Abstract
Imaging is required if complication is suspected in acute pyelonephritis to assess the nature and extent of the lesions, and to detect underlying causes. The current imaging modality of choice in clinical practice is computed tomography. Because of associated radiation and potential nephrotoxicity, CEUS is an alternative that has been proven to be equally accurate in the detection of acute pyelonephritis renal lesions. The aims of this study of 48 patients are to describe in detail the CEUS findings in acute pyelonephritis, and to determine if abscess and focal pyelonephritis may be distinguished. Very characteristic morphologic and temporal patterns of enhancement are described. These allow differentiation of focal pyelonephritis from renal abscess, and detection of tiny suppurative foci within focal pyelonephritis. The detection of abscesses is important because follow-up in 25 patients revealed a longer clinical course. Typical pyelonephritis CEUS features permit distinction from other renal lesions. As a whole, CEUS is an excellent tool in the work-up of complicated acute pyelonephritis, so it may be considered as the imaging technique of choice in the evaluation and follow-up of these patients who frequently are very young, so as to minimise radiation exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Acute pyelonephritis (APN) is a common disease which affects mostly young women. Diagnosis is usually made on the basis of clinical and laboratory findings [1], and imaging is not required unless complication is suspected or in high-risk patients (immunocompromised patients, diabetics and the elderly) [2]. Complication is suspected when there is no clinical response after 72 h of intravenous antibiotic treatment or when there is obstruction of the urinary tract. Grey-scale ultrasound may show findings in complicated pyelonephritis [3], but its sensitivity is lower than that of computed tomography (CT). A 2007 study demonstrated a very similar diagnostic performance of CT and contrast-enhanced ultrasound (CEUS) in the diagnosis of acute pyelonephritis [4]. But to date, a detailed description of the imaging findings of complicated pyelonephritis in CEUS has not been published. The aims of this study are to describe the different findings of complicated acute pyelonephritis in CEUS, and to determine if CEUS is able to distinguish focal pyelonephritis from abscess.
Materials and methods
Patients and study
Carried out over a 3-year period in a single centre, this is a retrospective study which comprises 48 patients (43 women, 5 men) with a mean age of 33 years (range 14–85), who underwent CEUS for the evaluation of complicated acute pyelonephritis. Most of the patients were previously healthy, except three renal transplant recipients, a liver transplant-recipient, a patient with only one kidney secondary to nephrectomy due to calculi, a patient with double excretory system and previous surgery, and a drug abuser. Informed consent of patients was waived. The presence of APN was correlated with clinical and laboratory findings. Four of the patients had urinary sepsis. The clinical improvement after antibiotic therapy, the disappearance of the lesions, the development of scars at CEUS follow-up; or concordant contrast-enhanced CT findings (15 patients) were considered as a confirmation of the pyelonephritic nature of the lesions. 25 patients participated in CEUS follow-up. The imaging follow-up schedule was performance of basal and CEUS 1 week and 1 month after diagnosis. In ten patients with persistence of lesions, CEUS was performed 3 months later.
Ultrasound technique
The machines used were Sequoia and S 2000 (Siemens-Acuson). Convex multifrequency probes and a specific CEUS technology (Cadence Contrast Pulse Sequencing, CPS) with low mechanical indexes (0.07–0.18) were employed. A sulphur hexafluoride-filled microbubble contrast agent (SonoVue, Bracco, Milan) was used. All of the patients underwent grey-scale basal sonography and power Doppler of both kidneys. Presence or absence of renal and perirenal lesions and urinary tract dilatation or obstruction were noted. Afterwards, CEUS of the suspect kidney in all of the phases, and CEUS of the contralateral kidney in the parenchymal phase were performed. Each patient received a 2.4 mL intravenous bolus of contrast in a peripheral vein through a 20 gauge cannula, flushed with 10 mL of saline. In six cases, a second dose was needed to study the contralateral kidney. The CEUS exploration lasted until most of the bubbles had disappeared from the kidney (mean 3 min).
CEUS image analysis
Two main enhancement phases were considered: a cortical phase, in which enhancement of the cortex was seen (15–30 s after contrast injection), and a parenchymal phase, in which enhancement of both cortex and medullae was seen (25 s—4m after contrast injection). For practical reasons, the parenchymal phase was separated into early parenchymal phase (25 s—1m) and late parenchymal phase (after 1m).
The explorations were performed by two radiologists with 4 year experience in CEUS and were stored (divided in 40 s clips) in the hard disc and DVD or PACS. The complete explorations were reviewed afterwards by both of the radiologists in consensus.
None of the studied lesions met the criteria of a simple cyst on baseline US. The CEUS diagnostic criteria for abscess were defined as the presence of areas of non-enhancement throughout the whole exploration, with or without an enhancing rim or septa. The CEUS imaging criteria to diagnose focal pyelonephritis were the presence of a cortical or corticomedullary focal wedge-shaped or round lesion, hypoechoic (less enhancing) as compared to the surrounding parenchyma. In all of the patients, the presence, number, location and size of the lesions as well as unilateral or bilateral involvement were evaluated. Also, temporal patterns of enhancement (enhancement and washout during the different phases) were analysed. In follow-up explorations, the previous ones were reviewed, so as to assess the change in size, appearance and enhancement pattern of the lesions.
Results
According to CEUS findings, the lesions were classified into five groups (Table 1). All of the findings were unilateral. In those patients in whom different types of lesions existed, the patient was considered as belonging to the group with the most severe lesions.
Abscess
In 10 patients, 16 medium (2.5–4 cm) or large (4–6 cm) renal abscesses were detected. Their shape was rounded or geographical. In all of these lesions, there was absence of enhancement throughout the whole exploration, with rim enhancement in two lesions and enhancing thick septa in 3 lesions (Figs. 1, 2). CEUS detected five more lesions. CT was performed in seven patients with good correlation.
Focal pyelonephritis and abscess are shown in the different phases of CEUS. m, medullae; s sinus. Pyelonephritis areas are always most conspicuous during the parenchymal late phase, as round or wedge-shaped cortical or corticomedullar hypoechoic areas (shown in the drawing as grey areas). These areas may be seen also during the cortical phase, and are less conspicuous during parenchymal early phase. Abscesses are always seen as anechoic areas (shown in the drawing as black areas), with or without rim or septal enhancement, alone or within areas of pyelonephritis.
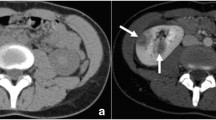
Renal abscess. 22 year old female transplant-recipient. A Grey-scale sonography of upper pole of transplanted kidney shows a heterogeneous round area of cortical thickening (black arrows) m, medullae; s sinus. It is impossible to distinguish focal pyelonephritis from abscess, and even from solid mass with this image. B Power Doppler sagittal view shows that this is a hypovascular area (arrowheads). C CEUS at 10 s shows abscess as an anechoic area with rim enhancement (black arrows). D CEUS follow-up 1 month later shows shrinkage of the lesion, without rim enhancement.
Focal pyelonephritis
In 14 patients, 22 areas suggestive of focal pyelonephritis were identified. These were wedge-shaped or rounded hypoechoic areas, located in the cortex or cortex and medullae with size ranging from 1.5 to 4 cm (Fig. 3). As for the enhancement temporal patterns, all of the lesions were best depicted during the late parenchymal phase, whereas the findings were variable in the rest of the exploration (Fig. 1). Most of them were initially hypoechoic (cortical or very early parenchymal phase), then isoechoic, to turn hypoechoic again in the late parenchymal phase. Some of the lesions were hypoechoic throughout the exploration, and some were only detected on the late parenchymal phase. Five new lesions were detected after CEUS.
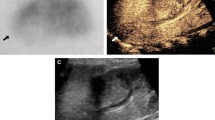
Focal pyelonephritis. A–C 17 year old female transplant-recipient. A Power Doppler shows a hypovascular cortical area (arrows). Underlying grey-scale image shows echogenic round area which abuts into the renal sinus. B CEUS image at 20 s shows focal pyelonephritis as a hypoechoic round area (white arrows). Slight inferior calix dilatation and urothelial thickening are depicted as well (black arrow). C 1 week later follow-up: the pyelonephritic focus has disappeared. D 21 year old female. CEUS 3m image depicts typical wedge hypoechoic cortical area in the upper pole of the right kidney (arrows).
Focal pyelonephritis with small abscess
In 14 patients, a combination of the two previously described enhancing patterns was seen. These lesions appeared as areas of focal pyelonephritis (with any of the aforementioned temporal patterns), but with areas of non-enhancement inside (n = 24), corresponding to small abscesses, most of them around 1 cm in size (range 0.8–3 cm) (Fig. 4). CEUS detected ten more lesions. Correlation with CT (eight cases) was good, but CT detected additional lesions in two patients. On the other hand, CEUS depicted better than CT very small abscesses (size around 1 cm).
Complicated focal pyelonephritis with small abscesses. 42 year old female with left nephrectomy and right ureteral obstructive lithiasis. A Grey-scale sonography. Transverse view of right kidney shows dilated calix and urothelial thickening (black arrow), and focal cortical thickening (white arrows), slightly hyperechoic in comparison to the rest of the cortex, slightly heterogeneous. B Power Doppler, transversal view. This area (white arrows) is hypovascular. C CEUS at 36 s, sagittal view, shows three small abscesses seen as anechoic foci (white arrows) within a slightly hypoechoic area of pyelonephritis (arrowheads). D CEUS at 2 min depicts more clearly the area of focal pyelonephritis as a hypoechoic area (white arrows) but the presence of the small abscesses is obscured.
Scar
In one patient, a scar related to previous pyelonephritis was the only finding. In another patient with focal pyelonephritis, there were scars related to previous APN. These were seen as focal thinning of the cortex, hypoechoic on CEUS.
Normal
In nine patients, CEUS showed normal enhancement, with no renal lesions. In all of these cases, no further imaging was performed (unless obstruction was present) and clinical resolution ensued.
Grey-scale ultrasound and power Doppler
Findings on basal grey-scale ultrasound and power Doppler and their correspondence to the CEUS diagnosis are shown in Table 2. The most common finding in complicated APN was a focal hyperechoic lesion, almost always with bulging of the cortex. This corresponded most frequently to focal pyelonephritis without abscess, but corresponded also to pyelonephritis with small abscess, and even to a medium size abscess (one case). Most medium or large abscesses were seen as heterogeneous or hypoechoic focal lesions with cortical bulging. All focal lesions appeared as hypovascular or avascular areas on power Doppler, but power Doppler was normal or non-evaluable in eight cases with focal pyelonephritis or focal pyelonephritis with abscess.
Other findings
Among those patients with parenchymal lesions on CEUS, pyonephrosis due to obstructive ureteral lithiasis was seen in two cases, one of them with a perirenal abscess. In five other cases, obstructive lithiasis was found.
In nine cases, parenchymal CEUS was normal. This excluded complicated pyelonephritis, but it did not exclude uncomplicated pyelonephritis. In two of these cases, the diagnosis was pyonephrosis; in another case acute diverticulitis was found.
Other findings included very frequently perirenal fluid and urothelial thickening and enhancement; and less commonly biliary bladder wall edema, pleural effusion and ascites.
Follow-up
Follow-up was performed in 25 patients. Resolution was very fast in focal pyelonephritis without abscess (days), without residual lesions. In all of the cases with medium size or large abscesses, the evolution was slow (more than a month), even though the patients were doing well clinically. The lesion gradually turned smaller and smaller, the rim enhancement disappeared, and ultimately ended in complete resolution or focal atrophic cortical lesions (scars) (three cases).The cases with small abscesses inside focal pyelonephritis had an intermediate evolution, with residual scarring in two cases.
Differential diagnosis
From an imaging standpoint, differential diagnoses included infarction, in two cases, and residual postpyelonephritic lesion (scar), in one case. One patient who had an abscess had previously had a lower pole infarction due to a critical stenosis in a lower pole segmental artery in the early posttransplantation period (Fig. 5). This was seen as non-enhancing areas within a large area of retarded and very diminished enhancement in the lower pole, with preservation of a cortical rim. In another patient who had multiple coalescent abscesses the possibility of multiple infarctions or septic emboli was considered because he was a drug abuser.
22 year old female transplant-recipient. Same patient as Fig. 2, some months earlier. m, medullae. A Grey-scale ultrasound. The lower pole is hypoechoic and heterogeneous (black arrows), and urothelial thickening (white arrow) is seen. B 12 s CEUS image shows segmental and interlobar arteries (arrowheads) and diminished and delayed enhancement of the lower pole (white arrows). C 18 s after the contrast injection, multiple very hypoechoic and anechoic wedge-shaped areas are seen which represent ischaemic areas with infarction (arrows). Note the presence of a cortical rim of enhancement (arrowheads), which is characteristic of infarction, due to preserved flow because of collateral cortical vessels. D Colour Doppler image shows aliasing in lower pole segmental artery with high velocity and spectral broadening that suggest stenosis of this artery. Distally, tardus parvus waves were seen (not shown).
Discussion
Imaging is necessary in complicated APN both to evaluate the presence and extent of renal and perirenal lesions, and to detect potentially treatable causes. Up till now, CT has been the preferred imaging technique to diagnose complications of acute pyelonephritis. CT is superior to grey-scale ultrasound and power Doppler [3] in detecting and characterizing APN lesions and has an excellent performance in identifying obstructive causes such as lithiasis or anatomic congenital alterations [2, 3]. But CT has associated an amount of radiation which is far from negligible (especially in this group of young patients, mostly women in bearing age), as well as potential contrast media nephrotoxicity. Mitterberger et al. [4] demonstrated CEUS and contrast-enhanced CT to be almost equally sensitive and specific for detecting renal parenchymal changes in APN. To date, there is very little written about CEUS in the evaluation of complicated pyelonephritis [5–8] and the EFSUMB 2008 guide does not include complicated APN as an indication for CEUS [9].To our knowledge, up till now the CEUS findings of APN have not been described in detail. This study describes typical CEUS enhancement features of the different parenchymal lesions, which make it possible to distinguish abscess from focal pyelonephritis, and to detect even very small abscesses within pyelonephritic areas.
Abscess enhancement findings in our study are concordant with CT findings (performed in eight patients with abscess) and coincident with those reported for CT, as expected [3]. An abscess is a necrotic cavity which contains pus and debris, so no enhancement is seen inside throughout all phases, or else enhancement of septa is seen if the abscess is partially liquefied. Peripheral rim enhancement in cortical phase may be seen. In our series, both rim enhancement and septal enhancement diminished during the parenchymal phase. These CEUS findings are similar to those described for hepatic abscesses [10], in which rim or septal enhancement diminishes during portal or late phase. In all of our cases CEUS clearly improved the performance of grey-scale sonography, since basal sonography of large abscesses was nonspecific in most of the cases. Just two of the cases showed lesions which were very suggestive of abscess on grey-scale US (hypoechoic with posterior sonic enhancement) and most of them were seen as heterogeneous lesions. Thus, basal sonography was not able to distinguish them from focal pyelonephritis or from other entities in most of the cases (Fig. 2).CEUS permitted assessment of the size, internal structure and extent of the abscesses, and detected five abscesses not detected by basal ultrasound.
Focal pyelonephritis appeared most frequently on grey-scale ultrasound as a hyperechoic focal lesion with cortical bulging. This represents the presence of numerous interfaces mostly due to the presence of abundant inflammatory cells in the interstitium and tubules [3, 11]. Besides the inflammatory infiltrate and edema, severe capillary damage has been demonstrated, with presence of intraluminal leukocyte and fibrin plugs [11].This explains the CEUS hypoechoic appearance of these lesions in comparison to the surrounding cortex, due to diminished enhancement. CEUS allows real-time assessment of renal perfusion, obtaining valuable temporal information (Fig. 1). Focal pyelonephritis areas are most conspicuous during the late parenchymal phase, because these areas (wedge or round-shaped) appear more hypoechoic during this phase. Even tiny lesions (smaller than 1 cm, may be detected). But care must be taken and all of the phases must be carefully analysed because during the late phase, if the pyelonephritis area is very hypoechoic, small abscesses may not be distinguished from the surrounding pyelonephritic area, or else the size of the abscesses may be overestimated (Fig. 4) Although it was not an objective of our study, we found a good correlation with CT findings (performed in seven cases with focal pyelonephritis); except in one case in which three lesions were missed by CEUS. This may be because some CT findings cannot be extrapolated to CEUS; such as the striated nephrogram or the delayed and persistent enhancement [12, 13]. Both of these signs occur because of underlying tubular obstruction; CEUS cannot detect them because US contrast is purely intravascular.
The CEUS temporal and morphologic features described above in complicated APN are quite typical, and usually differential diagnosis is not under consideration. Infarctions may be distinguished from focal APN because pyelonephritis has a typical temporal pattern of enhancement and always shows some degree of enhancement, whereas focal infarctions are seen as non-enhancing areas (although rarely there may be peripheral partially enhanced areas) (Fig. 5).Infarctions are usually wedge-shaped, whereas abscesses are round, geographical or coalescent. Also, infarctions usually do not produce cortical bulging, whereas large or medium size pyelonephritis, with or without abscess, usually does. Another distinctive feature is that infarctions may show a characteristic enhanced external border due to the presence of cortical collaterals [14]. In the long run, infarctions may produce scars indistinguishable from pyelonephritic scars. Pyelonephritis may repeat itself, especially in those cases with urologic anatomic alterations which cause reflux or obstruction, so pyelonephritic lesions may coexist with scars, as happened in one of our patients. Although scars are hypoechoic on CEUS, they are not difficult to distinguish from complicated APN, because they lack the characteristic temporal pattern and there is focal atrophy of the cortex. Other hypovascular lesions that can potentially mimic APN lesions in CT are renal lymphoma, lesions in autoimmune pancreatitis or Wegener granulomatosis, but there are scarce or no reports on CEUS findings in these entities [15–18].
Although it was not an aim of this study, CEUS proved to be very useful in the follow-up of these patients. During follow-up the radiological and the clinical course was longer in those patients with abscesses. Even though the course of small abscesses is usually benign, the clinical improvement is slower than when there is no abscess. The larger the abscess is, the longer the course of the disease, as well as the risk for kidney scarring. If parenchymal abscesses exist, a longer course of antibiotics or sequential therapy may be necessary [19]. Follow-up was done in all of the abscess patients, but not in all of the focal pyelonephritis patients, so it is possible that some scarring may have been missed.
An important factor to bear in mind is that US contrast media are not nephrotoxic and can be employed safely, even in patients with marginal renal function. Detection of complicated APN is of outmost importance in renal transplant recipients because it may be related with deterioration of graft function [8], but it is also very important to minimise the risk of nephrotoxicity. In these patients CEUS is very accurate due to the excellent visualization of the kidney.
Taking everything into consideration, it seems reasonable to choose CEUS first in complicated APN patients, and to perform CT in selected cases, so as to avoid radiation. In our institution, renal CEUS is now performed in the first place, with follow-up at 1 week and 1 month. Depending on the initial lesion (if there was abscess or not) and the evolution of the lesion, follow-up at 3 months and a year is done, so as to ascertain the complete resolution or the development of scars. CT or MR are performed if large renal abscesses or extrarenal collections are seen, because they perform better in the evaluation of perirenal or pararenal involvement; also if anatomic alterations are suspected. But even in these cases, follow-up is usually done with CEUS. Emphysematous pyelonephritis must always be evaluated and followed with CT [20]. DMSA scintigraphy remains the imaging method of choice in children [21], because US contrast agents are not approved for intravascular use in children.
Our study had some limitations. First of all, it is a retrospective study, so the analysis of clinical and imaging records had some inhomogeneity, and the follow-up schedule was not established from the beginning. In our study, the exploration throughout all phases was done on the symptomatic side and the contralateral kidney was examined for a short time during the early parenchymal phase. This makes it possible that small lesions (not abscesses) may have been unnoticed on the contralateral kidney, but we feel that the detection of these lesions would not have altered the patient management and evolution. Non-optimal kidney visualization due to patient body habitus or bowel gas is a potential CEUS limitation that was not frequent in this study, probably because of the young age and thin body habitus of most of the patients. Another limitation of this study is the absence of confirmation of the findings with CT in all patients. CT imaging is more panoramic and more sensitive than US in detecting calculi and underlying urinary tract anatomic abnormalities, so it is possible that some of these may have been unnoticed in those cases in which just CEUS was done.
Conclusions
Contrast-enhanced ultrasound is able to diagnose focal pyelonephritis and to distinguish focal pyelonephritis from abscess, with very typical findings. Because of absence of associated radiation and nephrotoxicity, CEUS should be considered the first imaging method for evaluating those patients suspected of complication in acute pyelonephritis. CT should be done when large abscess or extrarenal collections are seen, or when anatomic obstructive alterations or emphysematous pyelonephritis are suspected. The authors recommend CEUS as the method of choice for complicated APN follow-up.
References
Demertzis J, Menias CO (2007) State of the art: imaging of renal infections. Emerg Radiol 14:13–22
Stunell H, Buckley O, Feeney J, et al. (2007) Imaging of acute pyelonephritis in the adult. Eur Radiol 17:1820–1828
Craig W, Wagner B, Travis M (2008) Pyelonephritis: radiologic-pathologic review. RadioGraphics 28:255–276
Mitterberger M, Pinggera GM, Colleselli D, et al. (2007) Acute pyelonephritis: comparison of diagnosis with computed tomography and contrast enhanced ultrasonography. BJU Int 101:341–344
Quaia E (2007) Microbubble ultrasound contrast agents: an update. Eur Radiol 17:1995–2008
Setola SV, Catalano O, Sandomenico F, Siani A (2007) Contrast-enhanced sonography of the kidney. Abdom Imaging 32:21–28
Kim B, Lim HK, Choi MH, et al. (2001) Detection of parenchymal abnormalities in acute pyelonephritis by pulse inversion harmonic imaging with or without microbubble ultrasonographic contrast agent: correlation with computed tomography. J Ultrasound Med 20(1):5–14
Granata A, Andrulli S, Fiorini F, et al. (2011) Diagnosis of acute pyelonephritis by contrast-enhanced ultrasonography in kidney transplant patients. Nephrol Dial Transplant 26:715–720
Claudon M, Cosgrove D, Albrecht T, et al. (2008) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS)—update 2008. Ultraschall Med 29:28–44
Fontanilla T, Mendo M, Cañas T, et al. (2009) Diagnosis and differential diagnosis of liver abscesses using contrast-enhanced (SonoVue) ultrasonography. Radiología 51(4):403–410
Iványi B, Thoenes W (1987) Microvascular injury and repair in acute human bacterial pyelonephritis. Virchows Arch A 411(3):257–265
Kawashima A, Le Roy AJ (2003) Radiologic evaluation of patients with renal infections. Infect Dis Clin North Am 17:433–456
Kawashima A, Sandler CM, Goldman SM, Raval BK, Fishman EK (1997) CT of renal inflammatory disease. RadioGraphics 17:851–866
Bertolotto M, Martegani A, Aiani L, et al. (2008) Value of contrast-enhanced ultrasonography for detecting renal infarcts proven by contrast enhanced CT—a feasibility study. Eur Radiol 18(2):376–383
Takahashi N, Kawashima A, Fletcher JG, Chari ST (2007) Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology 242(3):791–801
Triantopoulou C, Malachias G, Maniatis P, et al. (2010) Renal lesions associated with autoimmune pancreatitis: CT findings. Acta Radiol 51:702–707
Ruiz E, Medina A, López G, et al. (2001) Multiple renal masses as initial manifestation of Wegener’s granulomatosis. AJR Am J Roentgenol 176:116–118
Urban BA, Fishman EK (2000) Renal lymphoma: CT patterns with emphasis on helical CT. RadioGraphics 20:197–212
Ramakrishnan K, Scheid D (2005) Diagnosis and management of acute pyelonephritis in adults. Am Fam Phys 71:5
Wan YL, Lee TY, Bullard MJ, Tsai CC (1996) Acute gas-producing bacterial renal infection: correlation between imaging findings and clinical outcome. Radiology 198:433–438
Boubakery A, Priory JO, Meuwly J-Y, Bischof-Delaloye A (2006) Radionuclide investigations of the urinary tract in the era of multimodality imaging. J Nucl Med 47:1819–1836
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fontanilla, T., Minaya, J., Cortés, C. et al. Acute complicated pyelonephritis: contrast-enhanced ultrasound. Abdom Imaging 37, 639–646 (2012). https://doi.org/10.1007/s00261-011-9781-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-011-9781-2