Abstract
3-Phenyllactic acid (PLA) is an antimicrobial compound with broad-spectrum activity against bacteria and fungi that could be widely used in the food industry and livestock feeds. Notably, d-PLA exhibits higher antibacterial activity, which gains more attention than l-PLA. In this report, the d-lactate dehydrogenase DLDH744 from Sporolactobacillus inulinus CASD was engineered to increase the enzymatic activities toward phenylpyruvate by protein structure-guided modeling analysis. The phenylpyruvate molecule was first docked in the active center of DLDH744. The residues that might tightly pack around the benzene ring of phenylpyruvate were all selected for mutation. The single site mutant M307L showed the highest increased activity toward bulkier substrate phenylpyruvate than the wild type. By using the engineered d-lactate dehydrogenase M307L expressed in Escherichia coli strains, without coexpression of the cofactor regeneration system, 21.43 g/L d-PLA was produced from phenylpyruvate with a productivity of 1.58 g/L/h in the fed-batch biotransformation process, which ranked in the list as the highest production titer of d-PLA by d-lactate dehydrogenase. The enantiomeric excess value of produced d-PLA in the broth was higher than 99.7 %. Additionally, the structure-guided design of this enzyme will also provide referential information for further engineering other 2-hydroxyacid dehydrogenases, which are useful for a wide range of fine chemical synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
3-Phenyllactic acid (PLA) is a broad-spectrum antimicrobial compound, which has been shown to inhibit the growth of a wide range of species, including yeasts and gram-negative and gram-positive bacteria (Lavermicocca et al. 2000, 2003; Schwenninger et al. 2008; Dieuleveux et al. 1998a; Ohhira et al. 2004). PLA has a low molecular mass and is hydrophilic, for which it diffuses into food and feed more easily (Dieuleveux et al. 1998a). Therefore, PLA has interesting potential as an antimicrobial agent in the food industry. The ability of PLA to act as a fungicide provides new perspectives for the possibility of using this natural antimicrobial compound to control fungal contaminants and extend the shelf life of food- and/or feedstuffs. Between the two optical isomers, d-PLA shows the higher antibacterial activity than l-PLA (Dieuleveux et al. 1998a). Furthermore, PLA is a promising building block for bio-based materials, as PLA can be polymerized into the biopolymer poly-PLA (Fujita et al. 2013). Unlike polylactic acid, poly-PLA exhibits high ultraviolet-absorbing properties due to the bulky aromatic side chain (Fujita et al. 2013).
Promising applications of PLA stimulated great endeavors in the development of synthesis strategies. PLA production has been reported in some studies with a variety of bacteria, including Lactobacillus, Leuconostoc, Bacillus, Weissella, Pediococcus, Aspergillus, and Geotrichum (Dieuleveux et al. 1998b; Valerio et al. 2004; Mu et al. 2009, 2012; Ndagano et al. 2011; Zheng et al. 2011). In the catabolism of lactic acid bacteria, phenylpyruvic acid (PPA) is converted to PLA by the lactate dehydrogenase (LDH). Therefore, several literatures reported the transformation of PPA to PLA by high expression of the native or site-mutated LDHs in Escherichia coli strains to enhance the productivity. However, to date, the production titer is not satisfactory, probably due to the low activity toward bulkier substrates such as PPA since normally the native activity of LDH toward PPA was much lower than that for pyruvate (Zheng et al. 2013; Li et al. 2014; Yu et al. 2014). DLDH744 is a novel d-lactate dehydrogenase recently identified from Sporolactobacillus inulinus. The complete tertiary structure revealed that the active center of DLDH744 is larger than that of the other reported LDHs (Zhu et al. 2015a). The enzyme was also confirmed to have d-phenyllactate dehydrogenase activity. And it is notable that the K m values for pyruvate and PPA were determined to be 3.4 ± 0.02 mM and 3.3 ± 0.08 mM, respectively, which indicates that DLDH744 have the same affinity for PPA with pyruvate (Zhu et al. 2015b). All the above results demonstrate that DLDH744 may have advantage in facilitating the production of d-PLA.
In this study, the DLDH744 was first engineered to increase the enzymatic activities toward PPA by protein structure-guided modeling analysis. The transformation process was fully optimized, and the high titer and optical purity of d-PLA were achieved. Thus, this study suggested a promising alternative for the production of chiral d-PLA from PPA.
Materials and methods
Bacterial strains and plasmids
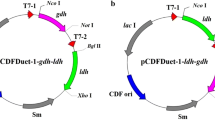
The gene of d-lactate dehydrogenase (DLDH744) from S. inulinus CASD was cloned from the genome (Yu et al. 2011). E. coli DH5α (Tiangen, Beijing, China) was used for gene cloning, and E. coli JM109 (DE3) (Dingguo, Beijing, China) was used for protein expression. Plasmids pMD 19-T (Takara, Dalian, China) and pET-28a (Novagen) were used as cloning and expression vectors, respectively.
Gene cloning, plasmid construction, protein expression, and purification
The cloning of DLDH744 gene and construction of recombinant plasmid pET-28a-DLDH744 were processed as described in our previous work (Zhu et al. 2015a). The recombinant plasmid was transformed into E. coli JM109 (DE3) for protein expression. The protein expression, purification, and desalination were performed as previous works (Zhu et al. 2015b). Protein concentration was determined by the Bradford method using bovine serum albumin as a standard (Bradford 1976).
Enzyme assays
The enzyme activity was assayed at 30 °C in 400 μl of 100 mM PBS (pH 5.5), 20 mM PPA or 10 mM pyruvate, 0.2 mM NADH, and the purified enzyme. The rate of NADH decrease was determined by measuring the absorbance change at 340 nm. One unit of enzyme activity was defined as the amount of enzyme that oxidates 1 μmol NADH per minute. The specific enzyme activity was measured as units per milligram of protein (Wang et al. 2014).
Molecular docking and site-directed mutagenesis
The molecular docking was performed based on the X-ray crystal structure of DLDH744 from S. inulinus CASD (PDB number 4XKJ) by using software AutoDock (version 4.2.6) according to the reference (Morris et al. 1998). Site-directed mutagenesis was performed using pET-28a-DLDH744 as the template. The nucleotide sequences of primers used for mutagenesis are shown in Table 1. The mutant plasmids was constructed using high-fidelity Q5 DNA polymerase (New England Biolabs, Beijing, China) (Urban et al. 1997). The resultant plasmids were digested with the restriction enzyme DpnI (Takara, Dalian, China) at 37 °C for 2 h. E. coli JM109 (DE3) cells were transformed with these plasmids, and the resulting strains were termed as R9L, Q51A, Y101L, N268L, F298Y, and M307L, respectively.
Effects of pH and glucose concentration on PLA yield
E. coli recombinant cells were cultured and induced as described above. The harvested induced cells were washed twice with 50 mM PBS (pH 7.0), then the cells were resuspended in 50 mM PBS containing 60 mM PPA at a final OD600 of 60 and incubated at 30 °C for 2 h. The effects of pH on PLA production was determined by comparing the PLA yield in bioconversion process at various pH (5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5). To determine the initial glucose effect on bioconversion yield, PLA production was processed in an initial glucose concentration range of 0–12.5 g/L.
Biotransformation of PPA to PLA by E. coli whole cells
The fed-batch bioconversion was conducted with PPA substrate feeding to avoid substrate inhibition. The bioconversion was initiated in 50 mM PBS (pH 7.5) at an OD600 of 80 containing 80 mM PPA and 5 g/L glucose at 30 °C; PPA was supplemented to maintain the initial concentration by one feeding. The fed-batch conversion was performed for 13.5 h. All experiments were performed in triplicate. The yield was calculated based on the produced PLA/added PPA (g/g, %).
Analytical methods
PLA and PPA were determined by using a high-performance liquid chromatography (HPLC) equipped with an Agilent Zorbax SB-C18 column (150 × 4.6 mm, 5 μm) and a UV detector at 210 nm. The mobile phase was 1 mM H2SO4 and acetonitrile with a ratio of 85:15 (v/v) at a flow rate of 0.7 ml/min at 30 °C. Glucose was measured with an SBA-40D glucose biosensor. d- or l-PLA was quantitatively measured by using HPLC with a chiral column (DAICEL CHIRALCEL OJ-RH) and a UV detector at 205 nm. The mobile phase was 0.1 % acetic acid and acetonitrile at a ratio of 90:10 (v/v), and the flow rate was 0.4 ml/min at 15 °C. The optical purity of PLA was defined as follows: d-PLA/(l-PLA + d-PLA) × 100 %.
Results
Modeling of phenylpyruvate in the active center of DLDH744
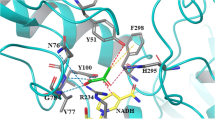
For engineering the DLDH744 to further increase the activity, the substrate PPA molecule was first modeled in the active center of DLDH744 (Fig. 1). The orientation of PPA corresponds to the orientation of pyruvate in the structure of d-lactate dehydrogenase from Aquifex aeolicus (Antonyuk et al. 2009) and is sterically possible. In the structural model, the PPA carboxylate group binds the side chain of Thr77 and Tyr101 and the main-chain nitrogen atoms of Ala78. The substrate PPA molecule is oriented face-on and directly adjacent to the nicotinamide ring of the coenzyme; thus, hydride ion transfer can easily occur during catalysis. In a previous report, His295 polarize the carbonyl group of pyruvate, making it prone to receiving or donating a hydride ion. This residue also acts as an acid/base group by donating (or abstracting) a proton to the pyruvate carbonyl group or the lactate hydroxyl group. In the structural model, His295 also forms a hydrogen bond with the carbonyl group of PPA to ensure the enzyme reaction can proceed well. This is also the first report to model the PPA molecule in the LDH structure.
Exploration of the key sites for improving LDH activity toward phenylpyruvate
A ternary structural model showed that a cluster of residues (Arg9, Gln51, Tyr101, Asn268, and Met307) with large and long side chains are tightly packed around the benzene ring of the modeled PPA molecule and led to larger steric hindrance (Fig. 2). Mutation of these residues to those residues with short side chains could reduce the steric hindrance around the C-3 group of PPA molecules and thus increase the enzyme activity toward PPA. Additionally, the amino acid sequence of DLDH744 was also aligned with that of a PPA reductase. The corresponding amino acid residue with Arg9 in the PPA reductase is Leu, which has a shorter side chain (Fujii et al. 2011). In order to improve the catalytic activity of DLDH744 toward PPA, residues Arg9, Tyr101, Asn268, and Met307 around the C-3 group were all selected to be mutated to Leu to facilitate the activity contrast. Gln51 was mutated to Ala (corresponding residue of Tyr52 in d-LDH from Lactobacillus pentosus) since it has been reported that the Ala replacement could increase the enzymatic activity toward PPA (Ishikura et al. 2005). Furthermore, according to the structure, residue Phe298 was located near the PPA C-3 group (Fig. 2). In our previous report, the main-chain nitrogen atom of Phe298 forms a hydrogen bond with the carbonyl group of nicotinamide ring, so Phe298 is essential for the coenzyme binding in DLDH744 (Zhu et al. 2015a). Interestingly, it is a Tyr residue in the equivalent position of d-HicDH, which was supposed to favor the binding of aromatic substrate (Tokuda et al. 2003). Therefore, the residue Phe298 in DLDH744 was also chosen to be mutated to Tyr to cover all the possibility.
Based on the above structural model, amino acids around the benzene ring of the substrate were chosen to be mutated. R9L, Q51A, Y101L, N268L, F298Y, and M307L mutants were constructed, and their enzyme activities toward pyruvate and PPA were determined (Fig. 3). Q51A, Y101F, and M307L mutants showed higher enzyme activity than wild-type DLDH744 toward PPA, especially the M307L mutant, which increased the original activity toward pyruvate more than 1.5 times. However, R9L, N268L, and F298Y mutants were less active than the wild type. Notably, the enzyme activities of these mutants toward pyruvate were also all lower than those of the wild type.
The kinetic parameters of the M307L mutant for pyruvate and PPA were further determined using NADH as the cofactor (Table 2). After mutation, the K m value for PPA was reduced, whereas that for pyruvate was increased. The V max with the M307L mutant using PPA as the substrate was increased to 7.15 U/mg. Moreover, the overall catalytic efficiency (k cat/K m) of the mutant was significantly increased. Then the production capacity of PLA by using M307L mutant was determined. Without optimization of the bioconversion conditions, E. coli JM109(DE3) harboring the wild-type enzyme DLDH744 could produce 1.11 ± 0.022 g/L PLA while strain E. coli JM109(DE3) harboring M307L mutant could produce 1.91 ± 0.037 g/L PLA under the same conditions. Since the native strain E. coli JM109(DE3) without introducing the enzyme DLDH744 only produced 0.133 ± 0.004 g/L PLA, it should be concluded that M307L mutant increased the production titer of PLA from PPA. Thereafter, the mutant M307L was used in the subsequent experiments for transforming PPA to PLA.
Optimization of bioconversion conditions
Since E. coli strain is susceptible to acidic stress, the initial pH of the medium should be an important factor that affects PLA production. Then the effects of pH on PLA production from PPA using resting cells of the recombinant E. coli strain with the mutant enzyme M307L were determined. PLA conversion yields at pH varying from 5.5 to 8.5 were examined. The high PLA yields were achieved in a broad range of pH from 7.0 to 8.5 while the highest PLA yield was obtained at pH 7.5 (Fig. 4).
Large-scale production of PLA from PPA using the enzymatic method requires large amounts of NADH and therefore has limited applications in the industry. In microbial conversion, NADH can be produced from low-cost cosubstrate glucose. Figure 5 shows the effect of glucose addition on whole-cell bioconversion. Addition of glucose at a low concentration of 2.5 g/L significantly improved the PLA production. PLA production titer reached a maximum value of 56 mM with 5 g/L glucose addition, and the yield was increased to 93.3 %.
Production of PLA by the whole cells by fed-batch biotransformation
To improve the d-PLA production, fed-batch process by feeding PPA during conversion was conducted under the above optimal conditions. d-PLA was rapidly accumulated with the decrease of PPA during the first 2.5 h, and PPA feeding was performed subsequently. Before feeding the substrate, the d-PLA accumulation reached 12.05 g/L from total PPA of 12.97 g/L at a volumetric productivity rate of 4.82 g/L/h, and the conversion ratio of PPA to d-PLA was 92.91 % (Fig. 6). With the feeding of the substrate PPA during the fed-batch conversion, the d-PLA concentration was continuously increasing. The highest PLA production reached 21.43 g/L after 13.5 h, and the final conversion ratio of PPA to d-PLA was 82.38 % (Fig. 6). We also tested the glucose addition with PPA feeding process, while there was no further positive effect on the production of PLA (data not shown). Considering substrate cost, only 5 g/L glucose was initially added. The volumetric productivity rate was 1.58 g/L/h, and the final optical purity of d-PLA reached a high value of 99.7 % (Fig. 7).
Discussion
PLA is an important class of organic acids with versatile applications. It is an ideal antimicrobial compound with broad-spectrum activity against both bacteria and fungi (Mu et al. 2012). Promising applications of PLA stimulated great endeavors in the development of its synthesis strategies. PLA has been reported to be produced by fermentation process. However, the chemical and enantioselective purities of PLA produced by fermentation are usually at moderate level and difficult to be purified (Mu et al. 2009). Asymmetric bioreduction of PPA by using dehydrogenases is regarded as one of the most promising solutions for the synthesis of PLA, especially optically pure PLA, due to its high efficiency, enantioselectivity, and easy operation (Zheng et al. 2013). Additionally, PPA is a feasible precursor for the large-scale production of PLA because it can be synthesized easily from hydantoin at a low cost. Many studies regarding engineering target enzymes have been reported while almost all the researches engineered the site of Tyr52 of d-LDH from L. pentosus. Tokuda et al. (2003) converted L. pentosus d-LDH into a d-hydroxyisocaproate dehydrogenase by replacing Tyr52 with a shorter Leu residue. Ishikura et al. (2005) enhanced the activity toward PPA of L. pentosus d-LDH through replacement of Tyr52 to Val and Ala. Zhu et al. (2015c) recently also replaced the Tyr52 residue of d-LDH from L. pentosus with small hydrophobic residues to enhance the PLA synthesis activity. Tyr52 and Phe299 of L. pentosus d-LDH were both selected for site-directed mutagenesis to abate the steric exclusion effect while only the Y52L mutant exhibited the best performance (Zheng et al. 2013). The above reports demonstrated the feasibility of improving d-LDH activity toward PPA by site-directed mutagenesis. Here, the d-lactate dehydrogenase DLDH744 from S. inulinus was selected as a starting target for improving the transformation capacity for PLA production, due to its high affinity for PPA which is different with all the previously reported LDHs. DLDH744 is much phylogenetically closer to d-hydroxyisocaproate dehydrogenase family, indicating DLDH744 might also have a larger space in the active center which might accept the larger substrates more efficiently (Zhu et al. 2015b).
To fully identify the key sites which might be critical for improving the activity toward PPA, the PPA molecule was first docked in the active center of DLDH744. The residues that might tightly pack around the benzene ring of PPA from the structure-guided analysis were all selected for mutation. Replacement of Gln51, Tyr101, and Met307 residues with small residues obviously increased the enzyme activity toward PPA, which may be due to the further excessive space around the C-3 group of substrate after mutation and, therefore, reduced the steric hindrance of substrate. However, the enzyme activities of Q51A/Y101F, Q51A/M307L, and Y101F/M307L double mutants as well as Q51A/Y101L/M307L triple mutant were also examined, while no double or triple mutant gives better activity toward PPA than the wild type (data not shown). Some studies have reported that LDHs changed the catalytic domain from the open to the closed conformation when it is catalyzing (Razeto et al. 2002). It implied that tight coupling between substrate and the enzyme was essential to the enzyme activity. This could also explain that, with the increase of activities toward PPA, the LDH activities toward pyruvate were reduced after mutating to the small residues. After mutation, excessive space around the C-3 group of substrate reduced steric hindrance of PPA but also decreased the closeness between pyruvate and the enzyme. So, the catalytic efficiency for PPA was increased, while the catalytic efficiency for pyruvate was decreased accordingly.
Furthermore, d-LDH is an NADH-dependent reductase, and the cofactor NADH was required during the substrate reduction. Yu et al. (2014) reported that exogenous addition of NADH restored the PLA production. While for the production of optically pure d-PLA with a large scale, the application of enzyme method involving the use of a d-LDH is limited by the high cost of cofactor (NADH) regeneration. In the whole-cell bioconversion system, NADH regeneration could be obtained by simple addition of much cheaper glucose (Zheng et al. 2011), which is vital to the industrial application to minimize the costs. The effect of glucose on PLA production was investigated, and the results indicated that only addition of 5 g/L glucose could significantly increase the yield. Besides the advantage in the coenzyme utilization, the whole-cell biotransformation has some superiority in reducing the influence of microbiological contamination and high concentration of PLA. Hence, DLDH744 mutant M307L were transformed into E. coli JM109 (DE3) strain, and a production titer of d-PLA of 21.4 g/L was achieved without coexpression of the cofactor regeneration system, which ranked in the list as the highest production titer of d-PLA by engineered d-LDH (Table 3). A high titer (37.3 g/L) of racemic PLA (the main isomer is l-PLA) produced from PPA by the mutant of Bacillus coagulans, with a very high concentration of 126 g/L glucose consumption during the fermentation, limited the economic feasibility of this process (Zheng et al. 2011). During the preparation of this manuscript, Xu et al. (2015) reported a novel phenylpyruvate reductase (LaPPR, d-3-phosphoglycerate dehydrogenase subfamily) from Lactobacillus sp. strain CGMCC 9967. By construction, the coexpression system with glucose dehydrogenase, as much as 100 g/L PPA, was asymmetrically reduced into d-PLA with a 91.3 % yield by addition of 1.5 equiv. glucose of PPA. The results suggest that LaPPR is also a promising biocatalyst for the synthesis of d-PLA. In our study, only 5 g/L glucose was required in the whole fed-batch process, which was the least consumption in comparison with other reports. This is probably due to predominant characteristic of DLDH744 that can use both NADH and NADPH as the coenzyme at equal efficiency (Zhu et al. 2015a).
In summary, the enzymatic activity of DLDH744 toward substrates with large aromatic group at C-3 was successfully improved by structured-guided mutation. Moreover, due to the high yield and high stereoselectivity of DLDH744 mutant M307L, the whole-cell catalysis system containing DLDH744 mutant was successfully applied to the direct synthesis of d-PLA. Thus, the process developed in this study could also be used as a promising alternative for the production of highly, optically pure α-hydroxy carboxylic acid.
References
Antonyuk SV, Strange RW, Ellis MJ, Bessho Y, Kuramitsu S, Inoue Y, Yokoyama S, Hasnain SS (2009) Structure of D-lactate dehydrogenase from Aquifex aeolicus complexed with NAD+ and lactic acid (or pyruvate). Acta Crystallogr Sect F: Struct Biol Cryst Commun 65:1209–1213
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Dieuleveux V, van der Pyl D, Chataud J, Gueguen M (1998a) Purification and characterization of anti-Listeria compounds produced by Geotrichum candidum. Appl Environ Microbiol 64:800–803
Dieuleveux V, Lemarinier S, Gueguen M (1998b) Antimicrobial spectrum and target site of D-3-phenyllactic acid. Int J Food Microbiol 40:177–183
Fujii T, Shimizu M, Doi Y, Fujita T, Ito T, Miura D, Wariishi H, Takaya N (2011) Novel fungal phenylpyruvate reductase belongs to d-isomer-specific 2-hydroxyacid dehydrogenase family. Biochim Biophys Acta 1814:1669–1676
Fujita T, Nguyen HD, Ito T, Zhou S, Osada L, Tateyama S, Kaneko T, Takaya N (2013) Microbial monomers custom-synthesized to build true bio-derived aromatic polymers. Appl Microbiol Biotechnol 97:8887–8894
Ishikura Y, Tsuzuki S, Takahashi O, Tokuda C, Nakanishi R, Shinoda T, Taguchi H (2005) Recognition site for the side chain of 2-ketoacid substrate in D-lactate dehydrogenase. J Biochem 138:741–749
Lavermicocca P, Valerio F, Evidente A, Lazzaroni S, Corsetti A, Gobbetti M (2000) Purification and characterization of novel antifungal compounds from the sourdough Lactobacillus plantarum strain 21B. Appl Environ Microbiol 66:4084–4090
Lavermicocca P, Valerio F, Visconti A (2003) Antifungal activity of phenyllactic acid against molds isolated from bakery products. Appl Environ Microbiol 69:634–640
Li X, Jiang B, Pan B (2007) Biotransformation of phenylpyruvic acid to phenyllactic acid by growing and resting cells of a Lactobacillus sp. Biotechnol Lett 29:593–597
Li L, Shin SY, Lee K, Han N (2014) Production of natural antimicrobial compound D-phenyllactic acid using Leuconostoc mesenteroides ATCC 8293 whole cells involving highly active D-lactate dehydrogenase. Lett Appl Microbiol 59:404–411
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Mu W, Chen C, Li X, Zhang T, Jiang B (2009) Optimization of culture medium for the production of phenyllactic acid by Lactobacillus sp. SK007. Bioresour Technol 100:1366–1370
Mu W, Yu S, Zhu L, Zhang T, Jiang B (2012) Recent research on 3-phenyllactic acid, a broad-spectrum antimicrobial compound. Appl Microbiol Biotechnol 95:1155–1163
Ndagano D, Lamoureux T, Dortu C, Vandermoten S, Thonart P (2011) Antifungal activity of lactic acid bacteria of the Weissella genus isolated from food. J Food Sci 76:M305–311
Ohhira I, Kuwaki S, Morita H, Suzuki T, Tomita S, Hisamatsu S, Sonoki S, Shinoda S (2004) Identification of 3-phenyllactic acid as a possible antibacterial substance produced by Enterococcus faecalis TH10. Biocontrol Sci 9:77–81
Razeto A, Kochhar S, Hottinger H, Dauter M, Wilson KS, Lamzin VS (2002) Domain closure, substrate specificity and catalysis of D-lactate dehydrogenase from Lactobacillus bulgaricus. J Mol Biol 318:109–119
Schwenninger SM, Lacroix C, Truttmann S, Jans C, Spörndli C, Bigler L, Meile L (2008) Characterization of low-molecular-weight antiyeast metabolites produced by a food-protective Lactobacillus-Propionibacterium coculture. J Food Protect 71:2481–2487
Tokuda C, Ishikura Y, Shigematsu M, Mutoh H, Tsuzuki S, Nakahira Y, Tamura Y, Shinoda T, Arai K, Takahashi O, Taguchi H (2003) Conversion of Lactobacillus pentosus D-lactate dehydrogenase to a D-hydroxyisocaproate dehydrogenase through a single amino acid replacement. J Bacteriol 185:5023–5026
Urban A, Neukirchen S, Jaeger KE (1997) A rapid and efficient method for site-directed mutagenesis using one-step overlap extension PCR. Nucleic Acids Res 25:2227–2228
Valerio F, Lavermicocca P, Pascale M, Visconti A (2004) Production of phenyllactic acid by lactic acid bacteria: an approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol Lett 233:289–295
Wang LM, Cai YM, Zhu LF, Guo HL, Yu B (2014) Major role of NAD-dependent lactate dehydrogenases in high optically pure L-lactic acid production by thermophilic Bacillus coagulans. Appl Environ Microbiol 80:7134–7141
Xu GC, Zhang LL, Ni Y (2015) Enzymatic preparation of D-phenyllactic acid at high space-time yield with a novel phenylpyruvate reductase identified from Lactobacillus sp. CGMCC 9967. J Biotechnol S0168–1656:30210–30218
Yu B, Su F, Wang L, Xu K, Zhao B, Xu P (2011) Draft genome sequence of Sporolactobacillus inulinus strain CASD, an efficient D-lactic acid-producing bacterium with high-concentration lactate tolerance capability. J Bacteriol 193:5864–5865
Yu S, Zhu L, Zhou C, An T, Jiang B, Mu W (2014) Enzymatic production of D-3-phenyllactic acid by Pediococcus pentosaceus D-lactate dehydrogenase with NADH regeneration by Ogataea parapolymorpha formate dehydrogenase. Biotechnol Lett 36:627–631
Yu S, Zhou C, Zhang T, Jiang B, Mu W (2015) Short communication: 3-phenyllactic acid production in milk by Pediococcus pentosaceus SK25 during laboratory fermentation process. J Dairy Sci 98:813–817
Zheng Z, Ma C, Gao C, Li F, Qin J, Zhang H, Wang K, Xu P (2011) Efficient conversion of phenylpyruvic acid to phenyllactic acid by using whole cells of Bacillus coagulans SDM. PLoS One 6:e19030
Zheng Z, Sheng B, Gao C, Zhang H, Qin T, Ma C, Xu P (2013) Highly stereoselective biosynthesis of (R)-α-hydroxy carboxylic acids through rationally re-designed mutation of D-lactate dehydrogenase. Sci Rep 3:3401
Zhu LF, Xu XL, Wang LM, Dong H, Yu B, Ma YH (2015a) NADP+-preferring D-lactate dehydrogenase from Sporolactobacillus inulinus. Appl Environ Microbiol 81:6294–6301
Zhu LF, Xu XL, Wang LM, Dong H, Yu B (2015b) The D-lactate dehydrogenase from Sporolactobacillus inulinus also possessing reversible deamination activity. PLoS One 10:e0139066
Zhu Y, Hu F, Zhu Y, Wang L, Qi B (2015c) Enhancement of phenyllactic acid biosynthesis by recognition site replacement of D-lactate dehydrogenase from Lactobacillus pentosus. Biotechnol Lett 37:1233–1241
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (31270108). BY is supported by the Youth Innovation Promotion Association, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Min Wang and Lingfeng Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, M., Zhu, L., Xu, X. et al. Efficient production of enantiomerically pure d-phenyllactate from phenylpyruvate by structure-guided design of an engineered d-lactate dehydrogenase. Appl Microbiol Biotechnol 100, 7471–7478 (2016). https://doi.org/10.1007/s00253-016-7456-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7456-1