.32 × 10−3 mm2/s; p < 0.0001), having completely merged with the ADC value of the healthy parenchyma (p = 0.71). The ADC values of the three foci still visible at the one-month follow-up are shown in Table 4. Patient 45 was the one with 5 APN foci at the diagnostic exam, all but one healed at follow-up.
All measurements, including those at the three lingering foci, showed an increase in ADC ranging from 6% to 326%.
Figure 2 illustrates a typical case of APN.
A typical case of APN: the top panel refers to the diagnostic DW-MRI (DWI: b 0, b 300, and b 600 s/mm2; ADC map) of an APN focus on the left side of the transplanted kidney. It appears as an area of high signal on the high-b-value image corresponding to an area of low signal intensity on the ADC map (arrows). The bottom panel shows the 1-month follow-up DW-MRI: the APN focus is no longer visible, neither on high-b-value image nor on the ADC map (arrowheads)
Relation ADC-WBC
At the acute stage, all patients had the white cell number and the value of serum creatinine well above physiological ranges, with a significant increase from the values at dismissal reported in Table 2: WBC = 10.6 ± 4.8 × 109/L (p = 0.004) and CRS = 3.38 ± 1.61 mg/dL (p = 0.005). Also the C-reactive protein (CRP) had skyrocketed to values several times higher than the physiological ones (74.5 ± 68.8 mg/L).
At the 1-month follow-up, the WBC, CRS, and CRP values of all patients had returned within the physiological range: in particular WBC showed a significant decrease from the values characterizing the acute phase (from 10.6 ± 4.8 × 109/L to 5.2 ± 1.6 × 109/L; p < 0.0001).
Figure 3 shows the plot of the ADC values (in case of multiple foci the average ADC) of the 22 patients against the WBC values, as measured at acute stage and follow-up; for clarity the 44 values have been grouped in 11 quadruplets represented in the plot by their mean values and standard deviations (horizontal error bars for WBC and vertical bars for ADC). The data show a moderate-to-good linear inverse correlation: Pearson’s linear coefficient is r = −0.64 with null probability p(r = 0) = 0.03; the regression coefficient Δ(ADC)/Δ(WBC) is −4.5 × 10−14 mm2L/s.
Discussion
Our study investigated and assessed the ability of ADC measurements in distinguishing APN foci from healthy parenchyma in transplanted kidneys. In transplanted kidneys, as well as in native kidneys, inflammatory foci appear as areas of impeded diffusion of water molecules with high signal intensity on DWI sequences and low-intensity signal on the corresponding ADC map. Our results showed that the 46 inflammatory foci had statistically lower ADC values (1.43 ± 0.32 × 10−3 mm2/s) than healthy parenchyma, as measured in the 22 patients with suspected APN (2.06 ± 0.16 × 10−3 mm2/s) and in the 26 patients without suspected APN (1.99 ± 0.14 × 10−3 mm2/s). The ADC ability to identify APN foci in transplanted kidneys, as estimated by the ROC curve, was excellent (95% CI for AUC 0.995–1), determining ADC < 1.9 × 10−3 mm2/s as the region typical of APN foci (PPV = 94% and NPV = 100%)
The role of diagnostic imaging in confirming a clinical suspicion of APN and its impact on treatment decisions has been reported by several studies [14–19]. In particular it was also emphasized that imaging is indispensable for confirming treatment effectiveness during follow-up [20]. DW-MRI with ADC allows functional evaluation of renal lesions and was proved to be reliable for diagnosing APN in native kidneys by several authors [14–17]. On the contrary, there are still relatively few studies in the literature on the use of DWI in transplanted kidneys, even if the initial results on its use for the assessment of early graft deterioration encountered in nearly 30% of renal allografts seem encouraging [21–24]. In particular, despite the pressing need of these patients for contrast-free MR imaging, to our knowledge there are no studies in which the feasibility and reproducibility of DW-MRI in detection and follow-up of APN in transplanted kidneys were analyzed.
The comparison between our transplanted kidneys data and the native kidneys data reported in the literature requires some caution, since the ADC values depend on the technical parameters and diffusion gradient used in the MRI. So for instance, the values for ADC in healthy parenchyma in native kidneys reported in the literature range from 1.60 to 2.65 × 10−3 mm2/s (500–1.300 s/mm2) [14, 15, 21, 23]. The comparison of our data on transplanted kidneys with the outcome of a study on native kidneys [14] carried out with a MR protocol similar to ours (gradient b values 0, 300 and 600 s/mm2) shows an interesting feature: the ADC for APN foci is very similar for transplanted and native kidneys (1.43 ± 0.32 × 10−3 vs. 1.46 ± 0.27 × 10−3 mm2/s; p = 0.91), whereas the ADC for healthy parenchyma is significantly lower in transplanted kidneys than in native ones (2.06 ± 0.16 × 10−3 vs. 2.16 ± 0.24 × 10−3mm2/s: p = 0.03). This difference is compatible with the results of Thoeny et al. [21], who reported that in transplanted kidneys the ADC values were almost identical in both medulla and cortex, while in native kidneys the ADC values were significantly higher in the cortex than in the medulla. The loss of a cortico-medullary differentiation, that the authors attribute both to the denervation of the transplanted kidneys and to the effects of immunosuppressive therapy, could indeed lead to the lower ADC values found for the parenchyma of transplanted kidneys.
At the 1-month follow-up, 19/22 (86%) patients had no visible foci. Forty-three out of the 46 originally diagnosed APN foci had by then become undistinguishable from the surrounding parenchyma, with a significant increase of their ADC values compared to the values recorded during the acute stage (1.94 ± 0.15 × 10−3 vs. 1.43 ± 0.32 × 10−3mm2/s; p < 0.0001). The remaining three patients had one focus that was still visible on DW-MRI, in spite of the increase in the measured ADC (see Table 4). The patient with a 90% increase in ADC was considered on the right track to healing and was not scheduled for a third MR examination. The two other patients, characterized at the acute stage by ADC values well below 1 × 10−3 mm2/s and a smaller increase (<20%) at follow-up, underwent a third MR examination 2 months later, which witnessed their definite trend toward a positive evolution.
It is indeed interesting to note that our study shows that the so-called “clinical recovery”, defined as the normalization of inflammatory laboratory data, does not necessarily correspond to the complete restitutio ad integrum as far as MR is concerned. At the 1-month follow-up, all 22 patients had normal laboratory data with a statistically significant decrease of WBC compared to the acute stage (5.2 ± 1.6 × 109/L vs. 10.6 ± 4.8 × 109/L; p < 0.0001); for three of them (14%), however, MR still witnessed the lingering of APN foci. It is not clear why some foci may persist despite laboratory resolution: a viable hypothesis is the lingering of edema and fibrosis that reduces the interstitial spaces hampering molecular diffusion.
It is also intriguing that the percentage amount of decrease in WBC from the acute stage to recovery approximately mirrors the corresponding increase in ADC values observed over the same period (−52% vs. +44%). The great variability in the WBC values does not, however, allow determining a sound correlation between ADC and WBC values.
This study has some limitations. First, it is a retrospective review of a limited number of examinations, even if taken from a basin of 340 possible interested individuals with transplanted kidneys; luckily for them, only a small percentage (6%) was stricken by APN. Furthermore, even if the sample is small, the topic has not yet been faced and debated in the literature. Second, the lack of the contrast media and the dynamic study did not allow us to investigate the possible presence of complicated APN foci.
In conclusion, we showed that DW-MRI represents the much needed reference approach for studying APN in patients with transplanted kidneys. In particular, the ADC value is a reliable marker, useful not only during the acute stage to evidence the existence of APN foci against healthy parenchyma, but also during the first few months of follow-up to monitor the path to healing induced by the appropriate medical therapy. DW-MRI, in combination with clinical findings, seems thus to be a feasible, safe, and rapid method to demonstrate and to monitor APN, especially when the administration of contrast media is contraindicated.
References
Säemann M, Hörl WH (2008) Urinary tract infection in renal transplant recipients. Eur J Clin Invest 38:58–65
De Souza RM, Olsburgh J (2008) Urinary tract infection in the renal transplant patient. Nat Clin Pract Nephrol 4:252–264
Castañeda DA, León K, Martín R, et al. (2013) Urinary tract infection and kidney transplantation: a review of diagnosis, causes, and current clinical approach. Transplant Proc 45:1590–1592
Nicolle LE (2014) Urinary tract infections in special populations: diabetes, renal transplant, HIV infection, and spinal cord injury. Infect Dis Clin North Am 28:91–104
Green H, Rahamimov R, Gafter U, Leibovitci L, Paul M (2011) Antibiotic prophylaxis for urinary tract infections in renal transplant recipients: a systematic review and meta-analysis. Transpl Infect Dis 13:441–447
Das CJ, Ahmad Z, Sharma S, Gupta AK (2014) Multimodality imaging of renal inflammatory lesions. World J Radiol 6:865–873
Bellin M-F, Stacul F, Webb JAW, et al. (2011) Late adverse reactions to intravascular iodine based contrast media: an update. Eur Radiol 21:2305–2310
Thomsen HS (2006) European society of urogenital radiology (ESUR) guidelines on the safe use of iodinated contrast media. Eur J Radiol 60:307–313
McDonald JS, McDonald RJ, Comin J, et al. (2013) Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology 267:119–128
Kim B, Lim HK, Choi MH, et al. (2001) Detection of parenchymal abnormalities in acute pyelonephritis by pulse inversion harmonic imaging with or without microbubble ultrasonographic contrast agent: correlation with computed tomography. J Ultrasound Med 20:5–14
Granata A, Andrulli S, Fiorini F, et al. (2011) Diagnosis of acute pyelonephritis by contrast-enhanced ultrasonography in kidney transplant patients. Nephrol Dial Transplant 26:715–720
Thomsen HS, Morcos SK, Almén T, et al. (2013) Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR contrast medium safety committee guidelines. Eur Radiol 23:307–318
Stacul F, van der Molen AJ, Reimer P, et al. (2011) Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol 21:2527–2541
Faletti R, Cassinis MC, Fonio P, et al. (2013) Diffusion-weighted imaging and apparent diffusion coefficient values versus contrast-enhanced MR imaging in the identification and characterisation of acute pyelonephritis. Eur Radiol 23:3501–3508
De Pascale A, Piccoli GB, Priola SM, et al. (2013) Diffusion-weighted magnetic resonance imaging: new perspectives in the diagnostic pathway of non-complicated acute pyelonephritis. Eur Radiol 23:3077–3086
Rathod SB, Kumbhar SS, Nanivadekar A, Aman K (2015) Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 56:244–249
Vivier P-H, Sallem A, Beurdeley M, et al. (2014) MRI and suspected acute pyelonephritis in children: comparison of diffusion-weighted imaging with gadolinium-enhanced T1-weighted imaging. Eur Radiol 24:19–25
Aoyagi J, Odaka J, Kuroiwa Y, et al. (2014) Utility of non-enhanced magnetic resonance imaging to detect acute pyelonephritis. Pediatr Int 56:e4–e6
Stunell H, Buckley O, Feeney J, et al. (2007) Imaging of acute pyelonephritis in the adult. Eur Radiol 17:1820–1828
Piccoli G, Colla L, Maass J, et al. (2005) Acute pyelonephritis: a new approach to an old clinical entity. J Nephrol 18:474–496
Thoeny HC, De Keyzer F (2011) Diffusion-weighted MR imaging of native and transplanted kidneys. Radiology 259:25–38
Park SY, Kim CK, Park BK, et al. (2014) Assessment of early renal allograft dysfunction with blood oxygenation level-dependent MRI and diffusion-weighted imaging. Eur J Radiol 83:2114–2121
Eisenberger U, Binser T, Thoeny HC, et al. (2014) Living renal allograft transplantation: diffusion-weighted MR imaging in longitudinal follow-up of the donated and the remaining kidney. Radiology 270:800–808
Wypych-Klunder K, Adamowicz A, Lemanowicz A, et al. (2014) Diffusion-weighted MR imaging of transplanted kidneys: preliminary report. Pol J Radiol 79:94–98
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Faletti, R., Cassinis, M.C., Gatti, M. et al. Acute pyelonephritis in transplanted kidneys: can diffusion-weighted magnetic resonance imaging be useful for diagnosis and follow-up?. Abdom Radiol 41, 531–537 (2016). https://doi.org/10.1007/s00261-015-0618-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-015-0618-2