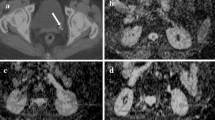
Abstract
Aims
This study aims to evaluate the (a) accuracy of conventional and diffusion-weighted-imaging (DWI) sequences in the diagnosis of acute pyelonephritis and (b) minimum apparent diffusion coefficient (ADC) values for the diagnosis of acute pyelonephritis and the differentiation of renal abscesses from acute pyelonephritis.
Materials and methods
Ultrasound, conventional MRI sequences, and DWI were used to evaluate the kidneys in 68 patients suspected to have acute pyelonephritis. Multiple similar regions of interest (ROIs) were placed over the renal parenchyma with visually identifiable diffusion restriction, over the non-diffusion-restricted renal parenchyma of affected kidneys and over the normal kidneys. Corresponding minimum ADCs were noted for analysis. Pyelonephritis was confirmed based on clinical criteria, laboratory findings, and by resolution/development of known complications of pyelonephritis.
Result
DWI showed the highest sensitivity(100%), while DWI read with T2-weighted imaging (both being positive) showed the highest specificity(100%) for the diagnosis of acute pyelonephritis in our population with a high baseline creatinine. The minimum-ADC of the nephritic diffusion-restricted area in patients with confirmed pyelonephritis was significantly lower than the minimum-ADC in patients without pyelonephritis [(0.934 ± 0.220, mean ± SD) vs (1.804 ± 0.404) × 10−3 s/mm2] (p < 0.001). ROC cut-off of minimum-ADC for the diagnosis of acute pyelonephritis was 1.202 × 10−3 s/mm2 (area under curve 0.978). The minimum-ADC of the abscesses were significantly lower when compared to the minimum-ADC of the nephritic diffusion-restricted portion of the same kidney [(0.633 ± 0.248) vs (0.850 ± 0.191) × 10−3 s/mm2] (p < 0.001).
Conclusion
DWI is an excellent stand-alone imaging tool that can be combined with conventional sequences for the diagnosis of APN even in patients with high serum-creatinine or other contraindications to intravenous contrast. Further, ADC values can be used to differentiate between renal abscesses and uncomplicated pyelonephritis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pyelonephritis, a renal infection of the parenchyma and pelvicalyceal system, has an annual worldwide incidence of about 10.5–26 million cases with a mortality rate of 7.3 to 16.5 deaths per 1000 patients [1,2,3]. Higher mortality rates are seen in diabetics, pregnant patients, and the immune-suppressed [1]. Imaging demonstrates the extent of the disease and identifies complications like papillary necrosis, abscess formation, and ureteric obstruction [4, 5]. Early diagnosis of this condition is important as it permits institution of early treatment and prevents complications.
Urine analysis and culture is the basis for diagnosing urinary tract infections (UTI) including acute pyelonephritis (APN). Biochemical tests have a sensitivity between 75 and 84% and a specificity between 82 and 98% for the diagnosis of UTI [3]. Clinical diagnosis of APN is suspected by signs of systemic inflammation like fever, chills, or malaise, with urinary frequency, urgency or dysuria, plus localizing signs of flank pain and tenderness. Urine culture is the cardinal confirmatory diagnostic test [1]. Uncertainties exist regarding the most cost-effective imaging strategies and appropriate treatment for patients with clinical pyelonephritis but non-confirmatory urine cultures [1]. Renal dysfunction may act as a confounding factor in the diagnosis of APN by reducing apparent-diffusion-coefficient (ADC) values [6]. This is postulated to be due to reduced perfusion, as well as due to reduced water diffusion due to underlying conditions like glomerulo-sclerosis, tubular atrophy, and interstitial fibrosis. Generally, ADC values in renal dysfunction are lower than 2.0354 (× 10−3 mm2/s) [6].
The authors conducted this study in a population with renal dysfunction as seen by the high mean creatinine among the study subjects. In our study, the authors included patients referred for imaging with a strong clinical suspicion of pyelonephritis who could not receive intravenous contrast. Previous studies were not conducted in study populations with a high serum creatinine. This study aimed to prove that diffusion-weighted imaging (DWI) has a high sensitivity and specificity for the diagnosis of APN, even in a population with renal dysfunction. However, the authors do express expected limitations of absolute ADC values given the variability between scanners and technique.
Aims and objectives
-
a)
To evaluate the accuracy of conventional and diffusion-weighted sequences in the diagnosis of APN,
-
b)
To evaluate the minimum-ADC values in the diagnosis of APN and in the differentiation of renal abscesses from APN.
Materials and methods
This prospective study was conducted on patients referred for MRI with a strong clinical suspicion of APN at a medical college hospital, after obtaining institutional ethics committee approval. Patients who did not undergo contrast enhanced CT imaging because of renal dysfunction or due to concerns about radiation were included. Exclusion criteria included patients with (a) prior surgical procedures on the kidney, (b) ureteric calculi on screening ultrasound, (c) congenital abnormalities of the kidney, (d) administered antibiotics before urine culture and (e) Emphysematous pyelonephritis.
Screening ultrasound was done using a convex probe of 2–5 MHz frequency on a GE Voluson 730 machine. Features like bulky kidney, perinephric free fluid, and renal parenchymal hypoechogenicity/hyper-echogenicity were considered to arrive at the diagnosis of pyelonephritis. After an initial ultrasound, axial and coronal conventional sequences and DWI were acquired on a 1.5 T MRI scanner (Signa HDxt® 1.5 T MRI scanner, GE Medical Systems). T2-FSE (TR-2330 ms, TE-87.86 ms, slice-thickness 4 mm, ET-1, matrix size-192 × 288, 320 × 224), T1-FSE (TR-7.62 ms, TE-4.20 ms, slice-thickness 4 mm, ET-1, matrix-size 192 × 288, 320 × 224), and FIESTA FATSAT images (TR-3.60 ms, TE-1.55 ms, slice-thickness 8 mm, ET-1, matrix-size 192 × 288, 320 × 224) were acquired in an axial and coronal plane. DWI (TR-6250 ms, TE-93.50 ms, slice-thickness 8 mm, ET-1, matrix-size 128 × 128) was acquired as a non-breath hold, single-shot-echo-planar-sequence with b = 0.800 s/mm2.
A radiologist with 4 years’ experience read the images in consensus with a radiologist with 13 years’ experience. In case of a discrepancy, the opinion of a radiologist with 25 years of experience was considered. The T2-weighted and DWI images were initially read independently of each other in that order (being blinded to the other sequence) and later together. The reader was blinded to the clinical details at the time of all the reads. The independent reading of DWI was for the evaluation of the fast DWI sequence solely as a screening tool — an approach to imaging that may be of use in a high volume emergency setting. Patients with T2 hyper-intensity of the renal parenchyma with/without features like perinephric free fluid were said to have pyelonephritis on T2-weighted imaging. Patients who showed renal parenchymal hyper-intensity on DWI with corresponding hypo-intensity on ADC images (true diffusion restriction) were said to have pyelonephritis on DWI. Analysis of the DWI sequence by definition includes the review of ADC images.
Minimum ADC was chosen as the parameter for analysis — this was chosen as a very sensitive parameter to detect the area of highest diffusion restriction. Fifteen ROIs (regions of interest) were placed over the region of diffusion restriction in the nephritic kidney. In the evaluation of minimum-ADC for APN alone, the ROIs were placed well away renal abscess during the evaluation. As far as possible, the ROIs were placed at the approximate junction of the renal cortex and medulla, always avoiding the edge of the kidney or the hilum. This was recorded as the minimum-ADC of the nephritic kidney.
Fifteen ROIs were placed over the normal kidney, 5 at each pole, and the minimum ADC thus obtained was recorded as the minimum-renal parenchymal ADC of the normal kidney.
In those patients with renal abscesses, the ADC of the renal abscess was derived by placing 15 ROIs over the region corresponding to the collection on conventional T2 FSE sequences. The edges of the abscess cavity were avoided during this evaluation. The minimum ADC thus obtained was recorded as the minimum-ADC of the abscess cavity.
Pyelonephritis was confirmed based on clinical criteria, laboratory findings, and by resolution/development of known complications of pyelonephritis as detailed below.
The diagnosis of APN was said to be confirmed if.
-
a)
Urine pus cells or cultures were positive, with a positive nitrite or leucocyte esterase test with presence of clinical symptoms of fever, costovertebral-angle pain, nausea, and vomiting with systemic features like an elevated CRP or an elevated WBC count
and
-
b)
If there was resolution of symptoms and imaging findings after antibiotic treatment or if sequelae of pyelonephritis were present on follow up imaging.
Contrast-enhanced studies were not used as a gold standard confirmatory test in our population with a high mean-creatinine because of the high risk of nephrogenic systemic fibrosis or contrast induced nephropathy. In our institution, for patients with eGFR < 30, type 2 contrast agents would be administered only if it potentially changed management and was unavoidable — with a high benefit to risk ratio.
With the above criteria in mind, 68 patients were included in this study with 2 patients excluded because of loss to follow up. Sixty-six patients (132 kidneys) were analyzed of which there were 44 males and 22 females. No patient had unilateral kidneys. Thirty-one patients had unilateral, and 11 had bilateral pyelonephritis (53 kidneys). Twenty-two patients were confirmed to have no pyelonephritis.
Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were calculated for ultrasound imaging, T2-weighted imaging alone, DWI-imaging alone, and for DWI read with T2-weighted images. Pearson’s chi-square test was applied to assess the significance of DWI, T2 imaging, and DWI + T2 imaging in the diagnosis of APN. Furthermore, the sensitivity and specificity of variables like perinephric free-fluid and bulky kidney on MRI were assessed for the diagnosis of APN.
The minimum-ADC of the parts of the kidney affected by uncomplicated pyelonephritis, the minimum-ADC in patients with renal abscesses, and the minimum-renal parenchymal ADC of normal kidneys were compared. Two-tailed t-test was applied to assess significance of minimum-ADC value in the diagnosis of APN. Graph of sensitivity vs 1-specificity was plotted to determine the ROC cut-off for the diagnosis of APN. Significance was assessed by using a 2-tailed t-test.
Results
The mean age of the study subjects was 49.8 years (SD 13.5 years). Mean serum creatinine among those with pyelonephritis was 3.709 mg/dL (SD 2.880 mg/dL) and those without pyelonephritis was 4.165 mg/dL (SD3.364 mg/dL). There was no significant difference in the mean of the serum creatinine levels between the two groups (t-test).
The most common microorganisms causing pyelonephritis were E coli, Candida, and Pseudomonas with relative frequencies as shown in Table 1. Three patients had polymicrobial infection which involved E. coli with one of the other organisms.
Of the 42 patients with APN, 25 (60%) had diabetes. Six of these diabetic patients had bilateral pyelonephritis, and no other underlying cause was detected for the renal infection in these patients. Of the remaining 24 patients who did not have pyelonephritis, 11 patients (46%) had diabetes. APN in 21 patients was attributed to diabetes (including 6 patients with bilateral pyelonephritis), 5 patients had a ureteric stricture, 3 patients had PUJ obstruction, and 2 patients were immunosuppressed due to HIV infection.
Twelve patients presented with renal abscesses, and 1 patient had 3 renal abscesses. In the patient with 3 renal abscesses, the abscess with the minimum ADC value was included in the analysis. Twenty-eight kidneys had features of or developed papillary necrosis despite treatment.
Visually detected bulky kidneys were found to have a much higher specificity and positive predictive value for the diagnosis of APN when compared to perinephric free fluid. However, both the variables had a low sensitivity and negative predictive value (Table 2). Table 3 shows the significance of different MRI sequences in differentiating APN from normal kidneys.
Among the MRI sequences, the highest sensitivity and NPV were seen with DWI alone or when DWI was read with T2-weighted images (hyper-intensity present on T2 weighted images or DWI). The addition of T2-weighted images to DWI resulted in a slight decrease in the specificity and PPV of the test. However, when both T2-weighted and DWI had to show hyper-intensity (true restriction) for the diagnosis of APN, the specificity and PPV rose to 100% with a drastic reduction in the sensitivity and the NPV (Table 4).
In kidneys affected by APN, the mean of the minimum-ADC 0.934 × 10−3 s/mm2, SD 0.220 × 10−3 s/mm2 (n = 53), was found to be lower than kidneys without APN 1.801 × 10−3 s/mm2, SD 0.404 × 10−3 s/mm2 (n = 79) (p < 0.001). ROC cut-off value of minimum-ADC for APN was 1.202 × 10−3 s/mm2 with a sensitivity of 95% and a specificity of 88.7% (Fig. 1; AUC of 0.978).
In kidneys with a renal abscess, the mean of the minimum-ADC from the abscess was 0.633 × 10−3 s/mm2, SD 0.248 × 10−3 s/mm2 (n = 12) which was significantly lower when compared to the non-abscess nephritic parts of the same kidney 0.850 × 10−3 s/mm2, SD 0.191 × 10−3 s/mm2. The mean of the minimum ADC was significantly lower in kidneys with renal abscesses when compared to (a) kidneys with only APN (p < 0.001) or (b) normal kidneys (p < 0.001).
Discussion
The role of imaging in APN is to demonstrate nature and extent of disease and to reveal complications like abscesses, pyonephrosis, and obstruction [4, 5]. MRI demonstrates complications like (a) obstruction, which may be secondary to papillary necrosis, (Fig. 2) (b) abscess formation, (Fig. 3) and (c) pyonephrosis [4, 7, 8]. Late complications like stricture formation and chronic pyelonephritis can also be diagnosed [9]
a–d A 41-year-old diabetic, with known chronic kidney disease presented with fever, chills, burning micturition, and flank pain. This patient had previously successfully undergone a pyeloplasty for PUJ obstruction. Coronal T2-w images of both the kidneys do not show abnormal T2 hyper-intensity. DWI shows diffusion restriction of the mid-pole of the left kidney and adjacent spleen (curved arrow). These features were suggestive of an acute pyelonephritis due to extension from a splenic abscess. Axial DWI shows diffusion restriction of the calyces of the right kidney representing “the ischemic phase” of papillary necrosis (arrow-head). d Follow-up MR urogram for a recurrence of flank pain after 4 months showed bilateral hydronephrosis and calyceal filling defects indicating frank papillary necrosis
a–c An 80-year-old non-diabetic female patient presenting with features of right flank pain and fever. T2 hyper-intensity of the mid and lower pole of the right kidney (small arrows) represents renal parenchymal edema. Figure 3 b shows an axial T2-w image of the right kidney with heterogeneous T2 hyper-intensity and a small perinephric fluid collection (long arrow). Figure 3 c shows diffusion restriction of the kidney and the perinephric collection (curved arrows) indicating pyelonephritis with a perinephric abscess
To the best of our knowledge, this is the first study where plain MRI without CECT or CEMR was used for the diagnosis of APN in a population of patients with high serum creatinine. In previous studies, either serum creatinine has not been significantly elevated or has been undocumented at the time of presentation [10,11,12]. In our study, the mean creatinine of patients was high in both the pyelonephritic (4.06 ± 0.325 mg/dL) and non-pyelonephritic group (3.735 ± 0.446 mg/dL) with no significant statistical difference. This was likely since the patients referred for MRI per our inclusion criteria could not undergo a contrast study because of a high baseline creatinine. Renal dysfunction evidenced by increased creatinine was also found to independently decrease the ADC value, thus acting as a confounding factor [6, 11, 13,14,15,16,17]. In a study by Yalçin-Şafak et al., the authors found that ADC values of less than 1.151 × 10−3 s/mm2 correlated strongly with chronic kidney disease of stage 3 and above [13]. The minimum ADC value of 0.934 × 10−3 s/mm2, SD 0.220 × 10−3 s/mm2 seen in pyelonephritis is 1 standard deviation below the mean ADC cut-off value of 1.151 × 10−3 s/mm2 seen in chronic kidney disease without acute pyelonephritis reported by Yalçin-Şafak et al. Though we do not have minimum-ADC values in renal dysfunction reported in literature for comparison, the authors believe that there could be significant overlap in the minimum ADC range of renal dysfunction and pyelonephritis. The caveat is that the ADC values are dependent on the scanner and show significant variability. Further, in a study on comparison of DWI with split-glomerular filtration rate, Xu et al. showed that impaired kidneys show lower ADC [14]. This was supported by a study on hydronephrotic kidneys by Toyoshima et al. [16]. Thoeny et al. in a case–control study showed that diffusion restriction is seen both in APN and in dysfunctional kidneys [15]. Our study, being conducted in a population with a high mean creatinine, can be used to validate the use of MRI with DWI, thus avoiding intravenous contrast for diagnosis of APN in patients with renal dysfunction.
The pathological definition of APN emphasizes the reliance on imaging or biopsy to demonstrate the presence of nephritis. Alternately, the clinical case definition uses clinical features, laboratory, and microbiological data [10]. The pathological gold standard is to demonstrate poorly enhancing areas of the renal parenchyma. However, the inability to use intravenous contrast resulted in the reliance on the clinical definition of pyelonephritis [18].
Ultrasound showed a sensitivity of about 45%, specificity of 91%, and a PPV of 77% for the diagnosis of APN in our study. A study by Nickavar et al. showed a lower sensitivity and specificity of 34% and 53%, respectively [19]. Previous studies have shown that ultrasound demonstrated abnormalities only in 20–24% of patients with pyelonephritis [20, 21]. The higher sensitivity and specificity was probably because, in our study, ultrasound was performed by a doctor who was not blinded to the clinical findings and the lab reports, which enabled a better diagnostic yield. Among the findings of perinephric free-fluid, bulky kidney and focally altered echogenicity, at least one finding was necessary to diagnose APN on ultrasound. The high index of suspicion in our screened patients may have contributed to the higher sensitivity and specificity in our study.
The authors found that DWI and T2-weighted imaging read independently or together were significant in differentiating kidneys with and without pyelonephritis (Fig. 4). This significance was supported by multiple studies [11, 12, 22]. The sensitivity, specificity, PPV, and NPV of T2-weighted imaging and DWI individually were found to be superior than ultrasound for the diagnosis of APN. The high sensitivity and NPV (both 100%) of MRI was supported by a study conducted by Henninger et al., where he used the clinical definition to define APN. Here, he showed that all patients affected by APN showed signal changes on DWI [22]. A study by Rathod SB showed that the sensitivity of DWI to detect pyelonephritis was 95.3% [23]. De Pascale et al. reported that DWI achieved a sensitivity of 95.2%, specificity of 94.9%, a PPV of 96.9%, and a negative predictive value of 92.3% [12]. Our study showed a marginally higher specificity and a similar PPV which could be due to technical advancements since the previous studies. Vivier et al. showed that the sensitivity and specificity of DWI were 100% and 93.5% [11]. This supports the higher sensitivity of DWI derived in our study. The slight difference in sensitivity, specificity, PPV, and NPV can also be attributed to (a) the patient sample with high mean creatinine, (b) smaller sample size, and (c) the use of the clinical case definition of APN instead of using contrast enhanced MRI. T2-weighted imaging had a lower sensitivity and NPV of 69.8% and 82.4%, respectively, when compared to DWI. Henninger et al. reported that only 14% of patients having APN showed obvious T2-weighted signal changes, while 81% of patients showed subtle increase in parenchymal signal [22]. This high percentage of subtle signal changes is probably responsible for the poorer sensitivity and NPV of T2-weighted imaging (Fig. 5). However, in our study, if both T2-weighted imaging and DWI were required to be positive for the diagnosis of APN, then the specificity and PPV rose to 100% at significant cost to the sensitivity and NPV.
a–c A 47-year-old male patient presenting with back pain, fever, and chills with a urine analysis showing numerous gram-negative bacilli. Coronal T2-w images of both kidneys show a subtle T2 hyper-intensity of the cortex (small arrows) and a well-defined perinephric collection (large arrow). DWI shows diffusion restriction of the renal parenchyma of both kidneys and the right-sided perinephric collection (curved arrows). The blood culture grew E. coli. A final diagnosis of bilateral pyelonephritis with a right perinephric abscess was made
A 62-year-old male patient with left-sided flank pain and acute on chronic kidney disease with urine culture showing growth of E. coli. a, b Coronal and axial T2-weighted images with left sided hydronephrosis and debris within the pelvicalyceal system (small arrow). There is no parenchymal T2 hyperintensity. Coronal diffusion–weighted image in c shows intense diffusion restriction (curved arrows). Follow-up imaging of this patient after 6 months with axial DWI (shown in Fig. 5d) and coronal T2-weighted images (not shown) shows significantly reduced diffusion restriction/significant response to antibiotics and interval resolution of hydronephrosis. Urine culture showed no growth on follow-up
DWI and contrast-enhanced MRI show high diagnostic agreement in multiple studies [11, 12, 22]. In our study, we used the “minimum-ADC” value among the 15 ROIs placed instead of using the “mean of the ADC” values. The minimum ADC of the diffusion restricted area in patients with confirmed pyelonephritis (mean value, 0.934 × 10−3 s/mm2, SD0.220 × 10−3 s/mm2) was significantly lower than the minimum ADC in patients without pyelonephritis (mean value (1.804 × 10−3 s/mm2, SD0.404 × 10−3 s/mm2) (p < 0.001). These “minimum-ADC” values are similar and consistently lower than the “mean ADC” values reported in the study by Rathod SB et al., where the authors reported the mean ADC of the renal cortical parenchyma in kidneys with pyelonephritis, with renal abscesses, and without pyelonephritis as (1.30 ± 0.13) × 10−3 s/mm2, (0.77 ± 0.08) × 10−3 s/mm2, and (2.10 ± 0.4) × 10−3 s/mm2, respectively (23). Further, the minimum-ADC of the abscesses in our study was significantly lower when compared to the minimum-ADC of the diffusion-restricted “nephritic” portion of the same kidney. This can be explained by the higher levels of restriction or immobility of water molecules occurring within an abscess cavity. Twelve kidneys (22.6%) out of 53 had renal abscesses, with one kidney having 3 renal abscesses. A study by Rollino et al. reported that 23.5% of their patients had renal abscesses, a prevalence rate similar to our study [24. ].
ROC cut-off of minimum-ADC for the diagnosis of APN was determined to be 1.202 × 10−3 s/mm2 (area under curve 0.978). In a study by Faletti et al., the ROC cut-off of the mean-ADC for the diagnosis of APN was 2 × 10−3 s/mm2 with area under the curve of 0.94 [25]. This ADC cut-off was higher than the minimum-ADC cut-off value of our study, which is expected, as mean values are larger than minimum values.
The non-contrast MRI sequences commonly used for the diagnosis of APN are T2-weighted sequences and DWI [11, 22]. T2-weighted images detect the renal parenchymal edema caused due to infection, while DWI uses the restricted free motion of water molecules within a given tissue to generate an image [17]. Vivier et al. showed that DWI was comparable to gadolinium enhanced MR imaging in the diagnosis of pyelonephritis and that along with T2-weighted imaging, it formed a comprehensive tool in the evaluation of pyelonephritis [11]. These findings are quantitatively proved by our study where we used T2-weighted sequences in concert with DWI to reliably diagnose APN without intravenous contrast (Fig. 6).
A 59-year-old male diabetic patient, who had chronic kidney disease secondary to bilateral ureteric strictures presented with bilateral flank pain and fever. Coronal T2-weighted MR images show T2 hyperintensity at the lower pole of both kidneys (short arrows) with bilateral hydronephrosis. Diffusion restriction of the renal parenchyma on both sides (curved arrows) is seen. Coronal 3D MRCP sequence showing filling defects (broken arrows) within the renal parenchyma representing papillary necrosis. MIP of a 3D MRCP image shows bilateral ureteric strictures (long arrow) causing hydroureteronephrosis — this hydronephrosis was treated with ureteral stenting. Follow-up images after 8 months show a shrunken right kidney and a left kidney with perinephric fat stranding. Bilateral ureteric stents are noted in situ.
MR imaging findings are similar to CT and include demonstration of renal edema, hemorrhage, renal enlargement, abscesses, and perinephric fluid collections. Inflammatory lesions and fluid collections follow the intensity of fluid [4]. MRI, by virtue of using urine itself as intrinsic contrast to image the urinary tract avoids the use of exogenous contrast for this purpose, with no administered radiation dose. Thus, it is a safer modality when compared to CT urogram where some renal function must be preserved in order to permit excretion of contrast [26,27,28,29]. MRI can also be safely used in patients with reduced renal function, in pediatric patients, women of reproductive age group, and in pregnant patients. To establish infection of the renal parenchyma, T2-weighted imaging alone was not sufficiently sensitive. The use of DWI in concert with T2-weighted imaging enables the diagnosis of APN with a high sensitivity and specificity without administration of intravenous contrast in a patient sample with renal dysfunction. This indicates the robustness of non-contrast MRI. The authors feel that this modality has been under-utilized, probably because of poor resolution in the early years of MRI, the time taken to schedule an MRI, and the scan time itself.
The ACR appropriateness criteria 2022 update gives various scenarios where CT with contrast, CT without contrast, MRI with contrast, and MRI without contrast may be appropriate. However, they do not consider the subgroup of patients with renal dysfunction under the scenarios provided [30]. The authors believe that the subset of patients with renal dysfunction must be considered under pyelonephritis, complicated patient (Variant 2 in the ACR appropriateness criteria). In the ER setting, a screening MRI of the renal system could be used with the following sequences: T2-FSE in an axial and coronal plane (TR-2330 ms, TE-87.86 ms, slice-thickness 4 mm, ET-1, matrix size-192 × 288, 320 × 224), DWI axial, and coronal plane (TR-6250 ms, TE-93.50 ms, slice-thickness 5–8 mm, ET-1, matrix-size 128 × 128, b = 0.800 s/mm2) with an added MR urogram thick slab sequences + 3-D MRCP sequence of the renal system if ultrasound or MRI shows evidence of hydronephrosis. *3D magnetic resonance cholangiopancreatography (MRCP) RTr ASSET (TR 3750 ms, TE 383 ms, slice thickness 1.6 mm, ET 64, matrix size 256 × 256), thick slab MR urogram sequence (TR 2566 ms, TE 1202 ms, slice thickness 60 mm, ET 1, matrix size 384 × 256)). The only caution for the use of MRI in suspected APN in the ER setting is for the diagnosis of an unsuspected or a small septic renal infarct. The differentiation of these renal infarcts from APN is especially hard without iv contrast. With contrast administration, wedge shaped hypo-perfusion or a cortical rim sign (cortical hyper-enhancement) makes the diagnosis of a septic renal infarct or an unsuspected incidental renal infarct more likely. Low signal on T2 is the only feature on non-contrast MRI that favors the diagnosis of a renal infarct over pyelonephritis — both show diffusion restriction! [31].
However, this study has a few limitations. We chose to manually apply ROIs to mimic how radiologists determine ADC values of lesions during reads. Also, there is a variability in ADC values between scanners and technique; each institution may have to tailor their ADC value cut-off as per their MRI vendor and scanning protocol. Also, the typical Gold Standard of contrast enhanced MRI could not be used given the study population; we had to use an alternate gold standard combining clinical features, laboratory values, and follow-up imaging.
In conclusion, DWI is an excellent stand-alone imaging tool that can be combined with conventional sequences for the diagnosis of APN even in patients with high serum-creatinine or other contraindications to intravenous contrast. Furthermore, ADC values can be used to differentiate between renal abscesses and uncomplicated pyelonephritis. APN can be diagnosed on MRI without the administration of intravenous contrast.
Data Availability
The dataset used for this study is publicly available [32].
References
Johnson JR, Russo TA (2018) Acute pyelonephritis in adults. N Engl J Med 378:48–59. https://doi.org/10.1056/NEJMcp1702758
Czaja CA, Scholes D, Hooton TM and Stamm WE (2007) Population-based epidemiologic analysis of acute pyelonephritis. Clin Infect Dis 45(3):273–280. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17599303
Ramakrishnan K, Scheid DC (2005) Diagnosis and management of acute pyelonephritis in adults. Am Fam Physician 71:933–942
Craig WD, Wagner BJ, Travis MD (2008) Pyelonephritis: radiologic-pathologic review. Radiographics 28:255–276
Chiew YF (1996) Candidal renal papillary necrosis: report of a case and review. Singapore Med J 37:119–121
Goyal A, Sharma R, Bhalla AS, Gamanagatti S, Seth A (2012) Diffusion-weighted MRI in assessment of renal dysfunction. Indian J Radiol Imaging 22:155–159
Chan JHM, Tsui EYK, Luk SH, Fung SL, Cheung YK, Chan MSM et al (2001) MR diffusion-weighted imaging of kidney: differentiation between hydronephrosis and pyonephrosis. Clin Imaging 25:110–113
Verswijvel G, Vandecaveye V, Gelin G, Vandevenne J, Grieten M, Horvath M et al (2002) Diffusion-weighted MR imaging in the evaluation of renal infection: preliminary results. JBR-BTR 85:100–103
Zielonko J, Studniarek M, Markuszewski M (2003) MR urography of obstructive uropathy: diagnostic value of the method in selected clinical groups. Eur Radiol 13:802–809
Piccoli G, Consiglio V, Deagostini M, Serra M, Biolcati M, Ragni F et al (2011) The clinical and imaging presentation of acute “non complicated” pyelonephritis: a new profile for an ancient disease. BMC nephrology 12:68
Vivier PH, Sallem A, Beurdeley M, Lim RP, Leroux J, Caudron J et al (2014) MRI and suspected acute pyelonephritis in children: comparison of diffusion-weighted imaging with gadolinium-enhanced T1-weighted imaging. Eur Radiol 24:19–25
De Pascale A, Piccoli GB, Priola SM, Rognone D, Consiglio V, Garetto I et al (2013) Diffusion-weighted magnetic resonance imaging: new perspectives in the diagnostic pathway of non-complicated acute pyelonephritis. Eur Radiol 23:3077–3086
Yalçin-Şafak K, Ayyildiz M, Ünel SY, Umarusman-Tanju N, Akça A, Baysal T (2016) The relationship of ADC values of renal parenchyma with CKD stage and serum creatinine levels. Eur J Radiol Open 3:8–11
Xu Y, Wang X, Jiang X (2007) Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging 26:678–681
Thoeny HC, De Keyzer F, Oyen RH, Peeters RR (2005) Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 235:911–917
Toyoshima S, Noguchi K, Seto H, Shimizu M, Watanabe N (2000) Functional evaluation of hydronephrosis by diffusion-weighted MR imaging. Relationship between apparent diffusion coefficient and split glomerular filtration rate. Acta radiol. 41:642–646
Reimer P, Parizel PM, Meaney JFM, Stichnoth FA (2010) Clinical MR imaging (Third Edition): a practical approach. Springer, Heidelberg, pp 1–23
Piccoli GB, Consiglio V, Colla L, Mesiano P, Magnano A, Burdese M et al (2006) Antibiotic treatment for acute “uncomplicated” or “primary” pyelonephritis: a systematic, “semantic revision.” Int J Antimicrob Agents 28:49–63
Nickavar A, Safaeian B (2015) Radiologic and clinical evaluation of children with first febrile urinary tract infection. Int J Pediatr Adolesc Med 2:24–28
Vourganti S, Agarwal PK, Bodner DR, Dogra VS (2006) Ultrasonographic evaluation of renal infections. Radiol Clin 44:763–775
June CH, Browning MD, Smith LP, Wenzel DJ, Pyatt RS, Checchio LM et al (1985) Ultrasonography and computed tomography in severe urinary tract infection. Arch Intern Med 145:841–845
Henninger B, Reichert M, Haneder S, Schoenberg SO, Michaely HJ (2013) Value of diffusion-weighted MR imaging for the detection of nephritis. Sci World J 2013:348105. https://doi.org/10.1155/2013/348105
Rathod SB, Kumbhar SS, Nanivadekar A, Aman K (2015) Role of diffusion-weighted MRI in acute pyelonephritis: a prospective study. Acta Radiol 56:244–249
Rollino C, Beltrame G, Ferro M, Quattrocchio G, Sandrone M, Quarello F (2012) Acute pyelonephritis in adults: a case series of 223 patients. Nephrol Dial Transplant 27:3488–3493
Faletti R, Cassinis MC, Fonio P, Grasso A, Battisti G, Bergamasco L et al (2013) Diffusion-weighted imaging and apparent diffusion coefficient values versus contrast-enhanced MR imaging in the identification and characterisation of acute pyelonephritis. Eur Radiol 23:3501–3508
Blandino A, Gaeta M, Minutoli F, Salamone I, Magno C, Scribano E (2002) MR urography of the ureter. Am J Roentgenol 179:1307–1314
Memarsadeghi M, Riccabona M, Heinz-Peer G (2005) MR urography: principles, examination techniques, indications. Radiologe 45:915–923
Leyendecker JR, Barnes CE, Zagoria RJ (2008) MR urography: techniques and clinical applications. Radiographics 28:23–46
Garcia-Valtuille R, Garcia-Valtuille AI, Abascal F, Cerezal L, Arguello MC (2006) Magnetic resonance urography: a pictorial overview. Br J Radiol 79:614–626
Smith AD, Nikolaidis P, Khatri G, Chong ST, De Leon AD, Ganeshan D, Gore JL, Gupta RT, Kwun R, Lyshchik A, Nicola R (2022) ACR Appropriateness Criteria® acute pyelonephritis: 2022 update. J Am Coll Radiol 19(11):S224–S239
Huynh AD, Sweet DE, Feldman MK, Remer EM (2022) Imaging of renal emergencies: review of infectious, hemorrhagic, vascular, and traumatic etiologies. Br J Radiol 95(1137):20211151
Pinto D (2023) Pyelonephritis data upload - repository.pdf. figshare. Dataset. https://doi.org/10.6084/m9.figshare.22201006.v1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pinto, D.S., George, A., Johny, J. et al. Role of MRI in the evaluation of acute pyelonephritis in a high-risk population with renal dysfunction: a prospective study. Emerg Radiol 30, 285–295 (2023). https://doi.org/10.1007/s10140-023-02122-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10140-023-02122-z