Abstract
In the last decade, attention to extreme environments has increased because of interests to isolate previously unknown extremophilic microorganisms in pure culture and to profile their metabolites. Microorganisms that live in extreme environments produce extremozymes and extremolytes that have the potential to be valuable resources for the development of a bio-based economy through their application to white, red, and grey biotechnologies. Here, we provide an overview of extremophile ecology, and we review the most recent applications of microbial extremophiles and the extremozymes and extremolytes they produce to biotechnology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biocatalysts are whole microbial cells or enzymes that can be used in biochemical reactions of modern biotechnology. Some of these reactions have optimized or even replaced existing processes (Wohlgemuth, 2010; Resch et al., 2011). Interest in biocatalysts has recently increased with the growth and development of biotechnology as a strategy towards attaining a bio-based economy. White (or industrial) biotechnology aims to resolve environmental and economic concerns associated with increasing energy and fuel demands and subsequent prices of petroleum-based products. It uses biocatalysts to convert renewable resources, such as wastes and byproducts, into fine chemicals, biopolymers, biomaterials, and biofuels. Grey (or environmental) biotechnology applies biocatalysts to bioremediate contaminated sites, while red (or medical/pharmaceutical) biotechnology exploits microorganisms to produce pharmaceuticals. To date, the majority of enzymes on the market are of bacterial or fungal origin, while few are derived from archaea, most of which have been produced by mesophilic microorganisms which are often inhibited under the extreme conditions of many industrial processes. Thus, the search for new sources of isolation, experimental procedures, and analytical methods is recently growing to identify robust biocatalysts. Specifically, extremophiles are receiving increasing attention; several have been obtained in pure culture, their genomes analyzed, and their enzymes characterized by either academic or industrial laboratories (Cárdenas et al., 2010; López-López et al 2014; Yildiz et al., 2015).
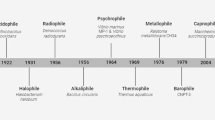
Extremophilic microorganisms thrive in the harsh environments where other organisms cannot even survive. Extremophiles are taxonomically widely distributed and are a functionally diverse group (Cowan et al., 2015) that includes thermophiles, psychrophiles, acidophiles, alkalophiles, halophiles, barophiles/piezophiles, metalophiles, and radiophiles. Extremophiles have the potential to produce biomolecules of high relevance for white, grey, and red biotechnological sectors. These microorganisms produce extremophilic enzymes (extremozymes) and protective organic biomolecules (extremolytes) that convey characteristics for survival in extreme environmental conditions. Here, we present an overview of the potential applications of these microorganisms and their products in biotechnology. We briefly describe the ecology of extremophilic prokaryotes and review the most recent reports on the application of extremozymes and extremolytes derived from extremophilic and extremotolerant microorganisms in various biotechnological processes.
Ecology and classification of extremophiles
Extremophiles are organisms that have adapted to thrive in ecological niches that are uninhabitable to others, for example, deep-sea hydrothermal vents, hot springs, solfataric fields, soda lakes, inland saline systems, solar salterns, hot and cold deserts, environments highly contaminated with nuclear waste or heavy metals, as well as lithic or rock environments. Psychrophiles are extremophiles that are adapted to extreme cold, and halophiles describe those that thrive in the presence of high salt concentrations; each type of microorganism uses different survival strategies to be successful in their environment (Oren 2013; De Maayer et al., 2014). Psychrophilic prokaryotes are widespread among bacteria and archaea and can be found within the genera Alteromonas, Halobacterium, Shewanella, Psychrobacter, Pseudoalteromonas, Arthrobacter, Colwellia, Gelidibacter, Marinobacter, Psychroflexus, Pseudomonas, Methanolobus, and Methanococcoides (De Maayer et al., 2014). In addition to adaptations for acidic environments, acidophiles are also typically adapted to environments with high temperatures, high salinity, or heavy metal concentrations because these conditions often co-occur, for example, in areas of acid drainage (Cárdenas et al., 2010; Navarro et al., 2013; Dopson and Holmes, 2014). Meanwhile, alkalophiles thrive in alkaline environments such as gypsum-based soils or soda lakes and are often halophiles. They encompass bacteria from different genera including among others Bacillus, Halomonas, and Pseudomonas (Sarethy et al., 2011) as well as archaea belonging to the genera Halalkalicoccus, Halobiforma, Halorubrum, Natrialba, Natronococcus, and Natronorubrum (Bowers and Wiegel, 2011). Deep-sea and deep subsurface environments host piezophiles (barophiles), a group of extremophiles that produce compatible solutes and polyunsaturated fatty acids and form multimeric and antioxidant proteins that enable them to survive under extremely high hydrostatic pressures (Kawamoto et al., 2011; Zhang et al., 2015). Most piezophiles are psychrophilic Gram-negative bacterial species that belong to the genera Shewanella, Psychromonas, Photobacterium, Colwellia, Thioprofundum, and Moritella, but some are archaea derived and can be found among the genera Thermococcus, Sulfolobus, and Pyrococcus (Zhang et al., 2015). Adaptation to high concentrations of heavy metals (otherwise essential as trace elements) allows metalophiles to thrive in metal-polluted sites (Johnson, 2014; Orell et al., 2013). Metalophiles are also acidophiles and include both bacteria from the genera Acidithiobacillus, Leptospirillum, Alicyclobacillus, Acidiphilium, Acidimicrobium, Ferrimicrobium, and Sulfobacillus and archaea from the genera Ferroplasma, Acidiplasma, Sulfolobus, Metallosphaera, and Acidianus (Johnson, 2014; Dopson and Holmes, 2014). In environments of high oxidative stress and radiation (UV, gamma, and X-rays), radiophiles thrive because of their ability to repair extensive DNA damage. Radiophiles are found among various microbial groups and species including bacteria from the genera Deinococcus, Bacillus, Rubrobacter, and Kineococcus, and the family Geodermatophilaceae and cyanobacteria including the genera Nostoc and Chroococcidiopsis (Brim et al., 2003; Gtari et al., 2012; Bagwell et al., 2008; Gabani and Singh 2013).
Potential applications of extremophilic/extremotolerant biocatalysts
Owing to their unique enzymatic features and physiological properties, the potential biotechnological applications of whole-cell extremophilic biocatalysts range from the bioremediation of toxic pollutants from water and/or sediments to the production of biomolecules for medical and industrial purposes. Because of their adaptation to high concentrations of heavy metals, metalophiles/acidophiles are currently being used for bioremediation and biomining (Navarro et al., 2013; Johnson 2014; Orell et al., 2013), while radiophiles are suited for application in the management of nuclear waste-polluted environments (Brim et al., 2003; Appukuttan et al., 2006). Applications can also be envisaged in agriculture where desert bacterial extremophiles that are able to cope with low water activity conditions can be used to improve the management of water by plants under drought stress (Marasco et al., 2012; Rolli et al., 2015).
In addition to entire microbial cells, extremozymes are enzymes that have developed molecular mechanisms (Hough and Danson 1999) of adaptation to extreme physico-chemical conditions that have relevant applications as biocatalysts in industrial biotransformation processes. Enzymes produced by psychrophiles have been shown to display high catalytic efficiency in the detergent and food industries and for the production of fine chemicals (Cavicchioli et al., 2011). Karan et al. (2013) reported on the purification and characterization of β-galactosidase from the cold-adapted haloarchaeon Halorubrum lacusprofundi. This enzyme was overexpressed in the model haloarchaeon, Halobacterium sp. NRC-1, and was shown to be active in high-salinity environments (with maximal activity in either 4 M NaCl or KCl) across a wide temperature range (−5 to 60 °C). Its functionality is conserved in the presence of 10–20 % (v/v) organic solvents, including methanol, ethanol, n-butanol, and isoamyl alcohol, suggesting its suitability for the synthesis of oligosaccharides under low water activity and cold temperatures.
The industrial potential for halophilic enzymes resides in their ability to be active and stable under low water activity and, in many cases, also in the presence of organic solvents (Raddadi et al., 2013; Datta et al., 2010). Examples of these extremozymes include polysaccharide-hydrolyzing enzymes of high relevance for the hydrolysis of cellulose, xylan, and starch (Raddadi et al., 2013; Bhalla et al., 2013; Du et al., 2013; Elleuche et al., 2014). For example, the extremotolerant cellulases produced by Paenibacillus tarimensis L88, an isolate obtained from the Sahara Desert in southern Tunisia, have been shown to have high functionality across a broad pH range (3.0 to 10.5), at high temperatures (80 °C) and high salt concentrations (up to 5-M NaCl) (Raddadi et al., 2013). Carboxymethyl cellulase activity has been detected in the presence of 40 % (v/v) 1-butyl-3-methylimidazolium chloride or 20 % (w/v) 1-ethyl-3-methylimidazolium acetate ionic liquids and was maintained after exposure to organic solvents, detergents, heavy metals, and even under high alkalinity. Paenibacillus tarimensis is an optimal candidate for the production of cellulases with promising applications in detergent, textile, and pulp and paper industries; it also has potential for simultaneous ionic liquid treatment and saccharification of lignocellulose in biorefinery processes (Raddadi et al., 2013). Some halophilic enzymes are lipolytic, such as lipases and esterases, such that they have the ability to hydrolyze long-chain acylglycerols (≥C10) and short-chain fatty esters (≤C10), respectively. These enzymes have a wide range of applications including the production of polyunsaturated fatty acids in the food industry or of biodiesel (Litchfield, 2011; Schreck and Grunden, 2014). For example, lipase from the halophilic bacterium Idiomarina sp. was shown to be highly active under a variety of harsh conditions including in the presence of organic solvents and high salt concentrations. Its application for biodiesel production from Jatropha oil in free or immobilized forms resulted in 80 and 91 % yields, respectively (Li et al., 2014).
Bacterial alkalophiles are mainly exploited for the production of enzymes that are widely applied in the detergent and laundry industries (Sarethy et al., 2011). Although the biotechnological potential of piezophiles is still poorly explored (Abe and Horikoshi, 2001; Mota et al., 2013; Lamosa et al., 2013), they may be valuable to the food industry in processes that require high pressures (Zhang et al., 2015). Moreover, piezophilic bacteria could be a source of essential fatty acids like, for example, omega-3-polyunsaturated fatty acids since these compounds are produced by the bacteria to stabilize the cell membrane under high pressure (Zhang et al., 2015).
Enzymes produced by radiotolerant microorganisms have been shown to be resistant to other stresses. For example, Shao et al. (2013) characterized lipases from the radiation-tolerant bacterium Deinococcus radiodurans expressed in Escherichia coli. Purified enzymes showed preference for short-chain esters, three of which were thermostable and retained their activities in the presence of surfactants and organic solvents.
Thermozymes are extremozymes produced by thermophilic and hyperthermophilic microorganisms. These enzymes are also often able to tolerate proteolysis and harsh conditions like the presence of denaturing agents and organic solvents as well as high salinity. Benefits of using thermozymes include reduced risk of contamination, lower viscosity, and higher solubility of substrates. Toplak et al. (2013) identified a gene coding for a subtilase termed proteolysin in the Gram-positive, anaerobic, thermophilic bacterium Coprothermobacter proteolyticus. By functionally expressing the gene into E. coli, the enzyme could be purified and identified as highly thermostable in the presence of organic solvents and detergents with a high level of activity across a wide pH range at high temperatures (up to 80 °C), making it a suitable candidate for application to thermophilic organic solid waste degradation (Toplak et al., 2013). This subtilase is a member of the serine protease family produced by Bacillus strains including the largest group of commercial proteolytic enzymes extensively used in food, textile, detergent, pharmaceutical, and leather industries. In addition, a thermostable nucleoside phosphorylase has been characterized from hyperthermophilic aerobic crenarchaeon Aeropyrum pernix K1 and has been used for the synthesis of nucleoside analogues used in antiviral therapies as an alternative to chemical synthesis (Zhu et al., 2013). Other thermozymes also include proteases like thermolysin used in the synthesis of dipeptides, pretaq protease used to cleanup DNA prior to PCR amplification, and starch-processing and DNA-processing enzymes (Bruins et al., 2001; Jayakumar et al., 2012).
In addition to the abovementioned extremozymes, other enzymes are also suitable for use in further industrial processes. For example, alcohol dehydrogenases can be used to synthesize building blocks for the chemical industry, such as optically active alcohols, or to synthesize cofactors such as NAD and NADP. Meanwhile, nitrile-degrading enzymes are of interest for the transformation of nitriles and carbon-carbon bond forming enzymes like aldolases, transketolases and hydroxynitrile lyases are useful in organic synthesis (Resch et al., 2011 Chen et al., 2009; Egorova and Antranikian, 2005; Demirjian et al., 2001).
Extremolytes and their biotechnological applications
Extremolytes are organic compounds that can constitute up to 25 % of dry cell weight accumulated in microorganisms exposed to stressful environmental conditions. Examples of extremolytes include several compounds of polyol derivatives (ectoine, hydroxyectoine, and betaine), carbohydrates such as trehalose and the mannose derivatives (mannosylglycerate [firoin] and mannosylglyceramide [firoin-A]), glucosylglucosylglycerate, glucosylglycerate (GG), and various amino acids (Borges et al., 2002; Lentzen and Schwarz, 2006; Singh and Gabani, 2011; Empadinhas and da Costa, 2011; Esteves et al., 2014, Alarico et al., 2013; Lamosa et al., 2013; Bougouffa et al., 2014). Several archaea accumulate negatively charged derivatives of inositol and glycerol such as phosphodiesters di-myoinositol-1,1′-phosphate and α-diglycerol phosphate or cyclic 2,3-diphosphoglycerate and trianionic pyrophosphate (Lentzen and Schwarz, 2006; Esteves et al., 2014). Several UV radiation-protective compounds have been isolated from UV-resistant extremophilic bacteria, for example, scytonemin, mycosporin-like amino-acids (MAAs), ectoines, bacterioruberin, and melanin (Singh et al., 2010; Gabani and Singh 2013; Rastogi and Incharoensakdi, 2014).
Extremolytes have primarily been used in cosmetics and have the potential for application to the pharmaceutical sector. The behavior of MAAs in the presence of UV radiation make them useful in UV-protective sunscreens in the cosmetics industry, and their potential application as preventative agents of UV radiation-induced cancers such as melanoma has also been suggested (de la Coba et al., 2009). In the future, MAA compounds may directly be implicated as therapeutic candidates. Scytonemin, a component in sunscreens (Soule et al., 2009), has also been suggested as a potential candidate for the development of a novel pharmacophore to produce protein kinase inhibitors such as antiproliferative and anti-inflammatory drugs (Singh and Gabani, 2011). The bacterioruberin produced by radioresistant microbes (Halobacterium and Rubrobacter) has been suggested to have application in preventing human skin cancer because it participates in repairing damaged DNA strands caused by ionizing UV radiation (Singh and Gabani, 2011). Choi et al. (2014) reported that the deinoxanthin isolated from the radioresistant bacterium D. radiodurans induced apoptosis of cancer cells, suggesting that this carotenoid could potentially be useful as a chemopreventive agent.
Extremolytes can also be used to stabilize macromolecules such as proteins and nucleic acids. Protein instability is a central challenge for administering therapeutic protein-based medicines, particularly in aqueous formulations. Owing to their ability to stabilize proteins in vivo and in vitro, extremolytes offer an attractive solution for the stabilization and storage of sensitive proteins in the absence of other protein stabilizers (Avanti et al., 2014). Moreover, extremolytes can inhibit protein misfolding and/or aggregation and, hence, are interesting candidates for the development of drugs for several diseases (Ryu et al., 2008; Faria et al., 2013; Kanapathipillai et al., 2005). For example, ectoine is currently used in skin care products (Pastor et al., 2010) and firoin and ectoine have recently been shown to reduce signal-dependent events resulting from exposure to carbon nanoparticles in vitro and in vivo, widening the fields of application for these compatible solutes. Such events include indeed the activation of mitogen-activated protein kinases or the upregulation of pro-inflammatory cytokines, apoptosis, and proliferation in lung epithelial cells, which could lead to lung cancer, chronic obstructive pulmonary disease, and fibrosis (Autengruber et al 2014). Furthermore, extremolytes have the potential for application in the food industry for the production of functional foods, food products that have an added positive health benefit by enhancing short-term well-being/performance ability or by the long-term mitigation of certain diseases (Cencic and Chingwaru, 2010). For example, in some cheeses that have been treated with Brevibacterium linens for surface ripening of the product, ectoine has been reported to accumulate (up to 89 mg/100 g of product) (Klein et al, 2007). Investigating whether ectoine accumulates in other fermented food products would be worthwhile towards evidencing extremolytes as functional food ingredients.
Concluding remarks and perspectives
Extremophilic/extremotolerant microbes have the potential to make a great impact on biotechnology via the compounds they produce (i.e., extremozymes and extremolytes) that enable them to thrive in harsh environments. The economic potential of extremozymes is considerable for their application to agriculture, food and beverages and feed, pharmaceutical, detergent, textile, leather, pulp and paper, and biomining industries. Although only a few extremozymes are currently being produced and used at the industrial level, the development of new industrial processes based on these enzymes is motivated by important results obtained in the field of extremophile research, the increasing demand of biotech industries for novel biocatalysts, and the rapid progress of new omics techniques such as metagenomics, proteomics, metabolomic gene-directed evolution, and gene/genome shuffling (Egorova and Antranikian, 2005; Ferrer et al., 2007). For example, extremozymes have been identified in metagenomes as overcoming bottlenecks related to the uncultivability of extremophiles in some cases (Ferrer et al., 2007; López-López et al 2014). To date, extremolytes have primarily been used in pharmaceutical and cosmetic sectors. At the industrial level, ectoine and its derivatives are produced using the “bacterial milking” process (Pastor et al., 2010), and research initiatives directed at developing additional strategies to improve the productivity of other compatible solutes are underway. For example, genetic engineering and encapsulation of glucosylglycerate (GG)-producing cyanobacteria in gels that aim to concentrate and secrete extremolytes into the extracellular environment have been performed (Tan et al., 2015). The authors report successful growth of Synechocystis and improved production and secretion of GG in the encapsulating gels after salt stress.
In addition to extremozymes and extremolytes, other metabolites, including exopolysaccharides (Raveendran et al., 2015), biosurfactants, biopolymers, and peptides, from extremophilic/extremotolerant microorganisms have great economic-industrial potential. For example, in agriculture, biosurfactants could substitute chemical surfactants as adjuvants in herbicide and pesticide formulations, enhance bioremediation of soils, or be applied to the biocontrol of phytopathogens owing to their antimicrobial activity and stimulation of plant defense (Sachdev and Cameotra, 2013). Moreover, biosurfactants could improve arid-zone soil structure and quality due to hydrophilization of soils, which improves wettability, and finally to reduced water infiltration. Subsequently, sustainable agriculture could be expanded in arid conditions. Radiophiles produce compounds with the potential for use as radioprotective drugs; however, because only a few studies of these microbes have been performed, their exploitation remains limited. Moreover, challenges associated with the specific nutritional needs and growing conditions of extremophiles have made their isolation and maintenance difficult; isolation of purified extremolytes is among the limiting factors in developing these compounds for therapeutic purposes.
In conclusion, extremophilic/extremotolerant microorganisms are sustainable resources that could be better exploited in several biotechnological sectors towards the development of a bio-based economy.
References
Abe F, Horikoshi K (2001) The biotechnological potential of piezophiles. Trends Biotechnol 19:102–108
Alarico S, Empadinhas N, da Costa MS (2013) A new bacterial hydrolase specific for the compatible solutes α-D-mannopyranosyl-(1→2)-D-glycerate and α-D-glucopyranosyl-(1→2)-D-glycerate. Enzym Microb Technol 52:77–83
Appukuttan D, Rao AS, Apte SK (2006) Engineering of Deinococcus radiodurans R1 for bioprecipitation of uranium from dilute nuclear waste. Appl Environ Microbiol 72:7873–7878
Autengruber A, Sydlik U, Kroker M, Hornstein T, Ale-Agha N, Stöckmann D, Bilstein A, Albrecht C, Paunel-Görgülü A, Suschek CV, Krutmann J, Unfried K (2014) Signalling-dependent adverse health effects of carbon nanoparticles are prevented by the compatible solute mannosylglycerate (firoin) in vitro and in vivo. PLoS One 9:e111485
Avanti C, Saluja V, van Streun ELP, Frijlink HW, Hinrichs WLJ (2014) Stability of lysozyme in aqueous extremolyte solutions during heat shock and accelerated thermal conditions. PLoS One 9:e86244
Bagwell CE, Bhat S, Hawkins GM, Smith BW, Biswas T, Hoover TR, Saunders E, Han CS, Tsodikov OV, Shimkets LJ (2008) Survival in nuclear waste, extreme resistance, and potential applications gleaned from the genome sequence of Kineococcus radiotolerans SRS30216. PLoS One 3:e3878
Bhalla A, Bansal N, Kumar S, Bischoff KM, Sani RK (2013) Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour Technol 128:751–759
Borges N, Ramos A, Raven ND, Sharp RJ, Santos H (2002) Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 6:209–216
Bougouffa S, Radovanovic A, Essack M, Bajic VB (2014) DEOP: a database on osmoprotectants and associated pathways. Database (Oxford). doi:10.1093/database/bau100
Bowers KJ, Wiegel J (2011) Temperature and pH optima of extremely halophilic archaea: a mini-review. Extremophiles 15(2):119–128
Brim H, Venkateswaran A, Kostandarithes HM, Fredrickson JK, Daly MJ (2003) Engineering Deinococcus geothermalis for bioremediation of high-temperature radioactive waste environments. Appl Environ Microbiol 69:4575–4582
Bruins ME, Janssen AE, Boom RM (2001) Thermozymes and their applications: a review of recent literature and patents. Appl Biochem Biotechnol 90:155–186
Cárdenas JP, Valdés J, Quatrini R, Duarte F, Holmes DS (2010) Lessons from the genomes of extremely acidophilic bacteria and archaea with special emphasis on bioleaching microorganisms. Appl Microbiol Biotechnol 88:605–620
Cavicchioli R, Charlton T, Ertan H, Omar SM, Siddiqui KS, Williams TJ (2011) Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol 4:449–460
Cencic A, Chingwaru W (2010) The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2:611–625
Chen J, Zheng RC, Zheng YG, Shen YC (2009) Microbial transformation of nitriles to high-value acids or amides. Adv Biochem Eng Biotechnol 113:33–77
Choi YJ, Hur JM, Lim S, Jo M, Kim DH, Choi JI (2014) Induction of apoptosis by deinoxanthin in human cancer cells. Anticancer Res 34:1829–1835
Cowan DA, Ramond JB, Makhalanyane TP, De Maayer P (2015) Metagenomics of extreme environments. Curr Opin Microbiol 25:97–102
Datta S, Holmes B, Park J, Chen Z, Dibble D, Hadi M, Blanch H, Simmons B, Sapra R (2010) Ionic liquid tolerant hyperthermophilic cellulases for biomass pretreatment and hydrolysis. Green Chem 12:338–345
de la Coba F, Aguilera J, de Galvez MV, Alvarez M, Gallego E, Figueroa FL, Herrera E (2009) Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical applications of algal UV-absorbing compounds. J Dermatol Sci 55:161–169
De Maayer P, Anderson D, Cary C, Cowan DA (2014) Some like it cold: understanding the survival strategies of psychrophiles. EMBO Rep 15:508–517
Demirjian DC, Morís-Varas F, Cassidy CS (2001) Enzymes from extremophiles. Curr Opin Chem Biol 5:144–151
Dopson M, Holmes DS (2014) Metal resistance in acidophilic microorganisms and its significance for biotechnologies. Appl Microbiol Biotechnol 98:8133–8144
Du Y, Shi P, Huang H, Zhang X, Luo H, Wang Y, Yao B (2013) Characterization of three novel thermophilic xylanases from Humicola insolens Y1 with application potentials in the brewing industry. Bioresour Technol 130:161–167
Egorova K, Antranikian G (2005) Industrial relevance of thermophilic Archaea. Curr Opin Microbiol 8:649–655
Elleuche S, Schröder C, Sahm K, Antranikian G (2014) Extremozymes—biocatalysts with unique properties from extremophilic microorganisms. Curr Opin Biotechnol 29:116–123
Empadinhas N, da Costa MS (2011) Diversity, biological roles and biosynthetic pathways for sugar-glycerate containing compatible solutes in bacteria and archaea. Environ Microbiol 13:2056–2077
Esteves AM, Chandrayan SK, McTernan PM, Borges N, Adams MW, Santos H (2014) Mannosylglycerate and di-myo-inositol phosphate have interchangeable roles during adaptation of Pyrococcus furiosus to heat stress. Appl Environ Microbiol 80:4226–4233
Faria C, Jorge CD, Borges N, Tenreiro S, Outeiro TF, Santos H (2013) Inhibition of formation of α-synuclein inclusions by mannosylglycerate in a yeast model of Parkinson's disease. Biochim Biophys Acta 1830:4065–4072
Ferrer M, Golyshina O, Beloqui A, Golyshin PN (2007) Mining enzymes from extreme environments. Curr Opin Microbiol 10:207–214
Gabani P, Singh OV (2013) Radiation-resistant extremophiles and their potential in biotechnology and therapeutics. Appl Microbiol Biotechnol 97:993–1004
Gtari M, Essoussi I, Maaoui R, Sghaier H, Boujmil R, Gury J, Pujic P, Brusetti L, Chouaia B, Crotti E, Daffonchio D, Boudabous A, Normand P (2012) Contrasted resistance of stone-dwelling Geodermatophilaceae species to stresses known to give rise to reactive oxygen species. FEMS Microbiol Ecol 80:566–577
Hough DW, Danson MJ (1999) Extremozymes. Curr Opin Chem Biol 3:39–46
Jayakumar R, Jayashree S, Annapurna B, Seshadri S (2012) Characterization of thermostable serine alkaline protease from an alkaliphilic strain Bacillus pumilus MCAS8 and its applications. Appl Biochem Biotechnol 168:1849–1866
Johnson DB (2014) Biomining-biotechnologies for extracting and recovering metals from ores and waste materials. Curr Opin Biotechnol 30:24–31
Kanapathipillai M, Lentzen G, Sierks M, Park CB (2005) Ectoine and hydroxyectoine inhibit aggregation and neurotoxicity of Alzheimer’s beta-amyloid. FEBS Lett 579:4775–4780
Karan R, Capes MD, DasSarma P, DasSarma S (2013) Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. BMC Biotechnol 13:3
Kawamoto J, Sato T, Nakasone K, Kato C, Mihara H, Esaki N, Kurihara T (2011) Favourable effects of eicosapentaenoic acid on the late step of the cell division in a piezophilic bacterium, Shewanella violacea DSS12, at high-hydrostatic pressures. Environ Microbiol 13:2293–2298
Klein J, Schwarz T, Lentzen G (2007) Ectoine as a natural component of food: detection in red smear cheeses. J Dairy Res 74:446–451
Lamosa P, Rodrigues MV, Gonçalves LG, Carr J, Ventura R, Maycock C, Raven ND, Santos H (2013) Organic solutes in the deepest phylogenetic branches of the bacteria: identification of α(1-6)glucosyl-α(1-2)glucosylglycerate in Persephonella marina. Extremophiles 17:137–146
Lentzen G, Schwarz T (2006) Extremolytes: natural compounds from extremophiles for versatile applications. Appl Microbiol Biotechnol 72:623–634
Li X, Qian P, Wu SG, Yu HY (2014) Characterization of an organic solvent-tolerant lipase from Idiomarina sp. W33 and its application for biodiesel production using Jatropha oil. Extremophiles 18:171–178
Litchfield CD (2011) Potential for industrial products from the halophilic Archaea. J Ind Microbiol Biotechnol 38:1635–1647
López-López O, Cerdán ME, Gonzalez-Siso MI (2014) New extremophilic lipases and esterases from metagenomics. Curr Protein Pept Sci 15:445–455
Marasco R, Rolli E, Ettoumi B, Vigani G, Mapelli F, Borin S, Abou-Hadid AF, El-Behairy UA, Sorlini C, Cherif A, Zocchi G, Daffonchio D (2012) A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS One 7:e48479
Mota MJ, Lopes RP, Delgadillo I, Saraiva JA (2013) Microorganisms under high pressure—adaptation, growth and biotechnological potential. Biotechnol Adv 31:1426–1234
Navarro CA, von Bernath D, Jerez CA (2013) Heavy metal resistance strategies of acidophilic bacteria and their acquisition: importance for biomining and bioremediation. Biol Res 46:363–371
Orell A, Remonsellez F, Arancibia R, Jerez CA (2013) Molecular characterization of copper and cadmium resistance determinants in the biomining thermoacidophilic archaeon Sulfolobus metallicus. Archaea 2013:289236
Oren A (2013) Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front Microbiol 4:315
Pastor JM, Salvador M, Argandoña M, Bernal V, Reina-Bueno M, Csonka LN, Iborra JL, Vargas C, Nieto JJ, Cánovas M (2010) Ectoines in cell stress protection: uses and biotechnological production. Biotechnol Adv 28:782–801
Raddadi N, Cherif A, Daffonchio D, Fava F (2013) Halo-alkalitolerant and thermostable cellulases with improved tolerance to ionic liquids and organic solvents from Paenibacillus tarimensis isolated from the Chott El Fejej, Sahara desert, Tunisia. Bioresour Technol 150:121–128
Rastogi RP, Incharoensakdi A (2014) Characterization of UV-screening compounds, mycosporine-like amino acids, and scytonemin in the cyanobacterium Lyngbya sp. CU2555. FEMS Microbiol Ecol 87:244–256
Raveendran S, Palaninathan V, Nagaoka Y, Fukuda T, Iwai S, Higashi T, Mizuki T, Sakamoto Y, Mohanan PV, Maekawa T, Kumar DS (2015) Extremophilic polysaccharide nanoparticles for cancer nanotherapy and evaluation of antioxidant properties. Int J Biol Macromol 76:310–319
Resch V, Schrittwieser JH, Siirola E, Kroutil W (2011) Novel carbon-carbon bond formations for biocatalysis. Curr Opin Biotechnol 22:793–799
Rolli E, Marasco M, Vigani G, Ettoumi B, Mapelli F, Deangelis ML, Gandolfi C, Casati E, Previtali F, Gerbino R, Pierotti Cei F, Borin S, Sorlini C, Zocchi G, Daffonchio D (2015) Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ Microbiol 17:316–331
Ryu J, Kanapathipillai M, Lentzen G, Park CB (2008) Inhibition of beta-amyloid peptide aggregation and neurotoxicity by alpha-d-mannosylglycerate, a natural extremolyte. Peptides 29:578–584
Sachdev DP, Cameotra SS (2013) Biosurfactants in agriculture. Appl Microbiol Biotechnol 97:1005–1016
Sarethy IP, Saxena Y, Kapoor A, Sharma M, Sharma SK, Gupta V, Gupta S (2011) Alkaliphilic bacteria: applications in industrial biotechnology. J Ind Microbiol Biotechnol 38:769–790
Schreck SD, Grunden AM (2014) Biotechnological applications of halophilic lipases and thioesterases. Appl Microbiol Biotechnol 98:1011–1021
Shao H, Xu L, Yan Y (2013) Thermostable lipases from extremely radioresistant bacterium Deinococcus radiodurans: cloning, expression, and biochemical characterization. J Basic Microbiol 54:984–995
Singh OV, Gabani P (2011) Extremophiles: radiation resistance microbial reserves and therapeutic implications. J Appl Microbiol 110:851–861
Singh SP, Klisch M, Sinha RP, Hader DP (2010) Genome mining of mycosporine-like amino acid (MAA) synthesizing and non-synthesizing cyanobacteria, a bioinformatics study. Genomics 95:120–128
Soule T, Garcia-Pichel F, Stout V (2009) Gene expression patterns associated with the biosynthesis of the sunscreen scytonemin in Nostoc punctiforme ATCC 29133 in response to UVA radiation. J Bacteriol 191:4639–4646
Tan X, Du W, Lu X (2015) Photosynthetic and extracellular production of glucosylglycerol by genetically engineered and gel-encapsulated cyanobacteria. Appl Microbiol Biotechnol 99:2147–2154
Toplak A, Wu B, Fusetti F, Quaedflieg PJ, Janssen DB (2013) Proteolysin, a novel highly thermostable and cosolvent-compatible protease from the thermophilic bacterium Coprothermobacter proteolyticus. Appl Environ Microbiol 79:5625–5632
Wohlgemuth R (2010) Biocatalysis: key to sustainable industrial chemistry. Curr Opin Biotechnol 21:713–724
Yildiz SY, Radchenkova N, Arga KY, Kambourova M, Toksoy Oner E (2015) Genomic analysis of Brevibacillus thermoruber 423 reveals its biotechnological and industrial potential. Appl Microbiol Biotechnol 99:2277–2289
Zhang Y, Li X, Bartlett DH, Xiao X (2015) Current developments in marine microbiology: high-pressure biotechnology and the genetic engineering of piezophiles. Curr Opin Biotechnol 33:157–164
Zhu S, Song D, Gong C, Tang P, Li X, Wang J, Zheng G (2013) Biosynthesis of nucleoside analogues via thermostable nucleoside phosphorylase. Appl Microbiol Biotechnol 97:6769–6778
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
This review article has been prepared following principles of ethical and professional conduct.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raddadi, N., Cherif, A., Daffonchio, D. et al. Biotechnological applications of extremophiles, extremozymes and extremolytes. Appl Microbiol Biotechnol 99, 7907–7913 (2015). https://doi.org/10.1007/s00253-015-6874-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6874-9